Abstract
It is well known that alongside senescence there is a gradual decline in cognitive ability, most noticeably certain kinds of memory such as working, episodic, spatial, and long term memory. However, until recently, not much has been known regarding the specific mechanisms responsible for the decline in cognitive ability with age. Over the past decades, researchers have become more interested in cAMP signaling, and its downstream transcription factor cAMP response element binding protein (CREB) in the context of senescence. However, there is still a lack of understanding on what ultimately causes the cognitive deficits observed with senescence. This review will focus on the changes in intracellular signaling in the brain, more specifically, alterations in cAMP/CREB signaling in aging. In addition, the downstream effects of altered cAMP signaling on cognitive ability with age will be further discussed. Overall, understanding the senescent-related changes that occur in cAMP/CREB signaling could be important for the development of novel drug targets for both healthy aging, and pathological aging such as Alzheimer's disease.
Keywords: Aging, Memory, Camp, CREB, Phosphodiesterase, Protein kinase A
1. Introduction
Aging, also known as senescence, can be defined according to the Dorland Medical Dictionary as “the process of growing old, especially the condition resulting from the transitions and accumulations of the deleterious aging processes.” The knowledge of the deleterious effects of senescence is not a recent or novel discovery; in fact, it has been known for thousands of years that certain biological processes become less efficient with increasing age while other processes remain intact (Berchtold and Cotman, 1998; Roman, 1999).
In particular, memory decline has been one of the most noted and observed changes correlated with increased biological age. However, different types of memory are affected to varying degrees, and not all aged individuals are affected (Rowe et al., 1998; Perls, 2006). Among the forms of memory, most commonly affected in aged individuals are working memory, episodic memory, spatial memory, and long term memory (LTM). The other forms of memory more commonly spared from the aging process include short term memory (STM), procedural memory, and semantic memory (Light, 1992). While STM and working memory are similar in that they both involve memory over a limited period of time, working memory differs from STM in that it requires the active manipulation of information being held in attention, like baking a cake or doing arithmetic. In contrast, STM is the simple maintenance of that information over a short period of time, such as remembering a phone number.
Perhaps the largest and arguably most studied form of senescent-related memory decline is that of spatial memory. Spatial memory decline is a phylogenetically conserved trait and has been observed in invertebrates (Münch et al., 2010), rodents (Barnes et al., 1980; Wyss et al., 2000), non-human primates (Lacreuse et al., 2005), and humans (Bruce and Herman, 1983; Moffat et al., 2001). Spatial memory, or the memory niche responsible for remembering one's environments and surroundings, is primarily managed by the hippocampus of the mammalian brain (Scoville and Milner, 1958), and indeed the hippocampal volume has been shown to be decreased with age (Convit et al., 1995; Mu et al., 1999; Driscoll et al., 2003). Because of this, the hippocampus has been the focus of many extensive studies involving senescent-related decline in spatial memory (Lister and Barnes, 2009), and will be a major area of focus in this review.
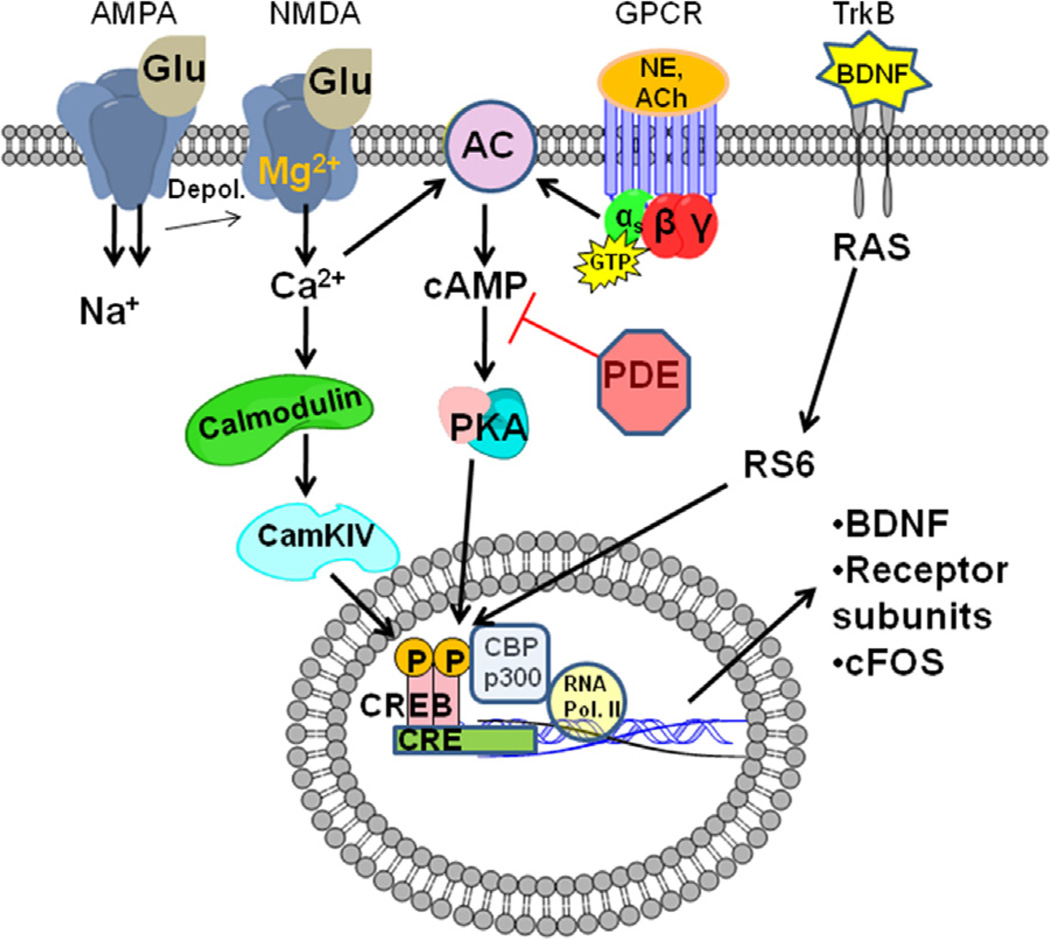
An additional current “hot spot” of memory research involves the protein CREB. There are two main subtypes of CREB (CREB1 and CREB2), and three different isoforms of CREB1 that are produced by alternative splicing (α, β, and δ). Of interest for this review is CREB1, which will be referred to hereafter simply as CREB. CREB is a transcription factor (Montminy MR, 1987) whose function in “learning” was first discovered in studies of Aplysia (Dash et al., 1990), and has now been extensively implicated in learning and memory (Bourtchuladze et al., 1994), long term potentiation (LTP) (Impey et al., 1996), and neuro-protection (Ao et al., 2006). Briefly, CREB is a ~46 kDa size protein residing in the nucleus that is capable of being activated by multiple pathways including cAMP/protein kinase A (PKA)-mediated signaling, Ca2+/CamKII/IV, or the MAPK/ERK pathway (Fig. 1). Upon activation, CREB is phosphorylated (pCREB) at its serine 133 residue, dimerizes with another pCREB, binds its cAMP response element (CRE) site on the DNA via a leucine zipper domain, and then recruits several co-activator proteins to assist with transcription. Among the co-activator proteins recruited are CREB binding protein (CBP), p300, and RNA polymerase II (Pol II). CBP and p300 both contain histone acetyl-transferase (HAT) ability, which relaxes the chromatin structure from histones, making it more readily available for transcription by Pol II (Johannessen et al., 2004; Carlezon et al., 2005). In regards to CREB kinetics, it is important to note that CREB may already be dimerized and bound to CRE sites under basal conditions, and simply becomes activated during phosphorylation. This is a controversial area and still needs further clarification, however, it is most likely a gray area with tissue-specific kinetics (Cha-molstad et al., 2004).
Fig. 1.
Simplified schematic of the cAMP/CREB signaling pathway. Multiple pathways are capable of converging to phosphorylate CREB and initiate gene transcription. For standard G-protein coupled receptor signaling (GPCR), binding of a ligand such as norepinephrine (NE) or acetylcholine (ACh) to its receptor activates the α-subunit, which then in turn activates adenylyl cyclase (AC). AC catalyzes the formation of cAMP from ATP in an energy dependent manner. Cyclic AMP activates protein kinase A (PKA) by binding to the regulatory subunits and relieving auto-inhibition. Once activated, the catalytic units of PKA are free to phosphorylate cAMP response-element binding-protein (CREB) in the nucleus. Once phosphorylated CREB binds cAMP response element (CRE) promoter sites on the DNA. CREB then recruits several co-activator proteins (CBP, p300) that have histone-acetyl-transferase activity which loosen the DNA, allowing RNA polymerase II to initiate gene transcription for products such as brain-derived neurotrophic-factor (BDNF) and certain receptor subunits. Phosphodiesterases (PDEs) hydrolyze cAMP and thus attenuate the effects of cAMP downstream. Glutamatergic signaling is also capable of activating the cAMP pathway through AMPA and NMDA receptors. Once the NMDA receptor is activated by AMPA induced depolarization, binding of glutamate, and expulsion of the magnesium ion, calcium flow through the NMDA receptor is capable of activating certain AC isoforms sensitive to calcium (I, III, VIII) which results in subsequent phosphorylation of CREB. In addition, CREB can also experience glutamatergic- and calcium-mediated phosphorylation via the Calmodulin-CamKIV pathway. Lastly, CREB is also capable of being phosphorylated via the mitogen-activated protein-kinase (MAPK) pathway which can be activated by binding of a trophic factor such as BDNF to its receptor tyrosine kinase b (TrkB).
Abbreviations : Glutamate (Glu), 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid (AMPA), N-methyl-D-aspartate (NMDA) receptor, depolarization (depol.), rat-sarcoma protein (RAS), ribosomal s6 kinase (RS6), RNA polymerase II (RNA Pol II), CREB binding protein (CBP).
There are also several proteins worth mentioning that are capable of modifying pCREB activity. The phosphatases PP1 and PP2A are both involved with negative regulation of pCREB, and have been shown to terminate its signaling (Wadzinski et al., 1993; Bito et al., 1996). In addition, the inducible cAMP early repressor (ICER), which is derived from the cAMP-responsive element modulator (CREM) gene via an alternative intrinsic promoter, is capable of inhibiting CREB activity by binding to the CRE domain. ICER is able to do this without activation of transcription because it lacks the activation and kinase inducible domains present on CREB, thus ICER competes with CREB for the CRE sites and acts as a negative regulator. Interestingly, the alternative intrinsic promoter for ICER contains a CRE site, and ICER transcription itself is driven by pCREB activation. Thus CREB is able to succinctly regulate its own activity by driving transcription of its repressors (Lonze and Ginty, 2002; Borlikova and Endo, 2009).
The CREB transcriptome has currently been identified at approximately 1663 CRE targets in humans and 1349 possible binding sites in mice, though the actual functional transcriptome differs drastically depending on the tissue and cell type (Conkright et al., 2003). Although the transcriptome of CREB may be vast, for the scope of this review we only focus on a few downstream targets such as brain derived neurotrophic factor (BDNF), c-fos, and Arc. BDNF in particular is a very potent neurotrophic molecule capable of eliciting strong effects on synaptic activity, memory, and neuronal growth; it can even elicit seizures if expressed at high enough levels in the brain (Croll et al., 1999). CREB is also highly expressed in the hippocampus, and for this reason makes it an ideal target to study when investigating the senescent brain.
Additionally, it is important to note the current views on neuronal loss in the aging brain. It used to be believed that the aging brain suffered immense neuronal loss, which caused the apparent decrease in volume seen in most aged brains, and the subsequent cognitive deficits. However, recent research shows this is actually not the case. In the majority of aged brains, the neuronal number remains intact, but neuronal density, the somatic size, and the total number of synapses are now generally believed to be reduced (Peters et al., 1998). However, certain sub-regions of the hippocampus have been shown to have no change in the synapse number in the aged brain, suggesting that the underlying cause for cognitive-related decline may be functional in nature in certain brain regions (Geinisman et al., 2004).
Recently, there has been an increase of research into cAMP/CREB signaling in the aging brain. However, there is still a lack of understanding of what ultimately causes the cognitive deficits observed with senescence. This review will focus on the changes in one intracellular signaling pathway in the aging brain, more specifically, alterations in the cAMP/ CREB pathway. The potential effects of altered cAMP/CREB signaling will then be correlated with various niches of hippocampal senescent research to attempt to answer the ultimate question; what causes senescent-related cognitive decline? Overall, understanding the underlying mechanisms is of particular importance to the identification of potential targets for treatment of cognitive deficits associated with not only healthy senescence, but dementia as well.
2. Senescent-related changes in cAMP levels and cAMP modulators
2.1. Cyclic AMP levels
Studies to date regarding senescent-related alterations in cAMP levels are not entirely consistent, and still need further investigation to accurately determine the changes that occur. However, the majority of research seems to agree that cAMP levels tend to decrease with age. It was first shown in the aged rat cerebral cortex that cAMP levels are highest around 2 months of age at 26 pmol/mg and then gradually decrease with senescence, reaching levels as low as 2 pmol/mg (Zimmerman and Berg, 1974). In a brief study looking at levels of cAMP in blood lymphocytes from aged individuals, it was found that both basal and trypsin-activated levels of cAMP were significantly lower in the aged cells (Birkenfeld and Ben-Zvi, 1984). In addition, Austin and colleagues have shown that cAMP levels in the aged cerebellum are 14% lower than young controls (Austin et al., 1978). This is consistent with another study, in which cAMP levels were found to be decreased in the gerbil cerebral cortex at 21 months of age compared to younger controls (Hara et al., 1992). However, controversial results also have been observed. For instance, cAMP levels were found to be higher in the striatum of aged rats (Sugawa and May, 1994). Also, though the scope of this review is focused on the cAMP signaling pathway, cGMP levels were also found to be drastically reduced in the hippocampus and cerebellum in rats after 12 months of age (Vallebuona and Raiteri, 1995). It is important to note that the alterations in cAMP levels could be site-specific, and should not be generalized to the rest of the brain or body. Due to the lack of investigation, there needs to be much more research done into the changes that occur with cAMP levels during senescence.
There are multiple factors that could contribute to a dysregulation of cAMP levels, some of which will be described in the sections below. A change in cAMP levels could be observed via changes in hormone or neurotransmitter levels, neurotransmitter receptor levels, adenylyl cyclase (AC) activity/expression, G protein activity/expression, or phosphodiesterase (PDE) activity/expression.
2.2. Neurotransmitters
Altered neurotransmitter levels could account for one of the mechanisms behind senescent induced alterations in cAMP levels. Indeed it has been shown that dopamine and norepinephrine levels are decreased in aged rats (Austin et al., 1978), acetylcholine decreased in age accelerated mice (Tomobe et al., 2007), and glutamate decreased in humans (Kaiser et al., 2005). Fluctuations of neurotransmitter levels with age would certainly result in a dysregulation of cAMP levels. However, due to the incredible diversity of G-protein-coupled receptor (GPCR) subtypes and their differing regulation of cAMP, a simplistic global increase or decrease in cAMP levels in the brain may not be realistic. For example, dopamine D1-like family receptors are linked to Gαs, which activates AC, while D2-like family receptors are linked to Gαi, which inhibits AC. These receptors are expressed in varying degrees in different brain regions and subsequently the effects of altered neurotransmitters on cAMP levels would be different in different regions (Weiner et al., 1991). In addition, AC has multiple isoforms that are activated and inhibited by different conditions. For example, AC isoforms V and VI, which are strongly expressed in the hippocampus, are actually inhibited by Ca2+/Calmodulin, and thus could be hypothesized to be less inhibited with decreased levels of glutamatergic signaling (Visel et al., 2006).
2.3. Phosphodiesterases
PDE activity has also been examined in the brains of aged subjects, and once again the results are contradictory. In one study, Stancheva and colleagues found a significant rise in activity of certain high Km PDEs in the cortex, striatum, hypothalamus, and hippocampus of aged (22 month old) rats (Stancheva and Alova, 1991). This rise in PDE activity should lead to increases in cAMP hydrolysis, and subsequent decreases in cAMP levels. It is important to note though that the activity of low Km PDEs were unchanged. An age-dependent increase in PDE4A expression has also been observed in primary cultures of cortical neurons (Hajjhussein et al., 2007). In contrast, in another study using PET imaging of conscious monkeys, PDE4 activity was found to be reduced in the aged monkey brain, but was less responsive to treatment with the D1 receptor agonist SCH23390, which decreased PDE4 activity to a greater extent in the striatum and frontal cortex of young monkeys (Harada et al., 2002). PDE4 activity was also shown to be decreased in the brains of aged rats, however interestingly enough this finding was not observed in the hippocampus (Tohda et al., 1996). Other studies have shown no age-related change in PDE expression (Ramos et al., 2003). While this review is focused mainly on the changes that occur in normal aging, it is interesting to note that the amyloid-beta found in Alzheimer's disease is capable of upregulating PDE4B, leading to increased production of TNF-α by microglial cells (Sebastiani et al., 2006). This makes it difficult to ignore the possible role that inflammation and PDEs may play in senescence and cognitive decline.
Due to the extensive complexity of PDE families, subtypes, splice variants, and their differential tissue expression, it becomes incredibly difficult to simplify or predict the changes that may occur in PDE with aging. In addition, PDEs are notorious for undergoing elaborate compensatory regulation (O'Donnell and Xu, 2012). While one may predict that PDE expression would be increased due to lower observed cAMP levels, it is very possible PDE expression could in fact be decreased as a compensatory mechanism in order to balance and restore lowered cAMP levels. Because of the complicated nature and compensatory mechanisms involved with PDEs, as well as a lack of overall investigation, much more research is needed in this area to correctly assess the role that PDEs play in normal, aging-induced memory loss.
2.4. Adenylyl cyclase and G-proteins
Arguably, one of the most direct manipulators of cAMP levels in the cell is AC. Indeed, the question of whether AC activity changes with senescence is an important issue, and has been examined in several studies. In one study looking at aged Fischer rat myocardium, it was found that both basal and stimulated AC activity was reduced by 30% (O'Connor et al., 1981). Senescent rabbits also showed decreases in AC activity in the anterior limbic cortex, frontal cortex, hypothalamus, and striatum (Makman et al., 1980). Other studies have also demonstrated reductions in AC activity with age (Sugawa and May, 1994). These results indicate that decreased AC may be related to aging.
It is not clear why AC activity is decreased with age; however, one possible explanation could be due to diminished AC expression. Indeed, several studies have shown decreased AC expression in aged-rat-brain regions such as the neocortex, thalamus, substantia nigra, nucleus accumbens (Araki et al., 1995), and hippocampus (Araki et al., 1995; Ramos et al., 2003). Consistent with this, AC mRNA is also decreased in the aged mouse brain. Specifically, AC I and IX were down regulated in the adult mouse hippocampus relative to young mice (Mons et al., 2004). AC I is of importance for its ability to be activated by the Ca2+/calmodulin pathway, an arguable necessity for the formation of LTM and LTP. One study looking at the individual subunits of AC in the myocardial tissue of aged rats found a decrease in the catalytic subunit activity (O'Connor et al., 1983).
Since AC is linked to most G protein-coupled receptors, a change in G protein expression could be responsible for observed alterations in cAMP levels. Decreases in Gαs protein levels result in lower levels of cAMP, while higher Gαs protein levels are responsible for increased cAMP production. Changes in Gαi protein levels could also lead to altered inhibitory activity on AC. It has been found that there is no change in any G protein expression or activation with increasing age in rat aortas (Chin et al., 1996a); however, Gαs expression was decreased in the kidneys from aged rats (Liang et al., 1993). A change in AC activity could also result from a change in the enzyme's affinity (Km) for cAMP, however this has also been found to be unchanged (O'Connor et al., 1981).
2.5. Summation of cAMP changes in the aged brain
It seems that the majority of research tends to show an overall decrease in cAMP and AC activity in aged animals; however, as described above, there are some studies showing a tendency to contradict these findings. One reason for these discrepancies could be species differences. While invertebrates and rodents may still be good models for primate senescence, there are species-specific traits that may affect the underlying genetics and their responses to senescence. This can be shown by looking at the aging gene expression patterns of rodents compared to primates. It has been found that while mice, monkeys, and humans share a small set of conserved gene expression changes, there is an observed evolutionary divergence that separates monkeys and humans from rodents. Monkeys and humans have decreased expression of certain neuronal genes like those involved with neuronal defense and plasticity, while mice in contrast have increased expression of these genes (Loerch et al., 2008).
In addition to the major species differences that might account for inconsistencies in the field of cAMP senescence, another explanation could be the mosaicism of phenotypes displayed by various aging tissues in the body, or even within the brain itself. It could be expected that while cAMP levels may fall in one region due to a decrease in AC activity or neurotransmitter levels, they may in fact be elevated in another region due to less inhibition of AC V/VI, or even increased levels via disrupted calcium homeostasis (Landfield, 1987). Interestingly, it has been shown that disruption of AC V actually leads to increased longevity, and has been proposed as a drug target for age-related cognitive decline (Chester and Watts, 2007).
One additional interesting piece of evidence to consider is the decreased responsiveness of aged individuals to drugs such as β-adrenergic agonists, and the relationship this has with high-salt diets and blood pressure. It has been shown that aged individuals with high-salt diets have decreased AC activity, and attenuated responsiveness to β-adrenergic agonists (Feldman et al., 1987). However, when a low-salt diet was fed, the responsiveness to these drugs was restored (Feldman et al., 1987). It has also been shown that there is reduced cerebral blood flow to certain parts of the aged brain, more specifically the dentate gyrus in both humans and rats (Small et al., 2004), an area of the brain important for both neurogenesis and spatial memory. Perhaps the low salt diet helps to temporarily allow increased nutrient flow to the aging brain from the otherwise normally constricted vasculature. This in turn could restore AC activity and cAMP to its normal endogenous levels and adrenergic responses are once again allowed to continue. In agreement with these previous findings is the fact that certain areas of the aged brain also have significantly decreased rates of glucose utilization, which could arguably result in lower levels of cAMP (Smith et al., 1980).
3. Altered cAMP levels could affect PKA and CREB homeostasis
As discussed in the previous section, the levels of cAMP are altered in aged subjects, possibly through decreased AC activity, modified PDE activity, altered hormonal levels, or more likely a combination of all these factors. Regardless of the mechanisms, it is clear that the aging brain has a disruption in the cAMP signaling pathway which leads to a downstream domino effect, in particular PKA and CREB as discussed in the following sections.
3.1. PKA activity and expression
Arguably, the most immediate effect of altered cAMP levels would be on PKA, and the majority of the literature shows that aging correlates with a decrease in PKA activity. First, PKA activity is decreased in the aged brains of Ceratitis capitata, the Mediterranean fruit fly (Laviada et al., 1997). PKA activity was also found to be decreased in the aged mouse liver, lung, spleen (Blumenthal and Malkinson, 1988), and senescent erythrocytes (Jindal et al., 1996). In addition, basal PKA and stimulated PKA are significantly reduced in the hippocampus and frontal cortex of senescent rats (Karege et al., 2001a, 2001b). A contradictory result was found when looking at the cerebro-vasculature of aged rats. In this study, cerebral microvessels of aged rats actually had a higher PKA activity when compared to young microvessels (Cashman and Grammas, 1995). Decreased PKA activity could result from a natural decrease in activation by lowered cAMP levels, or a change in expression and regulation. Indeed, it has been found that with increasing age there is an increase in the expression of PKA regulatory subunits. However, this was examined in the aortas of aged rats and needs further validation in the CNS (Chin et al., 1996a). Decreases in cAMP levels, and subsequent lowered activation of PKA should alter downstream signaling, and for the scope of this review we will focus next on CREB.
3.2. Alterations in CREB
CREB, as previously described, is a protein that has been extensively linked to LTM, LTP, and neuroprotection. Briefly, it was originally discovered that injection of CRE sites into Aplysia blocked long-term enhancement (Dash et al., 1990), a putative candidate for the molecular machinery behind LTM. Additional evidence for the role of CREB in memory came from studies with CREB isoform manipulation. Knocking down the CREB α/δ isoforms resulted in a dramatic decrease in LTM as well as LTP; however, short-term “enhancement” was still normal (Bourtchuladze et al., 1994). In addition, dominant negative expression of a mutant CREB in Drosophila produced similar abatements of LTM (Yin et al., 1994). CREB overexpression via viral vectors has also facilitated LTM in mice (Brightwell et al., 2007). Additional evidence supporting CREB as a target for memory enhancement is beyond the scope of this review and the reader is encouraged to investigate prominent reviews elsewhere on this subject (Johannessen et al., 2004; Carlezon et al., 2005).
Since CREB plays such a large role in learning and memory, and also is strongly expressed in the hippocampus (Deisseroth et al., 1996; Strömberg et al., 1999), it is an ideal candidate for further investigation in age-related memory decline. This has indeed been the case and there has been an abundance of recent research into CREB-related signaling decline with age. It has been shown that CREB binding protein (CBP) decreases with age in the cerebral cortex and hippocampus of rats (Chung et al., 2002). CBP is an integral part of CREB-mediated transcription. After phosphorylation of CREB, CBP and p300 are recruited to CREB where they assist with CREB-mediated transcription by the addition of their HAT ability. This allows the DNA to be opened up for transcription by RNA polymerase II. While there is yet no evidence for a change in CBP in humans with aging, mutations in CBP are known to cause Rubinstein-Taybi syndrome and mental retardation, hence the importance of CBP in cognition and development cannot be ignored (Petrij et al., 1995). There is also evidence for a significant reduction in the CRE binding by CREB in the aged rat brain (Asanuma et al., 1996). Specifically, the most marked differences were observed in the forebrain, hippocampus, and striatum. It is interesting to note that this decrease in binding was rescued by rolipram, a PDE4-specific inhibitor. This result indicates that the cellular machinery for CREB activation is still present; however, age induced decreases in cAMP levels may result in decreased CREB activation. This is further validated by findings that decreased pCREB levels in the aged hippocampus can be significantly increased with lotus seedpod extract (Xu et al., 2010). Decreased CREB expression and CREB-mediated transcription has even been discovered in vitro using senescent IMR (Institute for Medical Research) fibroblasts, a cell line well characterized for its use in the molecular causes of aging (Chin et al., 1996b). CREB staining intensity has also been shown to be significantly decreased in the motor neurons of the spinal cord in the aged rat (Matsumoto, 2000). Less pCREB in the CA1 region has been found in age-impaired animals after contextual fear training (Kudo et al., 2005); in contrast, total CREB remained unchanged. Additional studies showed decreased levels of pCREB in the hippocampus of aged rats (Ramos et al., 2003; Hattiangady et al., 2005). Interestingly, one study has shown an increase in pCREB levels in the prefrontal cortex (PFC) of aged rats (Ramos et al., 2003). It is important to note that the PFC plays a dominant role in the regulation of working memory, and that cAMP/CREB signaling may have vastly different effects in the PFC on memory than in the hippocampus (Ramos et al., 2003, 2006; Wang et al., 2011; Arnsten and Jin, 2012). This will be further discussed later on in this review.
In addition, spatially impaired aged mice have decreased levels of pCREB in certain brain regions and time points following the Morris water-maze (MWM) training compared to young controls. It is interesting to note that the pCREB levels of these impaired animals were significantly lower in the CA1 and dentate gyrus (DG) regions of the hippocampus 15 min after the last MWM session, but were not significantly different at later time points. The level of c-fos, a transcription factor which itself is regulated by CREB, was also decreased in these animals. Decreased time specific pCREB levels were mainly noted in these two regions of the hippocampus. However, diminished pCREB levels were also found in the amygdala and striatum at 15 and 60 min post-MWM, respectively. Upon further studies only in the amygdala the decreased pCREB levels were significant (Porte et al., 2008). It is worth mentioning that in addition to the hippocampus, the amygdala also plays an important role in cued fear conditioning, a form of memory that has also shown to decline in aged rats (Moyer and Brown, 2006).
Though it is clear CREB activity and expression decreased with age, it was not known what CREB subtypes were changing. CREB subtype expression is an important piece of the puzzle because, while CREB1 is only able to activate transcription, CREB2 is able to activate or inactivate transcription. A differential change in CREB subtype expression could result in paradoxical results, and undermine previous findings regarding reduced age-related CREB signaling. However, it has been found that in aged rats with impaired spatial memory, only hippocampal CREB1 is decreased, and there is no observed change in CREB2 (Brightwell et al., 2004).
In regards to proteins that have been found to modify CREB activity such as PP1/PP2A, and ICER, there have yet to be studies examining how they change with age. One can appreciate that this is certainly an important issue, as alterations in these modifying enzymes would certainly have a significant effect on subsequent CREB activity and transcription rate, and could play a prominent role in the effects of senescence on cognition. Hopefully future studies will shed light onto this area.
3.3. CREB in senescence-accelerated models
The previous evidence shows that decreased CREB expression is a fairly established finding in traditional models of aging, however, what about a senescence-accelerated model? There are 9 strains of senescence-accelerated mice, referred to as SAMP. Would the previous findings of CREB and spatial memory also occur in these mice? One study using the SAMP8 strain of accelerated mice found just that. At several months of age, these mice exhibit memory impairments in step through passive avoidance, and also have lower levels of pCREB after training than SAMR1 normal-aging mice (Tomobe et al., 2007). However, there was also a completely unexpected finding; untrained SAMP8 mice had much higher basal levels of pCREB than the SAMR1 mice. One explanation for this may be due to altered Ca2+ homeostasis caused by free radical damage, and subsequent activation of pCREB via the calmodulin-dependent pathway. Constitutively active pCREB in these animals may in fact partially contribute to the observed memory deficits. This is in agreement with other findings that while elevated pCREB levels are normally advantageous to memory, constitutively chronic enhancement of pCREB may in fact be detrimental to spatial memory (Viosca et al., 2009). This brings up an incredibly important point that like most biological cascades in the body, even an inherently “good” molecule like pCREB can cause damage if increased beyond normal endogenous levels for an extended period of time. The nootropic effects of pCREB levels may in fact follow an “inverted U-shape” pattern, with the most beneficial levels being slightly elevated, and significantly-outlying low and high levels being detrimental. This is an important piece of information to keep in mind when developing drug targets for the cAMP/CREB signaling pathway.
4. Immediate effects of altered CREB signaling
It has been shown that decreased levels of CREB with aging are fairly consistent findings. Obviously this change in CREB signaling would have dramatic downstream effects. Decreased CREB signaling could manifest as lowered levels of BDNF, c-Fos, Arc, certain types of receptors, as well as decreased LTP.
4.1. BDNF
BDNF is a neurotrophic molecule that has extensively been linked to LTP (Patterson et al., 1996, 2001; Korte et al., 1996, 1998; Figurov et al., 1996; Xu et al., 2000) and learning and memory (Hall et al., 2000). BDNF binds to the TrkB receptor (Xu et al., 2000) and can be released from neurons upon stimulation (Androutsellis-Theotokis et al., 1996; Kojima et al., 2001). The relationship between CREB and BDNF is cyclical; BDNF activates CREB transcription (Finkbeiner et al., 1997), which in turn increases BDNF transcription via the CRE promoter (Tao et al., 1998; Shieh and Ghosh, 1999). Indeed, it has been found that BDNF expression decreases with age in senescent rats (Croll et al., 1998; Schaaf et al., 2001), mice (Tsumamoto et al., 2002), and monkeys (Hayashi et al., 2001). BDNF-induced LTP has also been found to be decreased in aged rat DG alongside decreased TrkB and ERK activation (Gooney et al., 2004). A decrease in BDNF levels due to decreased CREB signaling would significantly affect neuronal health. A full-length discussion of changes in BDNF and their implications in aging is beyond the scope of this review, and has previously been covered (Halbach, 2010).
4.2. Glutamate receptors
Altered CREB signaling could affect levels of NMDA receptor expression in neurons through both BDNF and direct transcriptional control. BDNF has been shown to positively affect NMDA receptor expression and activity. This is accomplished by increasing NMDA-NR1, 2/A subunit transcription, movement of NR2B subunit transport to the membrane (the subunit which favors Ca2+ permeability), leading to increased single channel open probabilities via increased phosphorylation (Caldeira et al., 2007). Supporting this is the finding that there are decreased levels of NMDA receptors in the aged monkey hippocampus (Gazzaley et al., 1996). Also, several studies have shown decreases in NMDA responses in the aged rat brain (Gonzales et al., 1991; Pittaluga et al., 1993). NMDA receptor levels are positively correlated with spatial ability in the MWM (Adams et al., 2001).
In addition, CREB activation results in increased NMDA subunit transcription (Rani et al., 2005). Decreased NMDA receptor expression would hypothetically form a positive feedback loop, less CREB would be activated, and subsequently less NMDA receptor subunit transcription would occur. CREB can also regulate transcription of the AMPA subunit GluR1 (Borges and Dingledine, 2001; Olson et al., 2005). Dwindling CREB expression/activation could be responsible for decreased AMPA receptor expression, which could have dramatic effects on LTP. However, there is also opposing evidence for increased receptor expression in age-impaired animals; it has been shown that aged rats which perform worse in maze training actually have more NMDA receptor, α-adrenergic, and 5-HT1A receptors in the hippocampus (Topic et al., 2007). This may actually represent a compensatory mechanism due to decreasing levels of neurotransmitters, and also decreased noradrenergic and serotonergic innervation to the hippocampus seen in senescence (Ishida et al., 2000; Luellen et al., 2007).
4.3. LTP
LTP has been linked as a putative mechanism underlying memory. Briefly, there are two kinds of LTP: early phase LTP (E-LTP) which lasts minutes to hours, and late phase LTP (L-LTP) which can last much longer. L-LTP in particular, has the defining characteristic of requiring gene expression and protein synthesis (Frey et al., 1988; Castellucci et al., 1989; Nguyen et al., 1994). In addition, it has been known for some time that the formation of LTM also requires protein synthesis. Specifically, this has been shown through the application of transcription inhibitors after training regimens, while STM is intact, LTM is dramatically impaired (Barondes, 1968). Since LTM and LTP both require protein synthesis and CREB is a transcription factor, it is logical to assume that decreased CREB signaling could manifest as altered LTP and LTM. As mentioned before, CREB knockdown does dramatically impair LTP and LTM (Bourtchuladze et al., 1994). Additionally, it has also been shown that the expression of many immediate early genes (IEG) are decreased in aging (Porte et al., 2008; Penner et al., 2010; Marrone et al., 2010). Many IEGs are CREB-mediated and are also very important in LTP. As mentioned in the last section, decreased receptor expression could also significantly dampen the ability for LTP to form. Both AMPA and NMDA glutamate receptors are involved in LTP, and decreased transcription of their subunits in response to decreased CREB signaling could significantly affect L-LTP formation. Decreased LTP due to altered CREB signaling would certainly affect the ability to encode and consolidate new memories for LTM, and could account for many of the problems in age-related decline.
5. Dysregulation of cAMP/CREB signaling disrupts neuronal circuitry
This review has detailed many changes that occur in cAMP levels, AC, PDE, PKA, and the effect these alterations have on pCREB levels and subsequent transcriptional activity and downstream targets. However the question remains, what ultimately causes the weakening of memory in aged subjects? There is not a simple answer to this; however, one could argue that a dysregulation of cAMP/CREB signaling results in a disruption of neuronal circuitry.
As mentioned previously, for LTM to occur, the putative theory is that de novo protein synthesis needs to occur. Since CREB is a transcription factor, decreased CREB signaling would result in lowered protein synthesis. In support of this, protein synthesis has been shown to be significantly reduced in the aged brain (Ingvar et al., 1985). As mentioned earlier, there is a plethora of genes containing CRE promoter sites; thus, alterations in CREB signaling would most likely affect a multitude of additional downstream targets not mentioned here.
Another theory is that decreased melatonin synthesis in the brain could add to the aging phenotypes (Bondy and Sharman, 2007). Melatonin has been suggested to be extremely neuroprotective, and can induce antioxidant gene expression (Kotler et al., 1998), and attenuate inflammation (Sewerynek et al., 1995). Since norepinephrine induces melatonin synthesis in the pineal gland via CREB-mediated transcription (Maronde et al., 1997), decreased CREB signaling may result in the lowered melatonin levels in aged animals. Though beyond the scope of this review, the detrimental effects of inflammation and free radical damage to the homeostasis of the aged brain is certainly something to keep in mind, and indeed represents an active area of senescent research (Zhao et al., 2011).
An additional mechanism behind cAMP-associated LTM loss with aging could involve the sleep cycle. It has been known for some time that sleep is important for the consolidation of LTM (Fishbein, 1971). It is also well known that sleep is significantly disturbed in a large percentage of aged individuals (Crowley, 2011). However, recently it has been shown that sleep deprivation significantly lowers cAMP signaling in the hippocampus, and also raises PDE4 activity (Vecsey et al., 2009). In addition, it has been shown in aged rats that sequence reactivation, which is needed for the formation of LTM and transition from the hippocampus to the cerebral cortex, is also significantly impaired (Gerrard et al., 2008). This creates an environment detrimental for the formation of LTM. Thus aged subjects may now have handicaps both while they are awake (lowered cAMP levels), and also while they are sleeping (impaired sequence reactivation, increased PDE4 Activity).
Another huge component of decreased cAMP/CREB signaling may be on adult neurogenesis, which has been correlated with memory (Dupret et al., 2008) and also CREB signaling (Giachino et al., 2005; Herold et al., 2010; Li et al., 2011). Indeed, it has been shown that neurogenesis is decreased with age (Kuhn et al., 1996; Kempermann et al., 1998). Decreased CREB signaling may very well be responsible for the decreased neurogenesis seen with aging. Recently, it has been shown that neurogenesis can be restored in aged rats via a herbal cocktail therapy called nt-020, and that this restoration correlates with improved spatial memory (Acosta et al., 2010). Whether the compound exerted its effects through increased CREB signaling was not specifically examined in the study, however, it has been shown elsewhere that the ingredients of this compound (blueberries and green tea extract) do increase CREB activation and subsequent memory performance (Williams et al., 2008; Xu et al., 2010).
The effect of neurogenesis may very well have its actions through the effect of dentate gyrus (DG) filtering. The DG is believed to be the gate or filter into the hippocampus, and its efficient, strict homeostasis ensures accurate hippocampal circuitry (Patrylo and Williamson, 2007). In spatially impaired aged rats, granular cell integration is altered (Krause et al., 2008). Also, since DG mossy fibers project to both pyramidal neurons in the CA3 and interneurons, this could differentially affect CA3 activity. Indeed, CA3 place cells have age-dependent alterations that could affect the encoding of new information (Wilson et al., 2005). This correlates with the difficulty that aged subjects may have in the formation of LTM.
So far, this review has focused predominantly on senescent-induced dysregulation of cAMP/CREB signaling in the hippocampus, and the effect this has on the formation of LTM. From what has been presented so far, it could be assumed that a global decrease of cAMP/CREB signaling is occurring in the brain and that the best therapy would be to raise cAMP/CREB signaling globally. However, recent research by Arnsten and colleagues has begun to shed light on the incredible complexities of cAMP signaling on various brain regions and different kinds of memory (Arnsten and Jin, 2012). In particular, they have shown that activation of the cAMP/ CREB signaling pathway in the PFC may in fact be detrimental to working memory (Taylor et al., 1999; Ramos et al., 2003, 2006; Sava and Markus, 2008; Wang et al., 2011). Inhibition of cAMP signaling in the PFC of aged rats by administration of guanfacine, a selective α2 A adrenergic receptor agonist, actually improved working memory (Ramos et al., 2006). Since the α2A-adrenoceptor is coupled to Gαi, activation of this receptor by guanfacine results in inhibition of AC, and subsequently decreased production of cAMP. In addition, it has been shown that pyramidal neurons thought to be involved for the “delay” in working memory have decreased firing in the PFC of the aged monkey (Wang et al., 2011); these firing rates are restored by administering Rp-CAMPS (a PKA inhibitor). Furthermore, inhibition of cAMP-mediated ion channels with administration of ZD7288, a blocker of hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels, also enhanced the firing rates of these delay neurons. HCN channels play a major role in regulating the activity of the dendritic spines in which they are found in a process known as “dynamic connectivity” (Arnsten et al., 2010). With this new research brought to light, we can now see that there are fundamental differences between the neural mechanisms encoding LTM formation in the hippocampus, and working memory in the PFC. While raising cAMP levels in the hippocampus may be good for the formation of LTM, it is detrimental to working memory. These findings should not be surprising though, and as quoted by Arnsten “working memory requires the continuous and dynamic updating of memory buffers, whereas long-term memory consolidation involves changes that are static and long lasting” (Arnsten et al., 2005).
6. Correlations with functional imaging
Thus far the majority of discussion has focused on changes in the cAMP system with aging at the neuronal level and the subsequent effect on behavior. However, the question still remains; how do these changes in cAMP and downstream signaling affect behavior at a system- and brain region-specific level? Recently, there has been a surge of research characterizing the changes of the aging brain using advanced imaging techniques that may help answer this question. The following section will focus on age-induced alterations in brain activity, and how altered cAMP/CREB signaling may be a potential mechanism that helps explain these changes.
As mentioned earlier, it has been known for some time now the important role the hippocampus plays in the transition of STM to LTM, specifically dealing with learned facts and experienced events such as episodic and spatial memory. Because of this, the hippocampus is typically one of the most studied brain regions when it comes imaging studies dealing with aging and memory, and it has been found that there are many age-induced alterations in the hippocampus that correlate with behavioral changes. In one study looking at hippocampal activity using high resolution fMRI, Yassa and colleagues discovered the DG and CA3 area to be hypoactive during certain tasks (Yassa et al., 2011). Specifically, in this study they looked at hippocampal activity and the relation to pattern separation and pattern completion, both of which are necessary parts for differentiating novelty from similarity, or retrieving contextual memory from a “similar” cue respectively. In a series of presentations they displayed aged and young subjects either novel, repeated, or lure scenes (similar to a previous scene however with slight differences). As expected, they found no differences in hippocampal activity between young and old groups for scenes that were vastly different; however, as the similarity between scenes was increased (lure condition) they found that the aged group had a significant hypoactivation in the DG/CA3 regions. This suggests that they were unable to tell this was a “novel” scene and were more likely identifying it as a repeat, indicating a shift towards pattern completion over pattern separation. In addition, because the DG is heavily connected to the entorhinal cortex (EC) via the perforant pathway, high resolution diffusion tensor imaging (DTI) was used to investigate the connectivity between the two regions, as well as measure fractional anisotropy (a proposed measure of dendritic spine integrity) in the DG/CA3 subfields. They found that there was a decrease in perforant path integrity, as well as an interpreted decrease in DG/CA3 subfield dendritic spine integrity. This change in dendritic spine integrity could be a result of the decreased connections from the EC, or it could precede it via an unknown internal augmentation in the DG/ CA3 prior to degradation of the perforant pathway. It has also been suggested that hormonal changes with aging could affect the DG, as the DG has been shown to be particularly sensitive to changes in fluctuating hormones (Galea and McEwen, 1999).
In regards to relating these changes to alterations in cAMP/CREB signaling, several inferences can be made. First, in regards to a decrease of dendritic spine integrity in the DG/CA3, senescent-induced alteration in BDNF levels could play a significant role in this. As mentioned earlier, BDNF is an extremely neurotrophic molecule that plays a large role in the regulation of spinogensis. Decreased BDNF levels with age would most likely result in decreased dendritic spine integrity as shown in this study. In addition, cAMP/CREB molecular alterations in the EC could also account for perturbations in the perforant pathway resulting in the subsequent effects on the DG/CA3 subfields. In agreement, it has been shown that the EC does undergo molecular changes with age. In one particular study, it was found that reelin, a protein important for synaptic plasticity and highly expressed in EC layer II neurons (the layer which projects to the DG and CA3), is significantly reduced in aged animals (Stranahan et al., 2011). Interestingly, reelin has been shown to result in phosphorylation of CREB through activation of NMDA receptors (Chen et al., 2005). Many other studies have also shown a decrease in hippocampal activation with age as well (Mitchell et al., 2000; Daselaar et al., 2006; Woodward et al., 2010; O'Brien et al., 2010; Ta et al., 2012).
It should be noted that not all studies show hippocampal hypoactivity. Some studies have shown no change (Miller et al., 2008), and some have actually shown an increase with aging (Maguire and Frith, 2003). These differences in activations between young and aged groups bring up many questions and theories to help explain them such as the compensation and dedifferentiation theory. The compensation theory explains that the increased activity in aging might be due to the fact that more brain areas are needed to process a task, since the aging brain is less efficient. The dedifferentiation theory basically states that the aged brain is less controlled, and is not able to maintain specific, efficient operations in distinct in brain regions. The most likely explanation for these inconsistent observations however is due to methodological differences in the study. This brings up a very important point for imaging studies examining aging, and a recent review by Ferreira and Busatto paints a very eloquent picture on the incredible complexities one needs to consider when designing and interpreting these kinds of studies. The first thing to consider is the chosen memory behavioral task to illicit task-induced hippocampal activity. For example, the hippocampus is involved in many different kinds of memory and memory tasks, all of which have networks that might be differentially affected by aging. Thus the memory task which is picked for that certain study could have a significant effect on the subsequent activity observed. In addition, there are multiple forms of analysis which can be chosen such as region of interest analysis (ROI) which has an a priori based focus approach, independent component analysis (ICA) which focuses more on whole brain and “finds” regions which might be changing without a previous hypothesis, and finally graph theory methods. All of these analysis methods could slightly change the results and need to be carefully chosen with analyzing. In addition to just methodological design, there is an additional plethora of confounds to focus on as well such as age related neuro-vascular changes which may affect BOLD signal, “noise” due to heartbeat and respiration, loss of attention span in elderly adults, increased probability for the elderly to fall asleep while scanning, and also interference with other diseases common in the elderly such as diabetes and hypertension which have been shown to affect imaging results (Ferreira and Busatto, 2013). In addition to these confounds already mentioned, the researcher also has to keep in mind that not all aged individuals experience cognitive decline, and that if the purpose of the study is to determine brain activity changes in those vulnerable to aging they need to exclude those not affected to avoid diluting the significance. As shown, when designing an imaging study there are many factors to keep in mind in order to accurately investigate the intended hypothesis and avoid confounds.
As mentioned above, BDNF may play a role in the augmentation of brain activation with aging via influences on synaptic strength by dendritic spine growth. However, is there any evidence to support this theory? Recent research and identification of the BDNF Val66Met single nucleotide polymorphism (SNP) mutation suggest that there is. It has been discovered that approximately 3% of the Caucasian population, and 20% of the Asian population are homozygous for this allele (it is very low in African Americans). This mutation which causes the valine at site 66 to be switched to a methionine has been shown to cause a much lower secretion of BDNF, mislocalization of BDNF to the perinuclear region, and much poorer episodic memory (Egan et al., 2003).
Imaging work has shown just how important BDNF is to memory networks in the brain. It has been found that Met allele carriers have smaller hippocampal volumes (Molendijk et al., 2012). In addition, many studies have shown that the BDNF Met allele carriers have lowered hippocampal activation during encoding and retrieval tasks. In one study, Banner and colleagues tested Val carriers vs. Met heterozygote and homozygote allele carriers in a virtual radial arm maze task. Individuals were trained on this virtual eight-arm maze, to find objects hidden at the end of each arm, just like in a regular radial arm maze used on rodents with food. The goal was for subjects to remember what arms contained the objects (spatial memory), and also what arms had already been visited (working memory). There are two strategies for how this task can be solved. The first task involves the use of the hippocampus, spatial memory, and contextual cues. Individuals could learn to associate the arms containing the prize with landmarks in the environment, thus forming an internal “map” of where the various prizes were. The second strategy involves the use of the caudate nucleus and is considered habit learning. Subjects could remember once placed in the maze they turn a certain direction, and then remember a series of locomotor moves and turns to achieve the desired outcome. This learning does not involve the use of spatial cues nor the hippocampus. When they tested the Val vs. Met carriers, they found that Met carriers had much lower levels of hippocampal activation during the training and testing. In addition, not only did Met carriers have lower levels of hippocampal activity, but they actually had increased activation of the caudate nucleus that was dose dependent in parallel with the degree of Met alleles (Val/Met vs. Met/Met) carried (Banner et al., 2011). It is interesting that the Met carriers altered their strategy and brain activity for the task at hand, lending credence to the ability of the brain to be plastic and compensate for other areas that may be underperforming. Other studies have also shown a decrease of hippocampal activity in individuals carrying the Met allele as well (Hariri et al., 2003; Hashimoto et al., 2008). Studies exploring the BDNF Val66Met mutation have lent credibility to the role BDNF plays in memory and healthy brain activity and supports animal studies. As mentioned earlier, it has been shown in rodents that BDNF is decreased with aging, an effect that has also been observed in primates as well (Hayashi et al., 1997). While there is little data available to show how BDNF changes longitudinally with age in a human being, there are several studies which suggest that it does decrease just as in animal models. One study has found that in humans the levels of BDNF decrease in the blood with aging (Lommatzsch et al., 2005). While this was not a direct measurement of BDNF in neurons of the CNS, these results may be able to be extrapolated to represent BDNF levels of the brain. In support of this is a study that shows the level of BDNF in the blood and cortical neurons of the brain have a positive correlation (Karege et al., 2002) suggesting the blood serum BDNF levels may be a possible indicator of BDNF levels in the brain. Another study examining the post mortem tissue of humans ages 1 month to 86 years have found that there are indeed alterations in BDNF signaling with age. First, looking within hippocampal subregions they found there was a decrease of BDNF in the DG, although these results were not significant. Next looking at the BDNF receptor TrkB, they found that there was a significant correlation of TrkB expression with age, revealing a 37% decrease of expression in the DG from infancy to the aged group. They also examined the truncated version of the BDNF receptor TrkBTK- and found that it also significantly decreased in the CA3, and subiculum with age. Next, looking in the temporal cortex, they found that BDNF levels had a significant negative correlation with age, with levels reducing up to 33% in comparison to neonates. They found that this change in BDNF was most pronounced in layer 3 of the temporal cortex. Finally, they also saw that TrkB levels were significantly reduced in the temporal cortex with aging (Webster et al., 2006). Conversely, BDNF levels have not been shown to change in the dorsolateral prefrtontal cortex (dlPFC) with aging in human post mortem tissue (Webster et al., 2002). While this review has focused primarily on non pathological aging, it has also been shown that BDNF levels are reduced significantly in the parietal cortex (Holsinger et al., 2000) and hippocampus (Phillips et al., 1991) of Alzheimer's patients as well.
So far we have focused on task induced fMRI, or imaging involving looking at brain regions when they are active. However, a recent study of resting state fMRI, or analysis of the brain during times of resting, is beginning to shed new light on how the resting state functional connectivity (RSFC) and baseline activity of the brain changes with aging. Analysis of resting state fMRI gives a fantastic view into the RSFC and ways different brain regions are connected and activate each other. Resting state fMRI has identified several “networks” of the brain which can be active during times when no explicit tasks are being performed. The networks which have been identified include the primary sensory, language, attention, motor, and default mode network (DMN). Of these networks mentioned, the DMN is most pertinent to this review as it involves many brain regions involved with memory such as the posterior cingulate (PCC) and precuneus which can collectively be called the posteromedial cortex (PMC), the dorsal and ventral medial prefrontal cortex (dmPFC, vmPFC respectively), lateral parietal cortices, and medial temporal lobe (Mevel et al., 2011). In one study, the RSFC was examined between the BDNF Val and Met66 carriers. It was found that Met carriers had much lower functional connectivity between the hippocampus and neocortical areas than Val wild type carriers (Thomason et al., 2009). This is in agreement with a large number of aging studies which tend to show a decrease of RSFC in the DMN with age that correlates with loss of cognitive abilities (Ferreira and Busatto, 2013; Mevel et al., 2013). Thus, though it has not been shown directly that decreased BDNF with aging causes lower RSFC, the evidence suggests this may be a strong possibility. In fact, alterations in cAMP signaling could also be responsible for deficits in RSFC by additional mechanisms other than through BDNF such as changes in myelinogenesis. It has been shown that there is a loss of white matter tracts with aging which are an important component of RSFC (Ferreira and Busatto, 2013). Increasing cAMP levels, PKA activation (Vartanian et al., 1988) and subsequent phosphorylation of CREB (Gao et al., 2004) have all shown to be myelinogenic, thus lowered cAMP levels with aging could be partially responsible for alterations in white matter tracts which manifest as deficits in RSFC in the DMN.
Positron emission tomography (PET) data has also yielded interesting results in support of altered cAMP levels with aging. PET studies have shown there is a significant decrease in glucose uptake in the aged brain, particularly in areas important for memory such as the frontal and temporal lobes (Shen et al., 2012) and the anterior cingulate (Pardo et al., 2007). While no direct studies linking cAMP levels to glucose uptake have been performed in humans, it has been shown that cAMP levels most likely do play a role in glucose uptake and utilization (Gillies et al., 2008). Hence, lowered cAMP levels in the brain could be responsible for decreased glucose uptake as seen in PET, however additional studies are needed.
While there have been great strides in identifying the neural correlates behind changes in brain region activation with aging and the effect on memory, there is still much left to be explored. As suggested here, decreased BDNF levels with age due to lowered cAMP/CREB signaling could play a large role in these changes. However, due to current technological limitations, animal studies showing decreases in cAMP/pCREB levels with age in the hippocampus have yet to be confirmed in humans. While there is yet no empirical evidence to date showing changes in cAMP/pCREB in humans with age, animal data and mounting human BDNF data make the cAMP/CREB pathway a strong candidate for the development of future novel drugs.
7. Summary
In conclusion, senescent-induced dysregulation of cAMP/ CREB signaling significantly disrupts the homeostasis of the brain. This altered homeostasis most likely manifests early at the neuronal level, first as deficits in early- and late-phase LTP, and then followed by actual anatomical changes in synaptic strength via decreased spinogensis. These changes in synaptic strength could then alter activation of brain regions to a task such as the hypoactivity observed in the hippocampus in aged fMRI studies. These changes might not be merely limited to one brain region, as increased atrophy in one region could cause decreased RSFC with another, following a Hebbian “use it or lose it, wire together/fire together” philosophy. This is supported by the resting state fMRI data of which the majority shows a decrease in RSFC in the DMN with age. It is important to keep in mind this is an incredibly complex, multifactorial feedback system that becomes increasingly synergistic and progressively worse once homeostasis is disrupted. For this reason, it becomes difficult to differentiate compensatory changes from the actual initial changes that might be causing memory deficits. This is something that needs to be taken into account when planning future experiments for aging studies.
Also, because of the incredible complexity of aging, and the large inconsistencies that may arise from species- and tissue-specific differences, it becomes increasingly difficult to determine the sequence of events in aging; alas, the age-old question of what came first the chicken or the egg arises. A prime example of this is that decreased cAMP levels may be the causal effect of lowered neurotransmitter levels, cerebral blood flow, and receptor expression. However, it can very well be that neurotransmitter levels and receptor expression are lowered due to decreased cAMP signaling. Whatever the sequence of events may be, once disruption of homeostasis occurs, it is a downhill event that feeds upon itself. The events that cause these changes with aging have still yet to be identified.
An additional thought to keep in mind is that it should not be expected that cAMP and pCREB signaling will be deteriorated with age globally in every brain region; as discussed above, there are cases where signaling is higher. As shown, increased cAMP signaling is thought to be more beneficial in the hippocampus and LTM, while the opposite holds true in the PFC in regards to working memory. The important thing to keep in mind however is that any kind of extreme dysregulation of cAMP/CREB signaling in either direction is unnatural and disruptive to the brain, and could result in undesired manifestations represented through behavior as a change in cognitive ability and development of memory deficits.
Acknowledgments
This work was supported by research grants from NARSAD Young Investigator Award (2006, 2008 to H.T. Zhang) and NIA (AG031687 to H.T. Zhang).
REFERENCES
- Acosta S, Jernberg J, Sanberg C, Sanberg P, Small B, Gemma C, Bickford P. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010;13:581–588. doi: 10.1089/rej.2009.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M, Smith T, Moga D, Gallagher M, Wang Y, Wolfe B, Rapp P, Morrison J. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp. Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, McCormack WJ, Bradford HF, Stern GM, Pliego-Rivero FB. The depolarisation-induced release of [125I]BDNF from brain tissue. Brain Res. 1996;743:40–48. doi: 10.1016/s0006-8993(96)00981-x. [DOI] [PubMed] [Google Scholar]
- Ao H, Ko SW, Zhuo M. CREB activity maintains the survival of cingulate cortical pyramidal neurons in the adult mouse brain. Mol. Pain. 2006;2:15. doi: 10.1186/1744-8069-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Kato H, Fujiwara T, Itoyama Y. Age-related changes in bindings of second messengers in the rat brain. Brain Res. 1995;704:227–232. doi: 10.1016/0006-8993(95)01117-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Jin LE. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J. Biol. Med. 2012;85:45–58. [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: a new form of neuroplasticity. [accessed 05.11.12];Trends Cogn. Sci. 2010 14:365–375. doi: 10.1016/j.tics.2010.05.003. Available from∷〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2914830&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. [accessed 31.10.12];Trends Mol. Med. 2005 11:121–128. doi: 10.1016/j.molmed.2005.01.006. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15760770〉. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Nishibayashi S, Iwata E, Kondo Y, Nakanishi T, Vargas M, Ogawa N. Alterations of cAMP response element-binding activity in the aged rat brain in response to administration of rolipram, a cAMP-specific phosphodiesterase inhibitor. Brain Res. Mol. Brain Res. 1996;41:210–215. doi: 10.1016/0169-328x(96)00098-8. [DOI] [PubMed] [Google Scholar]
- Austin J, Connole E, Kett D, Collins J. Studies in aging of the brain. V. Reduced norepinephrine, dopamine, and cyclic AMP in rat brain with advancing age. Age. 1978;1:121–124. [Google Scholar]
- Banner H, Bhat V, Etchamendy N, Joober R, Bohbot VD. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. [accessed 08.03.13];Eur. J. Neurosci. 2011 33:968–977. doi: 10.1111/j.1460-9568.2010.07550.x. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3084505&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can. J. Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Cohen HD. Memory impairment after subcutaneous injection of acetoxycycloheximide. Science. 1968;160:556–557. doi: 10.1126/science.160.3827.556. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s. Neurobiol. Aging. 1998;19:173–189. doi: 10.1016/s0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Birkenfeld A, Ben-Zvi A. Age associated changes in intracellular cyclic adenosine monophosphate. Clin. Exp. Immunol. 1984;55:651–654. [PMC free article] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/8980227〉. [DOI] [PubMed] [Google Scholar]
- Blumenthal E, Malkinson A. Age-dependent changes in murine protein kinase and protease enzymes. Mech. Ageing Dev. 1988;46:201–217. doi: 10.1016/0047-6374(88)90125-x. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Sharman EH. Melatonin and the aging brain. [accessed 02.08.10];Neurochem. Int. 2007 50:571–580. doi: 10.1016/j.neuint.2006.12.014. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/17276551〉. [DOI] [PubMed] [Google Scholar]
- Borges K, Dingledine R. Functional organization of the GluR1 glutamate receptor promoter. [accessed 22.11.10];J. Biol. Chem. 2001 276:25929–25938. doi: 10.1074/jbc.M009105200. Available from: ( http://www.ncbi.nlm.nih.gov/pubmed/11340067). [DOI] [PubMed] [Google Scholar]
- Borlikova G, Endo S. Inducible cAMP early repressor (ICER) and brain functions. [accessed 07.03.13];Mol. Neurobiol. 2009 40:73–86. doi: 10.1007/s12035-009-8072-1. Available from∷ 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2699388&tool=pmcentrez&rendertype = abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brightwell J, Gallagher M, Colombo PJ. Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol. Learn. Mem. 2004;81:19–26. doi: 10.1016/j.nlm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Neve RL, Colombo PJ. Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn. Mem. 2007;14:195–199. doi: 10.1101/lm.395407. [DOI] [PubMed] [Google Scholar]
- Bruce P, Herman J. Spatial knowledge of young and elderly adults: scene recognition from familiar and novel perspectives. Exp. Aging. Res. 1983;9:169–173. doi: 10.1080/03610738308258447. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. [accessed 17.01.11];Mol. Cell Neurosci. 2007 35:208–219. doi: 10.1016/j.mcn.2007.02.019. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/17428676〉. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cashman R, Grammas P. cAMP-dependent protein kinase in cerebral microvessels in aging and Alzheimer disease. Mol. Chem. Neuropathol. 1995;26:247–258. doi: 10.1007/BF02815141. [DOI] [PubMed] [Google Scholar]
- Castellucci V, Blumenfeld H, Goelet P, Kandel E. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J. Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Cha-molstad H, Keller DM, Yochum GS, Impey S, Goodman RH. Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc. Natl. Acad. Sci. USA. 2004;101:13572–13577. doi: 10.1073/pnas.0405587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang T-S, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. [accessed (28.02.13];J. Neurosci. 2005 25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/16148228〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Watts VJ. Adenylyl cyclase 5: a new clue in the search for the “Foutain of Youth”? Sci. STKE. 2007;413:64–67. doi: 10.1126/stke.4132007pe64. [DOI] [PubMed] [Google Scholar]
- Chin J, Hiremath A, Hoffman B. cAMP signaling mechanisms with aging in rats. Mech. Ageing Dev. 1996a;86:11–26. doi: 10.1016/0047-6374(95)01676-7. [DOI] [PubMed] [Google Scholar]
- Chin J, Okazaki M, Frazier J, Hu Z, Hoffman B. Impaired cAMP-mediated gene expression and decreased cAMP response element binding protein in senescent cells. Am. J. Physiol. 1996b;271:C362–C371. doi: 10.1152/ajpcell.1996.271.1.C362. [DOI] [PubMed] [Google Scholar]
- Chung YH, Kim EJ, Shin CM, Joo KM, Kim MJ, Woo HW, Cha CI. Age-related changes in CREB binding protein immunoreactivity in the cerebral cortex and hippocampus of rats. Brain Res. 2002;956:312–318. doi: 10.1016/s0006-8993(02)03562-x. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Guzman E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for camp responsiveness Salk Institute for Biological Studies. Mol. Cell. 2003;11:1101–1108. doi: 10.1016/s1097-2765(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Convit A, Leon Mde, Hoptman M, Santi SDe, Rusinek H. Age-related changes in brain: I. Magnetic resonance imaging measures of temporal lobe volumes in normal subjects. Psychiatr. Q. 1995;66:343–355. doi: 10.1007/BF02238754. [DOI] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9813325〉. [DOI] [PubMed] [Google Scholar]
- Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. [accessed 23.0.13];Cereb. Cortex. 2006 16:1771–1782. doi: 10.1093/cercor/bhj112. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1810232&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P, Hochner B, Kandel E. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien R. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton D, Petropoulos H, Yeo R, Brooks W, Baumgartner R, Sutherland R. The aging hippocampus: cognitive, biochemical and structural findings. Cereb. Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest J-M, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PloS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2396793&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/12553913〉. [DOI] [PubMed] [Google Scholar]
- Feldman RD, Lawton WJ, Mcardle WL. Rapid publication low sodium diet corrects the defect in lymphocyte fiadrenergic responsiveness in hypertensive subjects. J. Clin. Invest. 1987;79:290–294. doi: 10.1172/JCI112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. [accessed 01.03.13];Neurosci. Biobehav. Rev. 2013 37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/23333262〉. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9390517〉. [DOI] [PubMed] [Google Scholar]
- Fishbein W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav. 1971;6:279–282. doi: 10.1016/0031-9384(71)90155-7. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann K, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/10199627〉. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15541310〉. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc. Natl. Acad. Sci. USA. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. Available from: 〈 http://www. pubmedcentral.nih.gov/articlerender.fcgi?artid=39772&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol. Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J. Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/18667620〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schütz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/16267218〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. [accessed 07.03.13];J. Nucl. Med. 2008 49(Suppl. 2):24S–42S. doi: 10.2967/jnumed.107.047258. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/18523064〉. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Brown L, Jones T, Trent R, Westbrook S, Leslie S. N-methyl-D-aspartate mediated responses decrease with age in Fischer 344 rat brain. Neurobiol. Aging. 1991;12:219–225. doi: 10.1016/0197-4580(91)90100-x. [DOI] [PubMed] [Google Scholar]
- Gooney M, Messaoudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. [accessed 07.10.10];Neurobiol. Aging. 2004 25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15465630〉. [DOI] [PubMed] [Google Scholar]
- Hajjhussein H, Suvarna NU, Gremillion C, Chandler LJ, Donnell JMO. Changes in NMDA receptor-induced cyclic nucleotide synthesis regulate the age-dependent increase in PDE4A expression in primary cortical cultures. Brain Res. 2007;1149:58–68. doi: 10.1016/j.brainres.2007.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach OVBU. Involvement of BDNF in age-dependent alterations in the hippocampus. [accessed 14.10.10];Front. Aging Neurosci. 2010 2:1–11. doi: 10.3389/fnagi.2010.00036. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/20941325〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hara H, Onodera H, Kato H. Effects of aging on signal transmission and transduction systems in the gerbil brain: morphological and autoradiographic study. Neuroscience. 1992;46:475–488. doi: 10.1016/0306-4522(92)90067-c. [DOI] [PubMed] [Google Scholar]
- Harada N, Nishiyama S, Ohba H, Sato K, Kakiuchi T, Tsukada H. Age differences in phosphodiesterase type-IV and its functional response to dopamine D1 receptor modulation in the living brain: a PET study in conscious monkeys. Synapse. 2002;44:139–145. doi: 10.1002/syn.10067. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/12890761〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Moriguchi Y, Yamashita F, Mori T, Nemoto K, Okada T, Hori H, Noguchi H, Kunugi H, Ohnishi T. Dose-dependent effect of the Val66Met polymorphism of the brain-derived neurotrophic factor gene on memory-related hippocampal activity. [accessed 01.03.13];Neurosci. Res. 2008 61:360–367. doi: 10.1016/j.neures.2008.04.003. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/18501457〉. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Mistunaga F, Ohira K, Shimizu K. Changes in BDNF-immunoreactive structures in the hippocampal formation of the aged macaque monkey. Brain Res. 2001;918:191–196. doi: 10.1016/s0006-8993(01)03002-5. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. [accessed 23.01.13];Brain Res. 1997 749:283–289. doi: 10.1016/S0006-8993(96)01317-0. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9138728〉. [DOI] [PubMed] [Google Scholar]
- Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. [accessed 14.09.10];Mol. Cell. Neurosci. 2010 46:79–88. doi: 10.1016/j.mcn.2010.08.008. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/20801218〉. [DOI] [PubMed] [Google Scholar]
- Holsinger RMD, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res. Mol. Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. Available from: 〈 http://linkinghub.elsevier.com/retrieve/pii/S0169328 × 00000231〉. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres E, Poser S, Chavkin C, Storm D. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Maeder P, Sokoloff L, Smith C. Effects of ageing on local rates of cerebral protein synthesis in Sprague-Dawley rats. Brain. 1985;108:155–170. doi: 10.1093/brain/108.1.155. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Shirokawa T, Miyaishi O, Komatsu Y, Isobe K. Age-dependent changes in projections from locus coeruleus to hippocampus dentate gyrus and frontal cortex. Eur. J. Neurosci. 2000;12:1263–1270. doi: 10.1046/j.1460-9568.2000.00017.x. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/10762355〉. [DOI] [PubMed] [Google Scholar]
- Jindal HK, Ai Z, Gascard P, Horton C, Cohen CM. Specific loss of protein kinase activities in senescent erythrocytes. Blood. 1996;88:1479–1487. [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol. Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Lambercy C, Schwald M, Steimer T, Cissé M. Differential changes of cAMP-dependent protein kinase activity and 3H-cAMP binding sites in rat hippocampus during maturation and aging. Neurosci. Lett. 2001a;315:89–92. doi: 10.1016/s0304-3940(01)02358-8. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/12147321〉. [DOI] [PubMed] [Google Scholar]
- Karege F, Schwald M, Lambercy C, Murama JJ, Cisse M, Malafosse A. A non-radioactive assay for the cAMP-dependent protein kinase activity in rat brain homogenates and age-related changes in hippocampus and cortex. Brain Res. 2001b;903:86–93. doi: 10.1016/s0006-8993(01)02409-x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9547229〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J. Neurosci. Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Korte M, Staiger V, Griesbeck O, Thoenen H, Bonhoeffer T. The involvement of brain-derived neurotrophic factor in hippocampal long-term potentiation revealed by gene targeting experiments. J. Physiol. Paris. 1996;90:157–164. doi: 10.1016/s0928-4257(97)81415-5. [DOI] [PubMed] [Google Scholar]
- Kotler M, Rodríguez C, Sáinz R, Antolín I, Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 1998;24:83–89. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Krause M, Yang Z, Rao G, Houston FP, Barnes CA. Altered dendritic integration in hippocampal granule cells of spatial learning-impaired aged rats. [accessed 13.12.10];J. Neurophysiol. 2008 99:2769–2778. doi: 10.1152/jn.01278.2007. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2652140&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Dickinson-Anson H, Gage F. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, Chennareddi L, Herndon JG. Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta) Behav. Neurosci. 2005;119:118–126. doi: 10.1037/0735-7044.119.1.118. [DOI] [PubMed] [Google Scholar]
- Landfield PW. “Increased calcium-current” hypothesis of brain aging. Neurobiol. Aging. 1987;8:346–347. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Laviada ID, Galve-Roperh I, Malpartida JM, Haro A. cAMP signalling mechanisms with aging in the Ceratitis capitata brain. Mech. Ageing Dev. 1997;97:45–53. doi: 10.1016/s0047-6374(97)01899-x. [DOI] [PubMed] [Google Scholar]
- Li Y-F, Cheng Y-F, Huang Y, Conti M, Wilson SP, O'Donnell JM, Zhang H-T. Phosphodiesterase-4D KnockOut and rna interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. [accessed 10.01.11];J. Neurosci. 2011 31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. Available from: 〈 http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.5236-10.2011〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Barnes J, Hanai H, Levine M. Decrease in Gs protein expression may impair adenylate cyclase activation in old kidneys. Am. J. Physiol. 1993;264:F770–F773. doi: 10.1152/ajprenal.1993.264.5.F770. [DOI] [PubMed] [Google Scholar]
- Light L. The organization of memory in old age. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Erlbaum, Hillsdale, NJ: 1992. p. 111. [Google Scholar]
- Lister JP, Barnes C. Neurobiological changes in the hippocampus during normative aging. Arch. Neurol. 2009;66:829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, Yankner BA. Evolution of the aging brain transcriptome and synaptic regulation. PloS One. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. [accessed 08.03.13];Neurobiol. Aging. 2005 26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15585351〉. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and Regulation of CREB Family Transcription Factors in the Nervous System CREB and its close relatives are now widely accepted. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Luellen B, Bianco L, Schneider L, Andrews A. Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes Brain Behav. 2007;6:482–490. doi: 10.1111/j.1601-183X.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. [accessed 05.03.13];Brain. 2003 126:1511–1523. doi: 10.1093/brain/awg157. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/12805116〉. [DOI] [PubMed] [Google Scholar]
- Makman M, Ahn H, Thal L, Sharpless N, Dvorkin B, Horowitz S, Rosenfeld M. Evidence for selective loss of brain dopamine- and histamine-stimulated adenylate cyclase activities in rabbits with aging. Brain Res. 1980;192:177–183. doi: 10.1016/0006-8993(80)91017-3. [DOI] [PubMed] [Google Scholar]
- Maronde E, Schomerus C, Stehle J, Korf H. Control of CREB phosphorylation and its role for induction of melatonin synthesis in rat pinealocytes. Biol. Cell. 1997;89:505–511. doi: 10.1016/s0248-4900(98)80006-3. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Satvat E, Shaner MJ, Worley PF, Barnes CA. Attenuated long-term Arc expression in the aged fascia dentata. [accessed 27.09.10];Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.022. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/20850902〉. [DOI] [PMC free article] [PubMed]
- Matsumoto A. Age-dependent changes in phosphorylated cAMP response element-binding protein immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Neurosci. Lett. 2000;279:117–120. doi: 10.1016/s0304-3940(99)00965-9. [DOI] [PubMed] [Google Scholar]
- Mevel K, Chételat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer's disease. [accessed 05.03.13];Int. J. Alzheimers Dis. 2011 2011:535816. doi: 10.4061/2011/535816. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3132539&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, Joie RLa, Villain N, Mézenge F, Perrotin A, Eustache F, Desgranges B, Chételat G. Age effect on the default mode network, inner thoughts, and cognitive abilities. [accessed 05.03.13];Neurobiol. Aging. 2013 34:1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23084083. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, Depeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl. Acad. Sci. USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2538895&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain. Res. Cogn. Brain. Res. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/10978709〉. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol. Aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Tol M-J, van, Penninx BWJH, Wee NJA, van der, Aleman A, Veltman DJ, Spinhoven P, Elzinga BM. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. [accessed 03.03.13];Transl. Psychiatry. 2012 2:e74. doi: 10.1038/tp.2011.72. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3309548&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N, Segu L, Nogues X, Buhot M. Effects of age and spatial learning on adenylyl cyclase mRNA expression in the mouse hippocampus. Neurobiol. Aging. 2004;25:1095–1106. doi: 10.1016/j.neurobiolaging.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Montminy MR. Binding of a nuclear protein to the cAMP response element of the somatostatin gene. Nature. 1987;328:175–179. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Moyer J, Brown T. Impaired trace and contextual fear conditioning in aged rats. Behav. Neurosci. 2006;120:612–624. doi: 10.1037/0735-7044.120.3.612. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40–90 years of age. Am. J. Neuroradiol. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Münch D, Baker N, Kreibich CD, Bråten AT, Amdam GV. In the laboratory and during free-flight: Old honey bees reveal learning and extinction deficits that mirror mammalian functional decline. PloS One. 2010;5:1–9. doi: 10.1371/journal.pone.0013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P, Abel T, Kandel E. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2905893&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S, Scarpace P, Abrass I. Age-associated decrease of adenylate cyclase activity in rat myocardium. Mech. Ageing Dev. 1981;16:91–95. doi: 10.1016/0047-6374(81)90036-1. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Scarpace P, Abrass I. Age-associated decrease in the catalytic unit activity of rat myocardial adenylate cyclase. Mech. Ageing Dev. 1983;21:357–363. doi: 10.1016/0047-6374(83)90052-0. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Xu Y. Evidence for global reduction in brain cyclic adenosine monophosphate signaling in depression. [accessed 11.12.12];Biol. Psychiatry. 2012 72:524–525. doi: 10.1016/j.biopsych.2012.07.017. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/22959119〉. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J. Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15944383〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. [accessed 15.03.13];NeuroImage. 2007 35:1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1913629&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo P, Williamson A. The effects of aging on dentate circuitry and function. Brain. 2007;163:679–696. doi: 10.1016/S0079-6123(07)63037-4. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in Basal Synaptic Transmission and Hippocampal LTP in BDNF Knockout Mice. Cell. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/11604144〉. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age-related changes in Arc transcription and DNA methylation within the hippocampus. [accessed 19.09.10];Neurobiol. Aging. 2010 :1–13. doi: 10.1016/j.neurobiolaging.2010.01.009. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2888808&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed]
- Perls TT. The different paths to 100. Am. J. Clin. Nutr. 2006;83:484S–487S. doi: 10.1093/ajcn/83.2.484S. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/16470017〉. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cereb. Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9651126〉. [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, Ommen GJ, van, Goodman RH, Peters DJ, Breuning MH. Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/7630403〉. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Fedele E, Risiglione C, Raiteri M. Age-related decrease of the NMDA receptor-mediated noradrenaline release in rat hippocampus and partial restoration by D-cycloserine. Eur. J. Pharmacol. 1993;231:129–134. doi: 10.1016/0014-2999(93)90693-c. [DOI] [PubMed] [Google Scholar]
- Porte Y, Buhot M-C, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice; Neurobiol. Aging. 2008;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AFT. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/14622586〉. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Stark D, Verduzco L, Dyck CHVan, Arnsten AFT. α2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn. Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani CSS, Qiang M, Ticku MK. Potential role of cAMP response element-binding protein in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene transcription in fetal mouse cortical cells. Mol. Pharmacol. 2005;67:2126–2136. doi: 10.1124/mol.104.007872. [DOI] [PubMed] [Google Scholar]
- Roman G. A historical review of the concept of vascular dementia: lessons from the past for the future. Alzheimer Dis. Assoc. Disord. 1999;13:s4–s8. doi: 10.1097/00002093-199912001-00002. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Spreekmeester E, Meaney MJ, Quirion R, Rochford J. Reactivity to novelty in cognitively-impaired and cognitively-unimpaired aged rats and young rats. Neuroscience. 1998;83:669–680. doi: 10.1016/s0306-4522(97)00464-8. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/9483551〉. [DOI] [PubMed] [Google Scholar]
- Sava S, Markus EJ. Activation of the medial septum reverses age-related hippocampal encoding deficits: a place field analysis. [accessed 17.02.11];J. Neurosci. 2008 28:1841–1853. doi: 10.1523/JNEUROSCI.4629-07.2008. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/18287501〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, Workel JO, Lesscher HM, Vreugdenhil E, Oitzl MS, de Kloet ER. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res. 2001;915:227–233. doi: 10.1016/s0006-8993(01)02855-4. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/11595212〉. [DOI] [PubMed] [Google Scholar]
- Scoville W-B, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg.Psychiatry. 1958;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani G, Morissette C, Lagacé C, Boulé M, Ouellette M-J, McLaughlin RW, Lacombe D, Gervais F, Tremblay P. The cAMP-specific phosphodiesterase 4B mediates Abeta-induced microglial activation. Neurobiol. Aging. 2006;27:691–701. doi: 10.1016/j.neurobiolaging.2005.03.024. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/15993984〉. [DOI] [PubMed] [Google Scholar]
- Sewerynek E, Abe M, Reiter R, Barlow-Walden L, Chen L, McCabe T, Roman L, Diaz-Lopez B. Melatonin administration prevents lipopolysaccharide-induced oxidative damage in phenobarbital-treated animals. J. Cell Biochem. 1995;58:436–444. doi: 10.1002/jcb.240580406. [DOI] [PubMed] [Google Scholar]
- Shen X, Liu H, Hu Z, Hu H, Shi P. The relationship between cerebral glucose metabolism and age: report of a large brain PET data set. [accessed 24.03.13];PloS One. 2012 7:e51517. doi: 10.1371/journal.pone.0051517. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3527454&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. Dev. Neurobiol. 1999;41:127–134. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/10504200〉. [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl. Acad. Sci. USA. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Goochee C, Rapoport S, Sokoloff L. Effects of ageing on local rates of cerebral glucose utilization in the rat. Brain. 1980;103:351–365. doi: 10.1093/brain/103.2.351. [DOI] [PubMed] [Google Scholar]
- Stancheva S, Alova L. Age-related changes of cyclic AMP phosphodiesterase activity in rat brain regions and a new phosphodiesterase inhibitor—nootropic agent adafenoxate. Gen. Pharmacol. 1991;22:955–958. doi: 10.1016/0306-3623(91)90237-z. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. [accessed 22.03.13];Cereb. Cortex. 2011 21:392–400. doi: 10.1093/cercor/bhq106. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3020582&tool= pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- Sugawa M, May T. Signal transduction in aging. Arch. Gerontol. Geriatr. 1994;19:235–246. doi: 10.1016/s0167-4943(05)80069-x. [DOI] [PubMed] [Google Scholar]
- Ta AT, Huang S-E, Chiu M-J, Hua M-S, Tseng W-Yi, Chen S-Ha, Qiu A. Age-related vulnerabilities along the hippocampal longitudinal axis. [accessed 22.03.13];Hum. Brain Mapp. 2012 33:2415–2427. doi: 10.1002/hbm.21364. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/21898676〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. Available from: 〈 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt= Citation&list_uids=9581763〉. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum S, Ubriani R, Arnsten AF. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/10479716〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Yoo DJ, Glover GH, Gotlib IH. BDNF genotype modulates resting functional connectivity in children. [accessed 07.03.13];Front. Hum. Neurosci. 2009 3:55. doi: 10.3389/neuro.09.055.2009. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2786303&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohda M, Murayama T, Nogiri S, Nomura Y. Influence of aging on rolipram-sensitive phosphodiesterase activity and [3H]rolipram binding in the rat brain. Biol. Pharm. Bull. 1996;19:300–302. doi: 10.1248/bpb.19.300. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/8850327〉. [DOI] [PubMed] [Google Scholar]
- Tomobe K, Okuma Y, Nomura Y. Impairment of CREB phosphorylation in the hippocampal CA1 region of the senescence-accelerated mouse (SAM) P8. Brain Res. 2007;1141:214–217. doi: 10.1016/j.brainres.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Topic B, Willuhn I, Zilles K, Huston JP, Haseno RU. Impaired maze performance in aged rats is accompanied by increased density of NMDA, 5-HT 1A, and a-Adrenoceptor binding in hippocampus. Hippocampus. 2007;77:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- Tsumamoto Y, Yamashita K, Okada K, Kurokawa T, Yamashita H, Mishima H. Regulation of BDNF and Its Receptor TrkB in retina of normal aged mice and senescence-accelerated mice. Invest. Ophthalmol. Visual Sci. 2002:43. [Google Scholar]
- Vallebuona F, Raiteri M. Age-related changes in the NMDA receptor/nitric oxide/cGMP pathway in the hippocampus and cerebellum of freely moving rats subjected to transcerebral microdialysis. Eur. J. Neurosci. 1995;7:694–701. doi: 10.1111/j.1460-9568.1995.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Sprinkle TJ, Dawson G, Szuchet S. Oligodendrocyte substratum adhesion modulates expression of adenylate cyclase-linked receptors. Proc. Natl. Acad. Sci. USA. 1988;85:939–943. doi: 10.1073/pnas.85.3.939. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=279672&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X, Descalzi G, Kim SS, Chen T, Shang Y, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signaling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viosca J, Malleret G, Bourtchouladze R, Benito E, Vronskava S, Kandel ER, Barco A. Chronic enhancement of CREB activity in the hippocampus interferes with the retrieval of spatial information. Learn. Mem. 2009;16:198–209. doi: 10.1101/lm.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Alvarez-bolado G, Thaller C. Comprehensive analysis of the expression patterns of the adenylate cyclase gene family in the developing and adult mouse brain. J. Comp. Neurol. 2006;496:684–697. doi: 10.1002/cne.20953. [DOI] [PubMed] [Google Scholar]
- Wadzinski BE, Wheat WH, Jaspers S, Peruski LF, Lickteig RL, Johnson GL, Klemm DJ. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol. Cell Biol. 1993;13:2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang X-J, Laubach M, Mazer JA, Lee D, Arnsten AFT. Neuronal basis of age-related working memory decline. [accessed 04.09.12];Nature. 2011 476:210–213. doi: 10.1038/nature10243. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3193794&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. [accessed 01.03.13];Gene Exp. Patterns. 2006 6:941–951. doi: 10.1016/j.modgep.2006.03.009. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/16713371〉. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res. Dev. Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/12480128〉. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, Dowd BFO, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc. Natl. Acad. Sci. USA. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Mohsen MAEl, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JPE. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Biol. Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/18457678〉. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. [accessed 28.06.10];J. Neurosci. 2005 25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/16033897〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N. Prediction of cognitive decline in healthy older adults using fMRI. J. Alzheimers Dis. 2010;21:871–885. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Chambless BD, Kadish I, Groen Tvan. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol. Aging. 2000;21:671–681. doi: 10.1016/s0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Rong S, Xie B, Sun Z, Deng Q, Wu H, Bao W, Wang D, Yao P, Huang F, Liu L. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:933–940. doi: 10.1093/gerona/glq094. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. [accessed 04.03.13];Proc. Natl. Acad. Sci. USA. 2011 108:8873–8878. doi: 10.1073/pnas.1101567108. Available from: 〈 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3102362&tool=pmcentrez&rendertype=abstract〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Wallach J, Vecchio MDel, Wilder E, Zhou H, Quinn W, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhao HF, Li Q, Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. [accessed 05.11.12];Neuroscience. 2011 183:189–202. doi: 10.1016/j.neuroscience.2011.03.048. Available from: 〈 http://www.ncbi.nlm.nih.gov/pubmed/21463662〉. [DOI] [PubMed] [Google Scholar]
- Zimmerman I, Berg A. Levels of adenosine 3',5'-cyclic monophosphate in the cerebral cortex of aging rats. Mech. Ageing Dev. 1974;3:33–36. doi: 10.1016/0047-6374(74)90003-7. [DOI] [PubMed] [Google Scholar]