Abstract
Preliminary work indicates that cognitive vulnerability to depression may be associated with variants of the serotonin transporter promoter polymorphism (5-HTTLPR) and the valine to methionine at position 66 (val66met) polymorphism of the brain-derived neurotrophic factor (BDNF) gene; however, existing reports come from small samples. The present study sought to replicate and extend this research in a sample of 375 community-dwelling children and their parents. Following a negative mood induction, children completed a self-referent encoding task tapping memory for positive and negative self-descriptive traits. Consistent with previous work, we found that children with at least one short variant of the 5-HTTLPR had enhanced memory for negative self-descriptive traits. The BDNF val66met polymorphism had no main effect but was moderated by maternal depression, such that children with a BDNF methionine allele had a heightened memory for negative self-descriptive traits when mothers had experienced depression during children's lifetimes; in contrast, children with a methionine allele had low recall of negative traits when mothers had no depression history. The findings provide further support for the notion that the 5-HTTLPR is associated with cognitive markers of depression vulnerability and that the BDNF methionine allele moderates children's sensitivity to contextual factors.
Cognitive accounts of depression (e.g., Beck, 1976) are among the most influential theories of depression etiology. These models have been investigated in many samples, including clinically depressed patients, high-risk individuals, and nonclinical groups, and in adults, adolescents, and children. Such investigations have yielded a substantial empirical literature supporting the role of cognitive mechanisms in depression vulnerability (for reviews, see Abela & Hankin, 2008; Joormann, 2009). Although theories of cognitive vulnerability encompass a broad array of processes, biases in thinking that are activated in response to stress are thought to hold particular significance as a proximal cause of the disorder. Numerous studies have provided evidence that such reactivity is a marker of depression risk (Scher, Ingram, & Segal, 2005). It is this specific aspect of cognitive vulnerability that is the focus of the present investigation.
Despite marked research interest in this topic, it is unclear how and why such reactivity develops (Hankin et al., 2009). A complete answer to such questions may require the integration of genetic and other neurobiological approaches with research on depressive cognition (Beck, 2008). Along these lines, several papers have implicated specific genes in shaping depressogenic information processing (Beevers, Scott, McGeary, & McGeary, 2009; Beevers, Wells, Ellis, & McGeary, 2009; Fox, Ridgewell, & Ashwin, 2009; Gibb, Benas, Grassia, & McGeary, 2009; Sheikh et al., 2008). These studies have primarily focused on a functional polymorphism in the 5′ promoter region of the serotonin transporter linked polymorphic region gene (5-HTTLPR, on chromosome 17q; Lesch, Bengel, Heils, & Sabol, 1996). Polymorphic variants are a long allele, comprising 16 copies of an approximately 22 base pair (bp) repeat unit, and a short allele, consisting of 14 copies of the repeat unit. The presence of one or two short alleles leads to diminished transcription, lower transporter levels, and reduced serotonin uptake, with functional effects on neural circuits relevant to depression (Hariri & Holmes, 2006; Lesch et al., 1996).
Several groups (Beevers, Scott, et al., 2009; Hayden et al., 2008) have provided evidence derived from relatively small samples linking the short allele of the 5-HTTLPR to heightened negative cognition following a negative mood induction. Such reactivity may be a cognitive marker of stress sensitivity, which would be consistent with other lines of research linking this polymorphism to biological indices of stress reactivity such as cortisol reactivity to laboratory stress and amygdala reactivity to threatening stimuli (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Gotlib, Joormann, Minor, & Hallmayer, 2008; Hariri et al., 2002; Munafò, Brown, & Hariri, 2008), self-reported neuroticism in adults (Munafò et al., 2009), and laboratory observations of heightened negative emotionality in children with other sources of temperamental risk for depression (Hayden, Klein, Sheikh, et al., 2010). It would also be consistent with recent animal work indicating that the 5-HTTLPR is associated with neurocognitive markers of stress reactivity (Blakely & Veenstra-VanderWeele, 2011). The many studies reporting direct associations of the 5-HTTLPR short variant with a variety of indices of stress reactivity and cognition show that this variant need not be moderated by environmental factors to have an effect on such outcomes (e.g., Caspi et al., 2010; Homberg & Lesch, 2011).1
The brain-derived neurotrophic factor (BDNF) gene is another candidate gene for cognitive reactivity to negative mood (Beevers, Wells, & McGeary, 2009; Hilt, Sander, Nolen-Hoeksema, & Simen, 2007). BDNF is a neural growth factor assigned a critical role in models of the pathophysiology of depression (Duman & Monteggia, 2006). The BDNF gene, located on chromosome 11p13, has a G to A single nucleotide polymorphism (SNP) at nucleotide 196 (rs6265) that results in a valine (val) to methionine (met) change at codon 66 (val66met). This substitution changes the 5′ pro-region of the human BDNF protein and appears to lower depolarization-induced secretion of BDNF, leading to a decrease in available BDNF and other negative neurobiological effects (Chen et al., 2004; Egan et al., 2003). Supporting the role of this gene in depression are associations between this polymorphism and reduced hippocampal volume (Pezawas et al., 2004) and elevated hypothalamic–pituitary–adrenal (HPA) axis activity (Schule et al., 2006). In addition, two studies have linked this gene to rumination (Beevers, Wells, & McGeary, 2009; Hilt et al., 2007).
The BDNF gene may also interact with experience in predicting depression risk, with several papers indicating that the association between this gene and negative outcomes depends on contextual adversity (Aguilera et al., 2009; Kaufman et al., 2006). However, it was recently argued that associations between the BDNF gene and outcomes may be more complex (Hayden, Klein, Dougherty, et al., 2010), in that the BDNF methionine allele may serve as a marker of differential susceptibility to the environment (Belsky & Pluess, 2009). Several authors have concluded that certain polymorphisms may not function simply as vulnerability genes but instead perform a more complex role by enhancing biological plasticity, increasing reactivity to both adverse and supportive contexts (Belsky & Pluess, 2009; Ellis & Boyce, 2008). Consistent with this, it was recently reported that the BDNF methionine allele was associated with child temperament in a context-dependent manner, such that children with at least one copy of the methionine allele had either very high or very low levels of negative emotionality, a putative depression endophenotype, depending on whether contextual adversity was present (Hayden, Klein, Dougherty, et al., 2010).
We sought to extend research on the role of the BDNF methionine allele in depression vulnerability by examining associations between the BDNF val66met polymorphism and cognitive reactivity to negative mood, testing whether this variant was moderated by an important contextual factor linked to children's cognitive vulnerability: maternal depression. We focused on maternal depression because it is thought to contribute to children's depressive cognitions through a wide array of mechanisms (Garber & Martin, 2002). However, it is unclear whether the risk associated with maternal depression is primarily tied to child exposure to the mother's depressive episode (e.g., because of maternal depressive behavior or its effects on parenting or the family environment) or to more enduring attributes of mothers that may exist prior to and/or persist after depression remits (e.g., direct genetic influences, traitlike cognitive biases, a propensity for stress generation, or dysfunctional parenting and family processes that are independent of clinical state). Although many experts in the field believe that the timing of maternal depression may differentially impact children's risk (Goodman & Brand, 2008), research on this question is limited. In the area of parenting, there is evidence that, although a past history of depression influences the quality of maternal caregiving, the effects of current depression are much stronger (Lovejoy, Graczyk, O'Hare, & Neuman, 2000). A recent study also reported associations between child depression risk, indexed via maternal depression occurring during children's lifetimes, and children's performance on a dot-probe task (Joorman, Talbot, & Gotlib, 2007). However, we know of no study that has specifically examined whether exposure to maternal depression influences the extent to which associations are found between maternal depression and children's cognitive reactivity. It may, therefore, prove informative to account for whether maternal depression has occurred during children's lifetimes in testing the moderation of child genetic risk stemming from BDNF val66met variation.
Thus, the present study builds on recent work on the genetic bases of early-emerging cognitive vulnerability to depression in several ways. A central goal of the current study is to replicate our previous finding showing a main effect of the 5-HTTLPR short allele on cognitive reactivity in 38 young children (Hayden et al., 2008), using a substantially larger sample. Although the psychiatric genetics literature has historically been characterized by replication failures, we have previously argued that the use of refined measures of biologically plausible phenotypes may play an important role in generating a more consistent body of literature (e.g., Hayden et al., 2007). By using an objective, developmentally sensitive measure of cognitive reactivity to negative mood (i.e., a self-referent encoding task) in a large sample of 6-year-olds, we hoped to capitalize on this possibility. Following Hayden et al. (2008), we chose to measure cognitive reactivity to negative mood, a specific aspect of depressogenic cognition (Scher et al., 2005), via children's performance on a self-referent encoding task (SRET), a widely used information processing task tapping memory for affectively charged word stimuli. Previous studies have shown that children with depression (Garber & Kaminski, 2000) and children of depressed mothers (Taylor & Ingram, 1999) exhibit abnormal performance on these tasks (see Scher et al., 2005, for a review). In addition, in two independent samples of young children, performance on a SRET showed moderate stability over multiple 1-year follow-ups and was significantly associated with self-reported depressive symptoms and attributional styles (Hayden et al., 2006; Hayden, Sheikh, et al., 2010). Of critical importance, the SRET places fairly minimal cognitive demands on participants and can therefore be completed even by relatively young children (Hayden, Klein, Durbin, & Olino, 2006). There are also very few self-report measures of cognitive vulnerability that have demonstrated reliability and validity when used in this age group. Even if adequate self-report measures of cognition were available, it has been argued that self-reports index the products of cognitive vulnerability, whereas performance-based tasks like SRETs tap underlying cognitive processes more directly (Mineka, Rafaeli, & Yovel, 2003). Such processes may map more closely onto genetic influences than the higher order cognition tapped by self-report (e.g., Nigg, Blasky, Stawacki, & Sachek, 2004). Based on our previous study (Hayden et al., 2008) and consistent with recent, comprehensive reviews by Caspi et al. (2010) and Homberg and Lesch (2011), which showed main effects of the 5-HTTLPR short variant on an array of stress-related and cognitive phenotypes, we did not posit that an association between the short variant and cognitive reactivity would require moderation to be detected.
In addition to the main effect of the 5-HTTLPR short allele, we predicted that the BDNF methionine allele would be associated with negative cognition in a context-dependent manner, such that it would be related to negative cognition only in the high-risk context of maternal depression; in low-risk contexts (i.e., the absence of maternal depression), we predicted that this same variant would be associated with low levels of negative cognition. This hypothesis was derived from recent research on the influence of this variant on other markers of childhood depression risk (Hayden, Klein, Dougherty, et al., 2010). In testing this latter hypothesis, we distinguished between mothers with no history of depression, those with a history of depression prior to the child's lifetime, and mothers with a history of depression during the child's lifetime, in order to explore whether direct exposure to maternal depression was necessary for moderation.
We tested these questions in a sample of young children for several reasons. First, clinically diagnosable depression is rare in early and middle childhood (Garber, Gallerani, & Frankel, 2009); hence, this period presents an opportunity to identify risk markers and processes substantially before the onset of depressive disorder. Second, there is compelling evidence that depressotypic information processing biases can be detected in young children (Hayden et al., 2006; Kujawa et al., 2011). Third, the earlier one can identify and understand the development of the cognitive biases associated with depression, the larger the window of opportunity for prevention or early intervention. This may be particularly important given evidence suggesting that some interventions are more effective in younger children than in older youth and adults, perhaps owing to greater neuroplasticity earlier in development (Pine, Helfinstein, Bar-Haim, Nelson, & Fox, 2009). This may be particularly relevant to interventions targeting cognitive biases, which appear to stabilize and become more traitlike in late childhood and early adolescence (Cole et al., 2008; Hankin, 2008).
Method
Participants
The initial sample consisted of 559 children and their parents. Of these, 476 children (254 males) contributed DNA and are included in this report. The mean age of the 476 children at the time of the baseline assessment was 42.2 months (SD = 3.1). Potential participants were identified via a commercial mailing list, and eligible families had a child between 3 and 4 years of age with no significant medical conditions or developmental disabilities and at least one English-speaking biological parent. Most of the participants came from middle-class families (M = 44.8; SD = 10.9), as measured by Hollingshead's Four Factor Index of Social Status (Hollingshead, 1975), and the vast majority (96.6%) of children came from two-parent homes. Children were of average cognitive ability (M = 103.1, SD = 13.7) as indexed by the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997). Most of the children were White (86.8%); 8.2% were Hispanic, and the remainder of the sample comprised an array of other ethnic groups. A follow-up assessment, during which cognitive reactivity to negative mood was measured using age-appropriate measures, occurred 30.3 months after baseline (SD = 5.0) when children were 72.4 months old (SD = 5.2).
Genetic assessment
At baseline, buccal cells were collected from children for genetic analysis. The Qiagen DNA Micro Kit (Valencia, CA) was used to extract genomic DNA to genotype children for the 5-HTTLPR and BDNF val66met polymorphisms. Following Chorbov et al. (2007), primers used for polymerase chain reaction (PCR) amplification of the 5-HTTLPR flanking region were 5′-GGCGTTGCCGCTCTGAATGC-3′ (forward) and 5′-GAGGGACTGAGCTGGACAAC CAC-3′ (reverse). PCR conditions were 5 min of initial denaturation at 94°C followed by 30 cycles of 30 s of denaturation at 94°C, 20 s annealing at 58°C and 20 s of extension at 72°C, and a final extension of 5 min at 72°C. A common SNP occurs at the sixth nucleotide (adenine to guanine; A to G) within the first of two extra 20 to 23 bp repeats in the long allele (rs25531) of the 5-HTTLPR. Some studies suggest that, of the long (L) alleles, only those containing the A SNP (LA) are high functioning with regard to promoter activity, whereas the long allele with the G SNP (LG) may exhibit the same transcriptional activity as the short allele (Hu et al., 2005). This SNP is part of a recognition site for the MspI restriction endonuclease (Fermentas), which cuts one base 5′ of the SNP when the G nucleotide is present and does not cut when the A nucleotide is present. The Invitrogen PCRx Enhancer System kit (Invitrogen) was used for PCR amplification, instead of the 7-deazaGTP (otherwise necessary to amplify the GC-rich region), which impairs the ability of MspI to completely digest the PCR product. PCR was followed by digestion of amplicons at 37°C overnight with 1 unit of MspI (Fermentas, Burlington, ON, Canada), yielding a 249 bp fragment (uncut LA allele), two fragments of 148 bp and 101 bp (cut LG allele), or a 206 bp fragment (short allele).
For the BDNF val66met polymorphism, a PCR restriction fragment length polymorphism protocol was used (Bueller et al., 2006). Primers for amplification were 5′-AAAGAAGCAAACATCCGAGGACAAG-3′ (forward) and 5′-ATTCCTCCAGC AGAAAGAGAAGAGG-3′ (reverse). PCR amplification conditions were 5 min of initial denaturation at 94°C, followed by 30 cycles of 30 s initial denaturation at 94°C, 20 s annealing at 58°C, 20 s extension at 72°C, followed by a final extension of 5 min at 72°C. PCR products were restriction digested with Hsp92II (Promega, Madison, WI). The G allele produces 57 bp and 217 bp fragments after Hsp92II digest, but the A allele produces three fragments of 57, 77, and 144 bp in length. PCRs were carried out using the Applied Bio-systems (Foster City, CA) thermal cycler Gene Amp 9700. Amplicons were separated on 6% polyacrylamide gels, stained with ethidium bromide, and visualized and documented by a ultraviolet imaging system (BioRad Labs, Mississauga, CA).
In our sample, 143 children (30%) were homozygous for the L/L allele of the 5-HTTLPR, 241 (51%) were heterozygous, and 92 (19%) were homozygous for the short allele. This distribution is in Hardy–Weinberg equilibrium (χ2 = 0.28, p > .58). Two children lacked sufficient DNA for the SNP at rs25531 to be analyzed. Of the remaining 474 children, 184 (39%) were homozygous for the short or the LG alleles, 236 (50%) had only one short or LG allele, and 54 (11%) had two LA alleles. With respect to the BDNF val66met polymorphism, 232 children (49%) were val/val homozygous, 216 (45%) were heterozygous, and 28 (6%) were met/met homozygous. Although not in Hardy–Weinberg equilibrium (χ2 = 5.95, p < .05), this distribution closely resembles those reported by Shimizu, Hashimoto, and Iyo (2004), Houlihan et al. (2009), and Petryshen et al. (2009), and it is consistent with reports of the rarity of the met/met genotype in White samples. We had 155 participants’ BDNF genotypes independently assayed by another laboratory; no discrepancies were found. Consistent with most investigations of these genes, analyses contrasted children with the L/L genotype to those with at least one short allele, and those with the val/val genotype to those with at least one methionine allele.
Maternal psychopathology
At the time of the children's baseline (age 3) assessment, mothers were interviewed by masters-level clinicians using the Structured Clinical Interview for DSM-IV, nonpatient version (First, Spitzer, Gibbon, & Williams, 1996). Based on audiotapes of 30 assessments, interrater reliability (indexed by kappa) for lifetime mood disorder was 0.93. Of the 476 children, we had diagnostic information on 458 of their mothers; of these, 126 mothers had a lifetime history of major depressive disorder (MDD) and 52 had a history of dysthymic disorder (DD). Considering the high level of familial aggregation between MDD and DD (Klein et al., 1995; Klein, Shankman, Lewinsohn, Rohde, & Seeley, 2004) and the evidence that individuals with DD almost invariably develop MDD (Klein, Shankman, & Rose, 2006), MDD and DD were collapsed into a single category reflecting maternal depressive disorder. Of the 154 mothers in this group, 66 (43%) had a single episode of major depression; the remaining 88 mothers (57%) had chronic MDD or recurrent MDD or dysthymia. As far as common comorbid diagnoses, 80 (52%) of the mothers with a depressive disorder had a lifetime history of anxiety disorder, and 50 (32%) had a lifetime history of substance use disorder. Mothers’ diagnoses of MDD or DD and depression chronicity were unrelated to children's 5-HTTLPR and BDNF val66met genotypes (ps > .23).
As mentioned previously, because of the lack of research addressing this issue, we had no a priori reason to predict whether any risk for elevated cognitive vulnerability associated with maternal depression would be dependent on whether mothers’ depression occurred during children's lifetimes. Therefore, to examine whether any influences were contingent on exposure to maternal depression, mothers were assigned to one of three groups based on their history of depression at baseline, ascertained when children were 3.5 years old: no maternal history of depressive disorder, a history of depressive disorder prior to the child's birth but not during the child's lifetime through age 3.5, and history of depressive disorder during the child's lifetime through age 3.5. Of the mothers with a depression history, 102 (66%) had a past depressive episode but not during the child's lifetime through age 3.5, and 52 (34%) had a history of either MDD or DD during the child's lifetime through age 3.5.
Cognitive reactivity to negative mood
Follow-up occurred 30.3 months later (SD = 5.0) when children were 72.4 months old (SD = 5.2). We elected to measure cognitive reactivity at this wave of data collection because this age is likely the youngest at which measures of cognitive vulnerability to depression with established validity can be used. Cognitive reactivity, indexed via a SRET, was measured for 375 of the 476 children in the baseline sample. Children for whom SRET data were and were not available did not differ in terms of 5-HTTLPR or BDNF genotype distribution, sex, maternal depression history, ethnic status, or Peabody Picture Vocabulary Test scores (ps < .26), although there was a non-significant tendency for those with SRET data to have higher Hollingshead scores, t (417) = 1.74, p = .08.
As previously reported (Hayden et al., 2008), depressogenic cognition was indexed via children's performance on a SRET, a widely used information processing task tapping memory for affectively charged word stimuli. A negative mood induction procedure (MIP) was administered via a sad video clip from a children's film (Brenner, 2000) prior to the SRET, as described in detail elsewhere (Hayden et al., 2006). It was previously shown that this mood induction is associated with increases in children's facial expressions of negative affect (Hayden et al., 2006). With the help of an experimenter, children rated their own mood both prior to and following the MIP using a 5-point scale in which higher numbers reflected better mood. Based on paired t tests, the MIP was associated with a significant reduction in children's moods, t (368) = 17.78, p > .0001. Change in mood was unrelated to whether mothers had a history of depressive disorder (p < .89) or to children's genotypes (ps < .74).
Next, children were presented with a series of 14 positive and 14 negative trait adjectives matched for frequency and selected for beginner reading level (Carroll, Davies, & Richman, 1971), along with two neutral content words not included in the analyses that were shown to the children at the beginning and the end to address primacy and recency effects. Adjectives were shown on flashcards and spoken aloud by the experimenter, followed by a self-referent question (“Is this word like you?”). The experimenter noted the child's response to each query. Because the proportion of positive and negative adjectives endorsed as self-descriptive was unrelated to children's BDNF and 5-HTTLPR genotypes (all ps < .18), these variables are not considered further. An incidental recall period immediately followed, in which children were asked to recall as many of the adjectives as possible. Two scores were calculated: a SRET positive information processing score (proportion of positive adjectives rated self-descriptive and recalled, relative to all adjectives rated self-descriptive) and a negative information processing score (derived in the same manner but using negative trait adjectives). Following the task, children were given several small prizes to reverse the effects of the MIP.
Following the MIP reversal, with the help of an experimenter blind to children's genotypes, children completed the Children's Depression Inventory (CDI; Kovacs & Beck, 1977), a self-report measure of depressive symptoms. The mean CDI score was low (M = 7.11, SD = 5.06) and consistent with means from community samples of children (e.g., Richey et al., 2009). Although the CDI is not usually used with children younger than 8 years of age, there is evidence supporting its validity in children as young as 5 (Ialongo, Edelsohn, & Kellam, 2001). CDI scores were unrelated to children's change in mood in response to the MIP (p < .68).
Results
Groups based on the presence of a 5-HTTLPR short allele and a BDNF methionine allele were not significantly different on any demographic variables, depressive symptoms at the time of the cognitive assessment, or maternal depression history (Table 1). During the free recall, some children did not recall any of the trait adjectives they had endorsed (N = 71; 19%), and hence they received scores of 0 on the SRET. The failure to recall any adjectives endorsed was unrelated to child genotype (ps < .43). Because SRET scores were not normally distributed and because statistical transformation does not address the issue of 0 scores, we used Mann–Whitney U tests to examine bivariate associations between genotype groups and SRET information processing (Table 1). Neither genotype group differed on SRET positive information processing. Considering this lack of association, in conjunction with the evidence favoring the centrality of the processing of negatively valenced stimuli in depression (e.g., Watters & Williams, 2011), positive information processing is not considered further here. In a replication of our previous finding (Hayden et al., 2008), children with at least one short allele of the 5-HTTLPR had significantly higher SRET negative information processing scores than children without a short allele.2 There was no association between BDNF genotype and SRET negative information processing.
Table 1.
SRET positive and negative information processing scores by child 5-HTTLPR and BDNF val66met genotypes
|
Child Genotypes
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
5-HTTLPR
|
BDNF val66met |
|||||||
| L/L |
L/S and S/S |
Val/Val |
Val/Met and Met/Met |
|||||
| Variable | M (SD) | N (%) | M (SD) | N (%) | M (SD) | N (%) | M (SD) | N (%) |
| Child sex (male) | 69 (48) | 185 (55) | 115 (50) | 139 (57) | ||||
| SES | 44.44 (11.09) | 44.92 (10.89) | 44.32 (10.61) | 45.21 (11.25) | ||||
| PPVT | 102.41 (13.32) | 103.45 (13.83) | 103.03 (14.22) | 103.24 (13.17) | ||||
| CDI | 6.92 (5.37) | 7.20 (4.92) | 7.32 (5.31) | 6.89 (4.80) | ||||
| SRET pos. ranka | 186.51 | 188.65 | 184.71 | 191.30 | ||||
| SRET neg. ranka | 174.91 | 193.72* | 193.07 | 182.90 | ||||
| Maternal depression | ||||||||
| No history | 84 (61) | 221 (69) | 145 (65) | 160 (69) | ||||
| Prior to child's birth | 37 (27) | 64 (20) | 55 (25) | 46 (20) | ||||
| During child's lifetime | 16 (12) | 35 (11) | 24 (11) | 51 (11) | ||||
Note: 5-HTTLPR, serotonin transporter linked polymorphic region; L, long allele; S, short allele; BDNF, brain derived neurotrophic factor; Val, valine; Met, methionine; SES, socioeconomic status, as indexed by Hollingshead's Four Factor Index of Social Status (Hollingshead, 1975); PPVT, Peabody Picture Vocabulary Test; CDI, Children's Depression Inventory; SRET, Self referent encoding task.
Mean ranks were tested using Mann–Whitney tests, because scores were not normally distributed because some children did not recal any affectively valenced adjectives that they had also endorsed; hence, they received a score of 0 on the relevant processing scale.
p < .05.
We next examined a model testing the main effects of children's 5-HTTLPR and BDNF polymorphisms, maternal depressive history, and the hypothesized BDNF × Maternal Depression interaction in predicting children's SRET negative information processing. We used general linear models in PASW Statistics 17.0 to conduct robust regressions for all multivariate analyses. This approach implements maximum-likelihood estimation procedures with robust standard errors that adjust for violations of normality. In order to minimize the number of tests conducted, we focused our analyses on testing effects for which relatively strong a priori evidence existed (e.g., a main effect of the 5-HTTLPR on stress-related and cognitive phenotypes). Similarly, following Aiken and West's (1991) recommendations regarding the inclusion and exclusion of higher order terms, we specifically included only interactions that were predicted based on prior theory (i.e., the BDNF × Maternal Depression interaction). Thus, we did not include a term reflecting the BDNF × 5-HTTLPR interaction in this model; because there is no substantive prior literature indicating that these variants interact to predict cognitive reactivity, we had no basis for a specific hypothesis. In Table 2, tests of the main effects of the 5-HTTLPR, the BDNF val66met polymorphism, and maternal depression are presented, and the effect of the Maternal Depression × BDNF interaction is also shown. The main effect of the 5-HTTLPR was significant (d = 0.25). Although the BDNF val66met polymorphism (d = 0.01) and maternal depression history were not directly associated with children's negative information processing, the BDNF × Maternal Depression interaction term was significant.3 Running this model with CDI scores treated as a covariate yielded comparable results, with the main effect of the 5-HTTLPR and the BDNF × Maternal Depression interaction remaining significant and virtually unchanged (ps > .03).
Table 2.
Effects of child 5-HTTLPR and BDNF val66met genotypes, maternal depression history, and the interaction between child BDNF val66met genotypes and maternal depression on children's SRET negative information processing scores
| Variable | Wald χ2 | df | p |
|---|---|---|---|
| 5-HTTLPR | 5.70 | 1 | .017 |
| BDNF val66met | 0.01 | 1 | .929 |
| Maternal depression history | 0.26 | 2 | .879 |
| BDNF val66met × Maternal Depression History | 8.25 | 2 | .016 |
Note: 5-HTTLPR, serotonin transporter linked polymorphic region; BDNF, brain derived neurotrophic factor; Val, valine; Met, methionine; SRET, Self-referent encoding task.
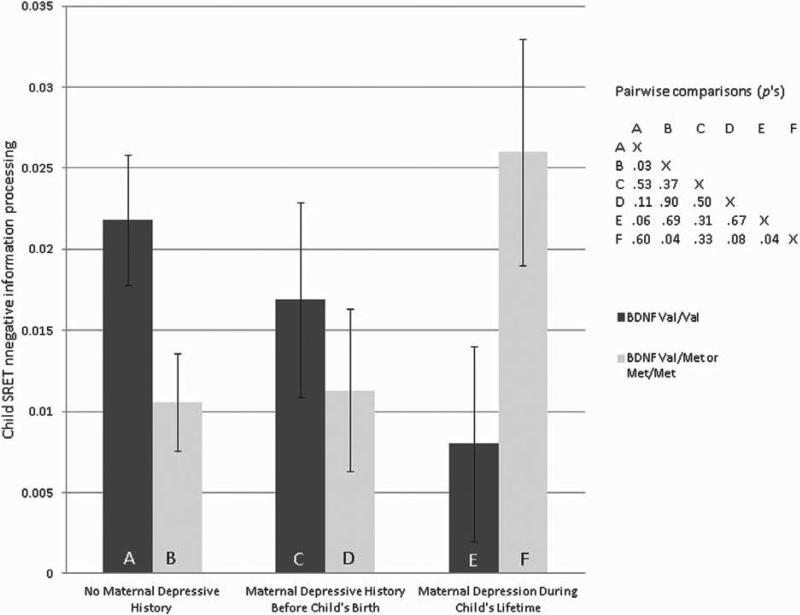
To understand the interaction, post hoc tests using the least significant difference method were used to compare children's estimated mean negative SRET scores across BDNF genotypes and maternal depression history (adjusted for other variables in the model, see Figure 1). Because we were testing a limited number of focused, a priori hypotheses (i.e., that the met/met group of children would show evidence for both high and low SRET negative information processing depending on maternal depression), rather than conducting exploratory analyses, we did not correct for multiple tests. Mean SRET negative information processing scores did not differ significantly between the val/val groups, regardless of maternal depression history, although there was a trend for children with the val/val genotype who did not have a maternal history of depression to have higher negative SRET scores than did val/val children with mothers with depression during their lifetimes. However, three of the other pairwise comparisons of mean scores on children's negative information processing were significant at p > .05. Children with a methionine allele who also had a mother who was depressed during their lifetime had significantly higher SRET negative information processing scores than did children with the same genotype with mothers who had no history of depression (d = 0.15). These children (i.e., those with a methionine allele who had a mother who was depressed during their lifetimes) also had higher SRET negative information processing scores than did children with the val/val genotype who had a mother who was depressed during their lifetime (d = 0.15). Finally, children with the val/val genotype who did not have a maternal history of depression had higher SRET negative information processing scores than did children with a methionine allele who did not have a maternal history of depression (d = 0.16). Thus, children with a methionine allele had either relatively high or low SRET negative information processing scores, depending on whether their mothers had been depressed during their lifetimes, when compared to children with the val/val genotype in the same contextual circumstances.
Figure 1.
Mean SRET negative information processing scores and standard errors by BDNF val66met genotype and maternal history of depression. Note: SRET negative information processing scores are the proportion of negative adjectives rated self-descriptive and recalled, relative to all adjectives rated self-descriptive.
Additional analyses
The mothers with depression during their children's lifetimes included some who also had depressive episodes prior to the child's birth. This latter subgroup could have more chronic/recurrent forms of depression, which could account for why their offspring had elevated SRET negative information processing scores when they also had a BDNF methionine allele. To see whether our findings would change when excluding this subgroup, we reran the model including only the mothers with depression onset during, but not prior to, the child's lifetime (N = 33). Despite the reduced sample size, there was a strong trend for the BDNF × Maternal Depression interaction (p = .06), and the pattern of SRET negative information processing scores was virtually identical to that presented in Figure 1.
To address concerns about population stratification, we reran our primary analysis using White participants only; results were consistent with those reported for the full sample. In particular, the BDNF × Maternal Depression interaction remained significant, χ2 (2, 323) = 15.37, p = .001, and plotting the interaction revealed the same pattern of findings reported in the primary analyses. Running the model with ethnicity as a covariate also yielded similar results, including a significant interaction, χ2 (2, 365) = 10.05, p = .001, and a similar pattern of relationships.
In addition, as noted previously, some reports suggest that only long alleles of the 5-HTTLPR that contain the A SNP (LA) are high functioning with regard to promoter activity and that the long allele with the G SNP (LG) may exhibit the same transcriptional activity as the short allele (Hu et al., 2005). Accordingly, all analyses were run contrasting children homozygous for the LA alleles to those children with an LG allele or an short allele; the main effect of 5-HTTLPR on negative SRET scores remained significant (p = .001), and other model parameters remained virtually unchanged including the BDNF × Maternal Depression interaction term, χ2 (2, 365) = 7.99, p = .02.
Finally, based on the previous literature (Caspi et al., 2010; Hayden et al., 2008; Homberg & Lesch, 2011), we did not posit moderation of the 5-HTTLPR by maternal depression. Nonetheless, given the widespread interest in 5-HTTLPR×Environment interactions, we conducted an exploratory analysis including the interaction term between the 5-HTTLPR and maternal depression in the full model. This interaction was not significant, χ2 (2, 365) = 3.04, p = .22. However, the main effect of 5-HTTLPR on negative SRET scores remained significant in this model (p = .001), as did the BDNF×Maternal Depression interaction term, χ2 (2, 365) = 9.67, p = .008.
Discussion
This replication of previous work (Hayden et al., 2008) in an independent, substantially larger sample adds to the burgeoning literature implicating the 5-HTTLPR short allele in a host of intermediate phenotypes for emotional disorders, including biases for emotional stimuli (Beevers, Ellis, Wells, & McGeary, 2010; Hayden et al., 2008), hypothalamic–pituitary–adrenal dysregulation (Gotlib et al., 2008), and neural reactivity to emotional stimuli (Hariri et al., 2005). Our findings also suggest a mechanism by which the 5-HTTLPR may increase depression vulnerability: via its associations with cognitive reactivity in the context of dysphoric mood, although the exact processes by which variation at this locus might influence cognition are admittedly unclear. We did not hypothesize that moderation by maternal depression was necessary for an association between the short allele and SRET performance to emerge, based on our previous finding, and the aforementioned other studies showing a main effect of this gene on other markers of depression risk (e.g., Beevers, Pacheco, Clasen, McGeary, & Schnyer, 2010; Caspi et al., 2010; Perepletchikova & Kaufman, 2011), including cognitive vulnerability (e.g., Beevers et al., 2009; Beevers, Wells, Ellis, & McGeary, 2009). Exploratory analyses provided no support for moderation. Moderation of 5-HTTLPR by stress in predicting other depression phenotypes has been reported by a few other, smaller studies (e.g., Gibb, Uhrlass, Grassia, Benas, & McGeary, 2009); our findings indicate that although such a pattern may emerge for other depression risk markers, it does not for children's SRET performance. This does not preclude such interactions for other outcomes relevant to children's mental health (e.g., Hankin et al., 2011).
Our findings also indicate that children with a methionine allele of the BDNF val66met polymorphism had a reduced memory for negative self-descriptive traits when their mothers had no history of depression, compared to children without a methionine allele who also had mothers without a depression history. In contrast, children with a methionine allele who had mothers with depression during their lifetimes had an enhanced memory for negative self-descriptive traits, compared to children with the val/val genotype with maternal depression during their lifetimes. Further, children without a methionine variant did not significantly differ from one another on SRET performance, regardless of mothers’ depression history (although there was a nonsignificant trend for the val/val group without a maternal depression history to have higher negative SRET scores than val/val children with a maternal history of depression during their lifetimes). In other words, our findings suggest that the BDNF methionine allele plays a role in both reduced and heightened memory for negative self-descriptive traits, depending on maternal depression. This is consistent with recent work in which the BDNF methionine allele was associated with child temperamental negative emotionality in a context-dependent manner (Hayden, Klein, Dougherty, et al., 2010). It is important to note that children's negative emotionality at age 3 and negative SRET scores were unassociated in general and in both BDNF genotype groups when examined separately (all ps <.34). In addition, including negative emotionality as a covariate in models did not change our findings, further indicating that negative emotionality does not mediate the effect of BDNF and context on children's performance on the SRET. Thus, the present findings provide further support that the methionine allele increases children's susceptibility to both positive and negative contextual factors (Belsky & Pluess, 2009; Ellis & Boyce, 2008) in relation to emerging depression risk indexed by independent emotional and cognitive markers.
More specifically, with respect to the present findings, the BDNF methionine allele appears to meet Belsky, Bakermans-Kranenburg, and van IJzendoorn's (2007) criteria for a differential susceptibility factor for children's cognitive reactivity to negative mood. As a final step in confirming this model, Belsky et al. suggest testing specificity by examining whether other genes produce the same pattern of findings with respect to other child outcomes. Although such analyses are beyond the scope of the present study, our findings are otherwise consistent with the criteria outlined by these authors.
The moderating effect of maternal depression on the BDNF-SRET negative processing scores was most pronounced when mothers’ depression occurred during children's lifetimes; children with mothers who were depressed only prior to their births had SRET negative processing scores similar to children with mothers who had never been depressed. This effect held even when limiting analyses to those mothers whose first episode occurred during their children's lifetimes. These results tentatively suggest that the moderating effect of mothers’ depression on the association between children's genotype and negative cognition is contingent on children's exposure to maternal depression. The processes that account for this effect warrant further investigation. Moreover, a limitation of the study is that we did not have interview-based information on maternal depression after children were 3 to 4 years old. We therefore cannot say whether exposure to maternal depression at older ages has the same effect. With respect to the group with depression before the child's birth, it is also important to note the possibility that some had unreported or subthreshold episodes during the child's lifetime. However, errors in ascertaining the timing of maternal depression would diminish, rather than enhance, our findings.
Our findings implicate variation in two genes in shaping children's early-emerging cognitive reactivity. These variants appear to shape risk through different processes, with one apparently having a direct effect (i.e., the 5-HTTLPR) in our sample and the other requiring contextual moderation (i.e., the BDNF val66met). Genetic influences on cognitive markers of depression risk are likely complex, involving an array of pathways that are currently poorly understood. However, ample evidence has accrued supporting functional, main/direct effects of the 5-HTTLPR on an array of cognitive and behavioral phenotypes that can be detected regardless of environmental context (Homberg & Lesch, 2011). It is intriguing that many of the cognitive phenomena linked to the short allele of the 5-HTTLPR are not maladaptive; for example, improved decision making, response inhibition, and reversal learning have all been directly linked to this variant (Crisan et al., 2009; Jedema et al., 2009; Roiser, Muller, Clark, & Sahakian, 2007), thus accounting for the frequency of the short allele in the population despite its role in increasing psychopathology risk. In contrast, although the BDNF val66met variant has been somewhat less well characterized in psychiatric genetics research, its role in neuronal plasticity and development (Boulle et al., 2012) suggests that environmental factors may be critical in causing potentially enduring changes in BDNF expression, particularly early in development. Although speculative, we submit that the BDNF val66met variant may be an especially potent contributor to phenotypic plasticity, shaping early-emerging phenotypes related to psychopathology risk in processes that are highly context dependent. Considering that BDNF is expressed at high levels in the limbic system and is also important for long-term potentiation, its plasticity in early development may have key relevance for shaping risk for disorders characterized by dysregulated emotion and memory bias, such as depression (Bekinschtein et al., 2008; Mao, Fibuch, & Wang, 2010; Pencea, Bingaman, Wiegand, & Luskin, 2001).
This research has a number of strengths. Of the emerging literature on the molecular genetics of cognitive vulnerability, the current study has by far the largest sample, although it is still relatively small for a genetic association study. However, our use of sophisticated measures of a cognitive phenotype and environmental risk (i.e., laboratory measures and structured clinical interviews) may have somewhat mitigated the need for a larger sample size by reducing measurement error (Moffitt, Caspi, & Rutter, 2005). We successfully replicated a previous finding from our group (Hayden et al., 2008) in a much larger sample and included tests of the effects of another plausible candidate for cognitive reactivity and a contextual moderator.
However, our study also had some limitations. Experts disagree on the extent to which population stratification, which can produce false associations, is a concern in studies such as ours (Hutchison, Stallings, McGeary, & Bryan, 2004), which used a relatively ethnically homogenous sample. It is possible that the genetic variants we examined are in linkage disequilibrium with other genes that influence SRET performance. Some analyses of subgroups had fairly small sample sizes (i.e., those considering the offspring of mothers with depression during their children's lifetimes in different genetic groups); this also limited our ability to conduct more exploratory analyses of gene–gene interaction and gene–gene–environment interaction. Children completed the CDI following the MIP and SRET, which raises the possibility that the task influenced CDI scores. However, because we gave children several small gifts to reverse the effects of the MIP and CDI scores were well within the normal range, this argues against this possibility. All children received a negative mood induction, because this is widely held to be an essential step in eliciting cognitive vulnerability (e.g., Miranda, Gross, Persons, & Hahn, 1998). However, the lack of a nondysphoric condition in our study means that we cannot speak to the question of the importance of the mood manipulation in facilitating the associations we found between our candidate polymorphisms and SRET performance. Similarly, we refer to performance on the SRET as indexing “cognitive reactivity” as is traditionally done in research on cognitive vulnerability (Scher et al., 2005), although we did not conduct a SRET prior to the mood induction to verify that task performance changed as a result of the induction. However, the large body of research showing that negative mood priming is essential to detecting differences in cognition when comparing depressed and at-risk individuals and those without depression risk (for reviews, see Segal & Ingram, 1994; Scher et al., 2005) suggests that tasks such as ours are indexing cognitive change in response to negative mood, that is, negative mood reactivity that is a marker of depression risk. Further, administering the task twice might itself have had unwanted effects on performance and would have been especially burdensome to our young sample. Finally, a complete test of the proposed model will require that participants continue to be tracked into the age of risk for the onset of depression.
A final limitation is that we did not conduct another clinical interview with mothers at follow-up; our study is therefore limited by the lack of diagnostic data on maternal depression for the mean 2.5 years between baseline and follow-up assessments. However, we have little reason to suspect that there were many new (incident) cases of depression occurring over this follow-up period: in a previous sample (Hayden et al., 2006), we found a very low rate of first lifetime episodes of MDD (N = 4) and DD (N = 0) over a 4-year follow-up period in a similar, community-dwelling group of 61 mothers roughly the same age as those in this study. Moreover, in the present sample, only 1% of mothers were currently depressed at the age 6 follow-up based on self-report data from the Diagnostic Inventory for Depression (Zimmerman, Scheeran, & Young, 2004), and all of these mothers had previous episodes of either MDD or DD based on the nonpatient version of the Structured Clinical Interview for DSM-IV. Hence, it seems unlikely that having clinical data on mothers across this 3-year gap would have significantly altered our findings.
The present study provides additional support for the notion that the 5-HTTLPR contributes to depression via its effects on cognitive reactivity that emerge relatively early in development. This research also lends support to previous work from our group indicating that the BDNF methionine allele increases children's sensitivity to context, and is thus linked to outcomes both good and bad. We have previously argued that children with this allele, by virtue of their enhanced responsivity to context, might be especially likely to derive benefits from targeted prevention (Hayden, Klein, Dougherty, et al., 2010). The present findings lend further support to this possibility and suggest that these children's depression-related cognitions may provide a particularly promising focus for preventative and early intervention efforts.
Acknowledgments
This research was supported by a Young Investigator award from the National Alliance for Research on Schizophrenia and Depression (to E.P.H.), General Clinical Research Center Grant M01-RR10710 (to Stony Brook University) from the National Center for Research Resources, and National Institute of Mental Health Grant R01 MH069942 (to D.N.K.).
Footnotes
Although there is clear evidence of direct effects on intermediate depression phenotypes, the association of 5-HTTLPR with depression more specifically may be moderated by life stress and adversity (Caspi et al., 2003; Karg, Burneister, Shedden, & Sen, 2011; Uher & McGuffin, 2010). However, these findings continue to be controversial (Duncan & Keller, 2011; Risch et al., 2009).
Examining the mean ranks (from a Kruskal–Wallis test) of negative SRET scores showed that children heterozygous for the 5-HTTLPR variants (M = 191.86) were similar to those homozygous for the short allele (M = 198.32) and had higher negative SRET scores than those of the L/L children (M = 174.91), supporting our decision to treat the short variant as dominant in analyses. Analyses using the three groups in the full model yielded results that were similar to those presented using the L/L and S/S + S/L groups. This pattern of findings is consistent with early work on this polymorphism (Lesch et al., 1996), which provided ample evidence for a functionally dominant effect of the short allele with respect to neuroticism-related traits. Similar analyses of the three BDNF genotypes were likewise supportive of our decision to contrast all children with a methionine allele to those without this variant; however, the small number of children in the met/met group limited the extent to which we had adequate power to test the specific effects of this genotype.
Effect sizes cannot be reported for maternal depression history and the BDNF × Maternal Depression interaction term because these are 2 df tests (i.e., as the overall interaction effect comprises two different regression parameters).
References
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. Guilford Press; New York: 2008. [Google Scholar]
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: New evidence of gene–environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. International Universities Press; Oxford: 1976. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Ellis AJ, Wells TT, McGeary JE. Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biological Psychology. 2010;83:260–265. doi: 10.1016/j.biopsycho.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Scott WD, McGeary C, McGeary JE. Negative cognitive response to a sad mood induction: Associations with polymorphisms of the serotonin transporter (5-HTTLPR) gene. Cognition and Emotion. 2009;23:726–738. [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Science USA. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Veenstra-VanderWeele J. Genetic indeterminism, the 5-HTTLPR, and the paths forward in neuropsychiatric genetics. Archives of General Psychiatry. 2011;68:457–458. doi: 10.1001/archgenpsychiatry.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle F, van den Hove DLA, Jakob SB, Rutten BP, Hamon M, van Os J, et al. Epigenetic regulation of the BDNF gene: Implications for psychiatric disorders. Molecular Psychiatry. 2012;17:584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Brenner E. Mood induction in children: Methodological issues and clinical implications. Review of General Psychology. 2000;4:264–283. [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta J-K. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B. Word frequency book. 1st ed. American Heritage; New York: 1971. [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen Z-Y, Patel PD, Sant G, Meng C-X, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. Journal of Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorbov VM, Lobos EA, Todorov AA, Health AC, Botteron KN, Todd RD. Relationship of 5-HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. American Journal of Medical Genetics. 2007;114B:830–833. doi: 10.1002/ajmg.b.30534. [DOI] [PubMed] [Google Scholar]
- Cole DA, Ciesla JA, Dallaire DH, Jacquez FM, Pineda AQ, La-Grange B, et al. Emergence of attributional style and its relation to depressive symptoms. Journal of Abnormal Psychology. 2008;117:16–31. doi: 10.1037/0021-843X.117.1.16. [DOI] [PubMed] [Google Scholar]
- Crisan LG, Pana S, Vulturar R, Heilman RM, Szekely R, Druga B, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Social, Cognitive, and Affective Neuroscience. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test 4. 3rd ed. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders—Non-patient edition. 1st ed. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proceedings of the Royal Society Biological Sciences. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Gallerani CM, Frankel SA. Depression in children. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. Guilford Press; New York: 2009. pp. 405–443. [Google Scholar]
- Garber J, Kaminski KM. Laboratory and performance-based measures of depression in children and adolescents. Journal of Clinical Child Psychology. 2000;29:509–525. doi: 10.1207/S15374424JCCP2904_5. [DOI] [PubMed] [Google Scholar]
- Garber J, Martin NC. Negative cognitions in offspring of depressed parents: Mechanisms of risk. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. American Psychological Association; Washington, DC: 2002. pp. 121–153. [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children's attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Uhrlass DJ, Grassia M, Benas JS, McGeary J. Children's inferential styles, 5-HTTLPR genotype, and maternal expressed emotion–criticism: An integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2009;118:734–745. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Brand SR. Parental psychopathology and its relation to child psychopathology. In: Hersen M, Gross AM, editors. Handbook of clinical psychology. Wiley; Hoboken, NJ: 2008. pp. 937–965. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Stability of cognitive vulnerabilities to depression: A short-term prospective multiwave study. Journal of Abnormal Psychology. 2008;117:324–333. doi: 10.1037/0021-843X.117.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer C, Jenness J, Young JF, Abela JRZ, et al. Differential susceptibility in youth: Evidence that 5-HTTLPR × Positive Parenting is associated with positive affect “for better and worse.”. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Oppenheimer C, Jenness J, Barrocas A, Shapero BG, Goldband J. Developmental origins of cognitive vulnerabilities to depression: Review of processes contributing to stability and change across time. Journal of Clinical Psychology. 2009;65:1327–1338. doi: 10.1002/jclp.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Durbin CE, Olino TM, Nurnberger JI, et al. Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: A multimethod association study. Psychiatric Genetics. 2007;17:135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, et al. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. Journal of Affective Disorders. 2008;107:227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Dyson MW, Durbin CE, et al. The role of brain-derived neurotrophic factor genotype, parental depression, and relationship discord in predicting early-emerging negative emotionality. Psychological Science. 2010;21:1678–1685. doi: 10.1177/0956797610385357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development and Psychopathology. 2006;18:409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Sheikh HI, Olino TM, Dougherty LR, Dyson MW, et al. The serotonin transporter promoter polymorphism and childhood positive and negative emotionality. Emotion. 2010;10:696–702. doi: 10.1037/a0019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Sheikh HI, Katsiroumbas P, Jordan P, Singh SM, Olino TM, et al. Early-emerging depressogenic information processing: Stability across time and associations with childhood individual differences and early contextual factors; Paper presented at the annual meeting of the Association for Cognitive and Behavior Therapies; San Francisco, CA. 2010. [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AA. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neuroscience Letters. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. 1975. Unpublished manuscript.
- Homberg JR, Lesch K-P. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Harris SE, Luciano M, Gow AJ, Starr JM, Visscher PM, et al. Replication study of candidate genes for cognitive abilities: The Lothian birth cohort 1936. Genes, Brain & Behavior. 2009;8:238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Edelsohn G, Kellam SG. A further look at the prognostic power of young children's reports of depressed mood. Child Development. 2001;72:736–747. doi: 10.1111/1467-8624.00312. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Molecular Psychiatry. 2009;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. Guilford Press; New York: 2009. pp. 298–321. [Google Scholar]
- Joorman J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.189. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic factor-5-HHTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Klein DN, Riso LP, Donaldson SK, Schwartz JE, Anderson RL, Ouimette PC, et al. Family study of early-onset dysthymia: Mood and personality disorders in relatives of outpatients with dysthymia and episodic major depression and normal controls. Archives of General Psychiatry. 1995;52:487–496. [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Rohde P, Seeley JR. Family study of chronic depression in a community sample of young adults. American Journal of Psychiatry. 2004;161:646–653. doi: 10.1176/appi.ajp.161.4.646. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Rose S. Ten-year prospective follow-up study of the naturalistic course of dysthymic disorder and double depression. American Journal of Psychiatry. 2006;163:872–880. doi: 10.1176/ajp.2006.163.5.872. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Beck AT. An empirical–clinical approach toward a definition of childhood depression. In: Schulterbrandt JG, Raskin A, editors. Depression in childhood: Diagnosis, treatment, and conceptual models. Raven Press; New York: 1977. pp. 1–25. [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, et al. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K, Bengel D, Heils A, Sabol SZ. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Mao LM, Fibuch EE, Wang JQ. Decoding BDNF-LTP coupling in cocaine addiction. Neuron. 2010;67:679–681. doi: 10.1016/j.neuron.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Rafaeli E, Yovel I. Cognitive biases in emotional disorders: Social– cognitive and information processing perspectives. In: Davidson R, Goldsmith H, Scherer K, editors. Handbook of affective science. Oxford University Press; Oxford: 2003. pp. 976–1009. [Google Scholar]
- Miranda J, Gross JJ, Persons JB, Hahn J. Mood matters: Negative mood induction activates dysfunctional attitudes in women vulnerable to depression. Cognitive Therapy and Research. 1998;22:363–376. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of Genetic Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. 5-HTTLPR genotype and anxiety-related personality traits: A meta-analysis and new data. American Journal of Medical Genetics. 2009;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: Results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. Journal of Neuroscience. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepletchikova F, Kaufman J. Genetic and environmental predictors of depression. In: Kendler KS, Jaffee S, Romer D, editors. The dynamic genome and mental health: The role of genes and environments in youth development. Oxford University Press; New York: 2011. pp. 272–293. [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Molecular Psychiatry. 2009;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. Journal of Neuroscience. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: Lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Schmidt NB, Lonigan CJ, Phillips BM, Catanzaro SJ, Laurent J, et al. The latent structure of child depression: A taxometric analysis. Journal of Child Psychology and Psychiatry. 2009;50:1147–1155. doi: 10.1111/j.1469-7610.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. International Journal of Neuropsychopharmacology. 2007;10:449–461. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schule C, Zill P, Baghai TC, Eser D, Zwanzger P, Wenig N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Ingram RE. Mood priming and construct activation in tests of cognitive vulnerability to unipolar depression. Clinical Psychology Review. 1994;14:663–695. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Hayden EP, Singh SM, Dougherty LR, Olino TM, Durbin CE, et al. An examination of the association between the 5-HTT promoter region polymorphism and depressogenic attributional styles in childhood. Personality and Individual Differences. 2008;45:425–428. doi: 10.1016/j.paid.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108:202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Watters AJ, Williams LM. Negative biases and risk for depression: Integrating self-report and emotion task markers. Depression and Anxiety. 2011;28:703–718. doi: 10.1002/da.20854. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Sheeran T, Young D. The Diagnostic Inventory for Depression: A self-report scale to diagnose DSM-IV major depressive disorder. Journal of Clinical Psychology. 2004;60:87–110. doi: 10.1002/jclp.10207. [DOI] [PubMed] [Google Scholar]