Abstract
Despite recent clinical guidelines recommending early initiation and widespread use of antiretroviral therapy (ART), many HIV-infected individuals are not receiving ART—in particular low-income, minority substance users. Few studies have examined psychological, as opposed to structural, factors related to not receiving ART in this population. Perceived capacity to tolerate physical and psychological distress, known as distress tolerance (DT), may be a particularly relevant yet understudied factor. The current study tested the relationship between self-reported physical and psychological DT and ART receipt among predominantly low-income, minority HIV-infected substance users (n=77). Psychiatric disorders, biological indicators of health status, ART use, structural barriers to health care, and self-reported physical and psychological DT were assessed. 61% of participants were receiving ART. The only factors that distinguished individuals not on ART were greater avoidance of physical discomfort, higher psychological DT, and higher CD4 count. Both DT measures remained associated with ART use after controlling for CD4 count and were associated with almost a two-fold decrease in likelihood of ART receipt. Current findings suggest higher perceived capacity to tolerate psychological distress and greater avoidance of physical discomfort are important factors associated with lower ART use among substance users and may be important intervention targets.
Introduction
Recent clinical guidelines recommend earlier initiation and more widespread antiretroviral therapy (ART) use for optimal HIV/AIDS outcomes, with current recommendations suggesting ART use for nearly all individuals diagnosed with HIV, regardless of disease stage.1,2 ART use is critical for viral suppression and slower disease progression and may also slow HIV transmission to others.3 Yet, a recent Centers for Disease Control and Prevention report estimated that only 45% of individuals diagnosed with HIV are receiving ART.4 In particular, low-income, minority substance users have consistently been identified as a high-risk group for not receiving ART.5–7 Increasing the proportion of individuals receiving ART, particularly in these high-risk groups, has important implications for HIV treatment and prevention.
Although a great deal of empirical attention has been paid to structural (e.g., transportation, cost) barriers to ART receipt, there has been less research on equally important psychological barriers (e.g., fear of side effects). This is despite evidence from a recent survey that among a sample of patients living with HIV/AIDS (n=158), 82% reported psychological barriers to receiving HIV treatment, including fear of physical side effects, fear of other people knowing, and stigma, as opposed to structural factors.8 Evidence indicates that patients face numerous forms of physical and psychological distress when considering ART use.9,10 As such, individual differences in one's perceived capacity to cope with or tolerate this distress may play an important role in predicting initiation and continuation of ART. Psychosocial models of HIV management recognize not only the disproportionate levels of distress experienced by individuals living with HIV/AIDS (e.g., related to living with a chronic condition, stigma, trauma), but also how individuals' response to distress is important in predicting optimal management of HIV/AIDS; for instance, previous research testing a stress coping model of HIV management among individuals on ART (n=322) found that greater avoidance in the face of negative affect predicted worse HIV outcomes over a 15-month period.11
One construct that captures individual differences in response to distress is distress tolerance (DT), conceptualized either as one's perceived capacity to withstand aversive physical and psychological states (i.e., assessed via self-report measures), or one's ability to withstand behaviorally psychological or physical distress (i.e., assessed via latency to discontinuation of a distressing task).12,13 Both physical and psychological DT have been conceptualized as multi-dimensional constructs; physical DT reflects both one's perceived capacity to tolerate physical discomfort and tendency to avoid physical discomfort,12 whereas psychological DT encompasses one's perceived tolerability of negative emotional states, appraisal and acceptability of negative emotions, levels of absorption by negative emotion, and capacity to regulate negative emotion.13
The DT construct maps closely onto existing psychosocial models of HIV management given the attention to perceived capacity to tolerate both physical and psychological states. Previous researchers have suggested the importance of examining DT in HIV management, as numerous stressors involved with HIV treatment may contribute to psychological and physical distress and perpetuate maladaptive coping responses among individuals with low psychological DT.14 Regarding the important outcome of ART receipt specifically, there are numerous forms of physical (i.e., side effects) and psychological (i.e., fear, stigma) distress associated with receiving ART. As such, individual differences in perceived capacity for tolerating such distress may distinguish individuals who delay ART receipt. Previous work in the area of HIV/AIDS has found a relationship between low psychological DT and both ART nonadherence and greater HIV symptom levels, such that individuals with lower psychological DT had greater ART nonadherence and symptom levels.15 However, DT has not been tested in relation to receipt of ART, only ART adherence, and more fine-grained analyses of the multiple dimensions of DT have not previously been examined in this relationship. In light of the numerous forms of distress associated with being on ART, this may be very relevant.
The current study aimed to expand upon existing stress coping models of HIV management to focus specifically on the construct of DT in order to improve our understanding of potential psychological barriers to ART receipt. Specifically, the current study tested the relationship between self-reported capacity to tolerate both physical and psychological distress and ART receipt among low-income, largely African American substance using individuals living with HIV/AIDS, a group at high risk for not receiving ART.5–7 Study hypotheses included that lower levels of self-reported physical and psychological DT would be associated with lower likelihood of ART receipt and that these associations would remain significant above and beyond the variance accounted for by other relevant factors that distinguish individuals based on ART use, including demographic characteristics, health status (CD4 count), and structural barriers to care.
Methods
Participants for the current study were recruited from a large urban residential substance abuse treatment center. Patients were referred by government agencies or mandated to treatment by the court system. Patients were required to have completed full detoxification and have a negative urine drug screen upon admission to the treatment facility. Regular urinalysis drug testing was conducted, and any substance use was grounds for program dismissal. Medication assisted therapy (MAT) was not provided at this treatment center (i.e., methadone, buprenorphine, vivitrol, or naltrexone for opioid dependence and naltrexone, vivitrol, acamprosate, and disulfiram for alcohol dependence).
Patients were recruited for the study after their first week in treatment and before completion of their third week. Eighty HIV-infected individuals were approached for participation; of those, three declined participation. Patients were in substance abuse treatment for a mean of 14.11 days (S.D.=8.67) at the time of the study assessment. During this time period, all patients in the facility received a standard intake interview that included an assessment of medical history and medication use. Patients were eligible for participation if they self-reported being HIV positive, which was also verified with treatment center medical records. Eligible patients were provided detailed information about the study and those interested provided informed consent. The importance of maintaining patient confidentiality and privacy was stressed throughout the study screening session. Additionally, treatment center staff members were not made aware of patients' study participation or refusal, and participation in the study did not affect patients' status in treatment. All study procedures were approved by the University Institutional Review Board.
Participants were administered the Structured Clinical Interview for DSM-IV (SCID-I)16 that included an assessment of current Axis I disorders, including all mood, anxiety, psychotic, and substance use disorders (assessed for alcohol, crack/cocaine, heroin, other opioid medications, cannabis, sedatives, stimulants, hallucinogens, and poly substance use). All Axis I disorders with greater than or equal to 5% prevalence in the sample were included in Table 1.
Table 1.
Comparison of Groups on Demographic Variables, Psychopathology, Health Status, Structural Barriers to Care
| Overall (n=77) | On ART (n=47) | Not on ART (n=30) | Statistic | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 45.40 (7.92) | 45.74 (7.83) | 44.82 (8.18) | t(75)=0.49 | 0.63 |
| Gender | χ2 (2)=3.06 | 0.22 | |||
| Male, % | 41.33 | 48.94 | 28.57 | ||
| Female, % | 50.67 | 44.68 | 60.71 | ||
| Transgender, % | 8.00 | 6.38 | 10.71 | ||
| Marital status | χ2 (2)=1.22 | 0.54 | |||
| Single, % | 62.67 | 57.45 | 71.43 | ||
| Separated/divorced, % | 26.67 | 29.79 | 21.43 | ||
| Married, % | 9.33 | 10.64 | 7.14 | ||
| Race | χ2 (3)=3.48 | 0.32 | |||
| White, % | 1.33 | 2.13 | 0.00 | ||
| Black, % | 94.67 | 93.62 | 96.43 | ||
| Hispanic, % | 2.67 | 4.26 | 0.00 | ||
| Native American, % | 1.33 | 0.00 | 3.57 | ||
| Heterosexual, % | 73.00 | 73.91 | 71.43 | χ2 (2)=0.09 | 0.96 |
| ≤High school/GED, % | 78.67 | 78.72 | 78.57 | χ2 (1)=0.00 | 0.99 |
| Total annual income <$10,000, % | 78.67 | 80.85 | 75.00 | χ2 (1)=0.36 | 0.55 |
| Unemployed, % | 89.33 | 85.11 | 96.43 | χ2 (1)=2.36 | 0.12 |
| Has a PCP, % | 95.95 | 97.83 | 92.86 | χ2 (1)=1.11 | 0.29 |
| Has a health insurance plan, % | 93.24 | 95.65 | 89.29 | χ2 (1)=1.12 | 0.29 |
| Homeless, % | 11.69 | 8.51 | 16.67 | χ2 (1)=1.18 | 0.28 |
| Prescribed psychotropic medication, % | 51.32 | 46.81 | 58.62 | χ2 (1)=1.00 | 0.32 |
| Psychopathology* | |||||
| PTSD, % | 12.33 | 13.64 | 10.34 | χ2 (1)=0.18 | 0.68 |
| MDD, % | 20.00 | 15.56 | 26.67 | χ2 (1)=1.39 | 0.24 |
| GAD, % | 6.67 | 8.70 | 3.45 | χ2 (1)=1.22 | 0.27 |
| Bipolar I, % | 14.47 | 10.87 | 20.00 | χ2 (1)=0.79 | 0.38 |
| Hal dependence (past year), % | 6.49 | 10.64 | 0.00 | χ2 (1)=3.41 | 0.07 |
| Cannabis dependence (past year), % | 6.67 | 6.67 | 6.67 | χ2 (1)=0.00 | 0.99 |
| Crack/cocaine dependence (past year), % | 53.25 | 48.94 | 60.00 | χ2 (1)=0.90 | 0.34 |
| Alcohol dependence (past year), % | 31.58 | 30.43 | 33.33 | χ2 (1)=0.07 | 0.79 |
| Opioid (heroin) dependence (past year), % | 25.33 | 24.44 | 26.67 | χ2 (1)=0.05 | 0.83 |
| Health status | |||||
| CD4 count, mean (SD) | 491.21 (300.86) | 442.36 (256.73) | 573.21 (353.08) | t(75)=− 1.89 | 0.06 |
| Viral load (Log10), mean (SD) | 2.46 (1.14) | 1.97 (0.83) | 3.67 (0.89) | t(75)=−7.37 | 0.0001 |
| Years since HIV diagnosis, mean (SD) | 10.30 (7.39) | 10.74 (6.99) | 9.11 (8.52) | t(73)=.75 | 0.46 |
| Structural barriers | |||||
| Access to care, mean (SD) | 75.60 (20.56) | 78.47 (16.88) | 70.88 (25.11) | t(74)=1.56 | 0.12 |
| Patient–doctor relationship, mean (SD) | 39.16 (7.43) | 40.20 (6.49) | 37.46 (8.62) | t(74)=1.55 | 0.13 |
All current Axis I diagnosis with≥5% prevalence in current sample are listed. GAD, generalized anxiety disorder; Hal, hallucinogen; MDD, major depressive disorder; PCP, primary care physician; PTSD, post-traumatic stress disorder.
To assess biological measures of health status, participants' CD4 count and viral load were obtained within a 3-month window of the study assessment (mean=9.57 days before; S.D.=30.35 days). Viral load was highly skewed (skewness=4.08, S.E.=0.29) and as such was log10 transformed.
Structural barriers to health care were assessed using a 9-item scale measuring access to care, including affordability, convenience, and availability (higher scores indicate greater access)17 and the patient-doctor relationship questionnaire (PDRQ-9; higher scores indicate a more favorable patient-doctor relationship).18 In addition to basic demographic information, participants self-reported years since HIV diagnosis, whether they had a primary care provider (PCP) and a health insurance plan, and whether they were homeless prior to substance abuse treatment entry. They also self-reported whether they were currently receiving ART (yes/no) and/or psychotropic medication, both of which were verified with treatment center medical records.
Perceived capacity to tolerate physical distress was measured using the 5-item self-report Discomfort Intolerance Scale (DIS),12 which measures individuals' perceived capacity to withstand uncomfortable but nonpainful bodily sensations. The DIS has two subscales: (1) Intolerance of physical discomfort (e.g., “I can tolerate a great deal of physical discomfort”); and (2) Avoidance of physical discomfort (e.g., “I take extreme measures to avoid feeling physically uncomfortable”). Higher scores on the subscales indicate greater intolerance of physical discomfort and greater avoidance of physical discomfort. Internal consistency of both subscales in the current sample were acceptable (Avoidance: α=0.77; Intolerance: α=0.73), which is consistent with other studies that have used the DIS in a similar population (i.e., among heavy substance users).19,20 To test specific relationships of the components of self-reported physical DT and ART use, all analyses were conducted using the separate subscales in line with previous recommendations using the DIS.21
Perceived capacity to tolerate psychological distress was assessed using the 15-item self-report Distress Tolerance Scale (DTS),13 which assesses perceived ability to withstand negative emotional states. The DTS has four subscales: (1) Tolerance of distress (e.g., “I can't handle feeling distressed or upset”); (2) Appraisal of distress (e.g., “My feelings of distress or being upset are not acceptable”); (3) Absorption of attention by negative emotion (e.g., “When I feel distressed or upset, I cannot help but concentrate on how bad the distress actually feels”); (4) Regulation efforts to alleviate distress (e.g., “When I feel distressed or upset I must do something about it immediately”). Higher scores on the subscales indicate greater perceived capacity to tolerate psychological distress, less negative appraisal of distress, less absorption by negative emotion, and less need to immediately regulate distress. Internal consistency of DTS subscales ranged from fair to acceptable in the current sample (Tolerance: α=0.75; Absorption: α=0.75; Regulation=0.62; Appraisal=0.68), which is consistent with other studies that have administered the DTS among largely minority HIV positive substance users.14,15,22 To test specific relationships of the components of self-reported psychological DT and ART use, all analyses were conducted using the separate subscales, which is also in line with previous recommendations.13
Statistical analyses overview
First, group status was determined based on ART use status (yes/no), which was self-reported and verified with treatment center medical records. Next, independent samples t-tests and chi square tests were used to compare the two groups on demographic information, psychopathology, health status (CD4 count and viral load, years living with HIV and PCP status), structural barriers to care, patient–doctor relationship, and self-reported physical and psychological DT. Any significant differences between groups on demographic or clinical information were to be included as covariates in subsequent analyses, which follow from statistical recommendations to select covariates based on relationships with the main outcome.23 As a conservative approach to identify potential covariates, group differences were assessed at p<0.10. Group differences in the DT subscales were also examined to determine which scales to use as the independent variable (IV) in the model. To identify the contribution of DT to ART receipt, a logistic regression analysis was conducted with ART receipt as the dependent variable (0=ART use; 1=no ART use), entering any group differences in demographic or clinical information as covariates in the first step, and in the second step, entering any relevant DT measures (i.e., subscales of the DIS or DTS) that differed across groups.
Results
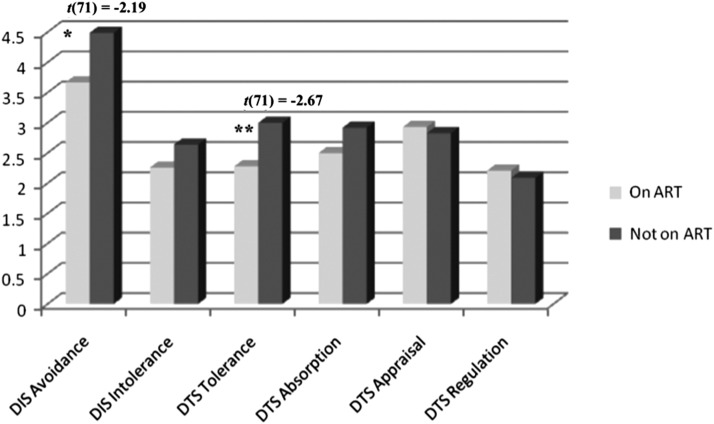
Of the final sample (n=77), 94.7% were Black, 73% heterosexual, 50.7% women, 78.7% had less than or equal to a high school degree or GED, and 78.7% had a total annual income of less than $10,000. Regarding ART use, in total, 61% (n=47) of the sample was receiving ART and 39% (n=30) was not receiving ART. Individuals not receiving ART had slightly higher CD4 counts (t(75)=−1.89, p=0.06) and higher viral load (viral load (t(75)=−7.37, p<0.0001). Group differences in viral load were not included as a covariate, as changes in viral load are the direct biological consequence of ART; group differences in viral load support credibility of the distinction of the two groups. CD4 count was included as a covariate in subsequent analyses. Regarding group differences in the DT subscales, individuals not receiving ART had higher self-reported avoidance of physical discomfort, (t(71)=−2.19, p<0.05) and higher self-reported psychological distress tolerance (t(71)=−2.67, p<0.01). All other comparisons were nonsignificant (see Table 1 and Fig. 1).
FIG. 1.
Group differences in self-reported physical and psychological DT based on ART use. Note: *p<0.05; **p<0.01; DIS, Discomfort Intolerance Scale (High Scores, Lower Tolerance; Greater Avoidance); DTS, Distress Tolerance Scale (High Scores, Higher Tolerance); DIS Intolerance: t(71)=−0.87, p=0.66; DTS Absorption=t(71)=−1.53, p=0.25; DTS Appraisal t(71)=0.48, p=0.24; DTS Regulation=t(71)=0.51, p=0.64.
To identify the contribution of the relevant DT subscales to ART receipt, a logistic regression analysis was conducted with ART receipt as the dependent variable (0=ART use; 1=no ART use) controlling for CD4 count, the only group difference using the one-tailed test. CD4 count was entered in the first step, and in the second step, the DIS Avoidance scale and DTS Tolerance scale were entered. Given potential issues of collinearity of the two measures of DT, we examined the correlation between subscales. No DIS or DTS subscales were significantly correlated; the DIS Avoidance the DTS Tolerance subscales that were entered into the model were uncorrelated (r=−0.09; p=0.46). In the final model, both DT variables remained significant after controlling for CD4 count (Table 2). Specifically, greater avoidance of physical discomfort and higher perceived capacity to tolerate psychological distress reduced the likelihood of being on ART almost two-fold (DIS Avoidance Subscale: [O.R.=1.59, p<0.01]; DTS Tolerance Subscale: [O.R.=1.84, p<0.01]).
Table 2.
Logistic Regression Analysis with Avoidance of Physical Discomfort and Psychological DT Predicting ART Use
| B | SE | Wald | OR (95% CI) | p | |
|---|---|---|---|---|---|
| Step 1 | |||||
| CD4 count | 0.002 | 0.001 | 3.60 | 1.00 (1.00–1.001) | 0.06 |
| Step 2 | |||||
| DIS Avoidance | 0.46 | 0.18 | 6.33 | 1.59 (1.11–2.28) | 0.01 |
| DTS Tolerance | 0.61 | 0.25 | 6.10 | 1.84 (1.13–2.99) | 0.01 |
DIS, Discomfort Intolerance Scale; DTS, Distress Tolerance Scale; DV, ART use status (0=on ART; 1=not on ART).
Discussion
The current study examined the relationship between perceived capacity to tolerate both physical and psychological distress and ART receipt among individuals living with HIV/AIDS entering a large, urban substance abuse treatment center. Results indicate that a substantial proportion of the sample was not using ART at treatment entry (39%), which is particularly problematic given recent recommendations to initiate ART earlier and across a more widespread range of disease stages,1,2 as well as evidence suggesting the potential utility of ART for secondary prevention.3 Study hypotheses were partially confirmed. As hypothesized, individuals who were more avoidant of physical discomfort were significantly less likely to be receiving ART; however, contrary to study hypotheses, higher perceived capacity to tolerate psychological distress (i.e., being more able tolerate psychological distress) also was associated with lower likelihood of receiving ART. The only other variable that distinguished individuals based on ART receipt was CD4 count, such that individuals not receiving ART had significantly higher CD4 counts. This is in line with recent evidence demonstrating that individuals with higher CD4 counts have increased odds and greater numbers of ART treatment interruptions.24
The finding that higher self-reported avoidance of physical discomfort was associated with approximately a two-fold decrease in the likelihood of receiving ART is in line with previous work pointing to patients' fear of side effects being a real barrier to ART use.8 Individuals who perceive themselves as highly avoidant of physical discomfort may not want to initiate or accommodate to a new treatment regimen. It is interesting that the groups differed only on discomfort avoidance and not actual perceived tolerance of physical discomfort. This can be understood given previous research indicating that discomfort avoidance, as opposed to intolerance, is more highly associated with poor psychological outcomes; it has been suggested that avoidance represents a more “extreme reaction” then intolerance, as avoidant individuals do not even enter a potentially uncomfortable scenario.21
High self-reported psychological DT also was associated with approximately a two-fold decrease in the likelihood of receiving ART. This is contrary to the directionality of previous findings that that low levels of self-reported psychological DT are problematic for ART adherence, substance use, and other poor outcomes.13–15 One explanation of this finding is that individuals with high psychological DT may be more able to deal with distress of inevitable disease progression without appropriate medical treatment. Although counterintuitive, this is in line with the idea of “distress overtolerance,” which suggests that high psychological DT may be maladaptive when individuals tolerate a high degree of distress “in a manner that does not fit with long-term values or interests.”25 In the present study, individuals with high levels of psychological DT may have been more likely to tolerate the uncertainty and distress of an inevitably worsening health status, which may be associated with a delay in initiating or re-initiating ART. However, these conclusions are speculative and require further empirical attention and replication. Additionally, study findings suggest that appraisal of, degree of absorption by, and regulation of distress are not as strongly related to ART receipt as is actual perceived tolerance of distress; however, these findings also need to be replicated, particularly incorporating a multi-method, behavioral measurement of DT.
Findings should be interpreted in light of study limitations, including reliance on self-reported measurement of key study variables, cross-sectional design, effects with relatively low magnitude, and a small sample size. Yet, it is worth noting that the population targeted—low-income, largely African American substance users living with HIV—are difficult to recruit and are at very high risk for poor HIV outcomes and continued transmission; thus, even recruiting a small sample cross-sectionally in this population has important public health implications. Although also a strength of the current study, the fact that the current sample was comprised of almost exclusively African American individuals—with approximately half of the sample being female and three-fourths of the sample identifying as heterosexual—generalizability of findings to other HIV-infected individuals, such as Caucasian men who have sex with men or nonsubstance-using patients should be done with caution.7 Finally, the study assessment was limited in that it did not distinguish between individuals who were ART naïve vs. had discontinued ART. This is an important distinction that must be made in future replications of this work. The assessment also did not include duration of ART use or a more comprehensive behavioral assessment of DT. Next steps include replicating the current findings using a larger sample size, prospective design, and more comprehensive, multi-method assessment of ART use and DT.
Despite these limitations, current findings have important clinical implications, including the need to focus on psychological barriers to ART receipt, substance abuse treatment as a clinical setting to identify individuals not receiving ART, and the potential utility of physical and psychological DT measures as screening tools for mechanisms underlying lack of ART receipt among substance users. Additionally, future intervention efforts aimed at increasing ART use may consider targeting one's capacity for facing and tolerating physiological and psychological distress associated with initiating and persisting with an ART regimen, for instance, using acceptance-based approaches that target avoidance of uncomfortable thoughts, feelings, and/or bodily sensations.26 If findings continue to replicate, DT may be an important intervention target for increasing ART use among difficult to reach, low-income African American substance users living with HIV/AIDS.
Acknowledgments
This work was supported by National Institute of Drug Abuse Grants R01DA022974 (PI: Daughters) and R36DA034513 (PI: Magidson).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thompson MA. Aberg JA. Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 Recommendations of the International Antiviral Society—USA Panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Clinical guidance across the continuum of care: Antiretroviral therapy. Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection. Jun, 2013. http://www.who.int/hiv/pub/guidelines/arv2013/art/arv2013_chapter07_low.pdf. [Jul 8;2013 ]. pp. 90–154.http://www.who.int/hiv/pub/guidelines/arv2013/art/arv2013_chapter07_low.pdf
- 3.Cohen MS. Chen YQ. McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Vital signs: HIV prevention through care and treatment: U.S. CDC MMWR. 2011;60:618–623. [Google Scholar]
- 5.Chander G. Himelhoch S. Fleishman JA, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21:655–663. doi: 10.1080/09540120802459762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisels L. Steinberg J. Tobias C. An investigation of why eligible patients do not receive HAART. AIDS Patient Care STDs. 2001;15:185–191. doi: 10.1089/10872910151133701. [DOI] [PubMed] [Google Scholar]
- 7.Thames AD. Moizel JM. Panos SE, et al. Differential predictors of medication adherence in HIV: Findings from a sample of African American and Caucasian HIV-positive drug-using adults. AIDS Patient Care STDs. 2012;26:621–630. doi: 10.1089/apc.2012.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seekins D. Scibelli A. Juday T. Stryker R. Das A. Barriers to accessing HIV testing, care, and treatment in the United States. Presented at the XVIII International AIDS Conference; Vienna, Austria. Jul 18–23;2010 ; Abstract THPE0624. [Google Scholar]
- 9.Chesney MA. Smith AW. Critical delays in HIV testing and care: The potential role of stigma. Am Behav Sci. 1999;42:1162–1174. [Google Scholar]
- 10.Harding R. Molloy T. Easterbrook P. Frame K. Higginson I. Is antiretroviral therapy associated with symptom prevalence and burden? Int J STD AIDS. 2006;17:400–405. doi: 10.1258/095646206777323409. [DOI] [PubMed] [Google Scholar]
- 11.Weaver KE. Lllabre MM. Duran RE, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on HAART. Health Psych. 2005;24:385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt NB. Richey JA. Fitzpatrick KK. Discomfort intolerance: Development of a construct and measure relevant to panic disorder. J Anxiety Disord. 2006;20:263–280. doi: 10.1016/j.janxdis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Simons J. Gaher R. The Distress Tolerance Scale: Development and validation of a self-report measure. Motiv Emot. 2005;29:83–102. [Google Scholar]
- 14.Brandt CP. Zvolensky MJ. Bonn-Miller BO. Distress tolerance, emotion dysregulation, and anxiety and depressive symptoms among HIV+ individuals. Cog Therapy Res. 2013:1–10. [Google Scholar]
- 15.O'Cleirigh C. Ironson G. Smits JAJ. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behav Ther. 2007;38:314–323. doi: 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First MB. Spitzer RL. Gibbon M. Williams JBW. NY: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- 17.Cunningham WE. Andersen RM. Katz MH, et al. The impact of competing subsistence needs and barriers on access to medical care for persons with HIV receiving care in the U.S. Med Care. 1999:37. doi: 10.1097/00005650-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Van der Feltz-Cornelis CM. Van Oppen P. Van Marwijk HW. De Beurs E. Van Dyck R. A patient-doctor relationship questionnaire (PDRQ-9) in primary care: Development and psychometric evaluation. Gen Hosp Psychiatry. 2004;26:115–120. doi: 10.1016/j.genhosppsych.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Buckner JD. Keough ME. Schmidt NB. Problematic alcohol and cannabis use among young adults: The roles of depression and discomfort and distress tolerance. Addict Behav. 2007;32:1957–1963. doi: 10.1016/j.addbeh.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsui JI. Herman DS. Kettavong M, et al. Chronic pain and hepatitis C virus infection in opioid dependent injection drug users. J Addict Dis. 2011;30:91–97. doi: 10.1080/10550887.2011.554775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt NB. Richey JA. Cromer KR. Buckner JD. Discomfort intolerance: Evaluation of a potential risk factor for anxiety psychopathology. Behav Ther. 2007;38:247–255. doi: 10.1016/j.beth.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Magidson JF. Listhaus AR. Seitz-Brown CJ, et al. Rumination mediates the relationship between distress tolerance and depressive symptoms among substance users. Cog Therapy Res. 2013:1–10. doi: 10.1007/s10608-012-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabachnick BG. Fidell LS. Analysis of Covariance. Using Multivariate Statistics. 5th. Pearson Education, Inc.; Boston, MA: 2007. pp. 195–242. [Google Scholar]
- 24.Adakun SA. Siedner MJ. Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. JAIDS. 2013;62:317–321. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch TR. Mizon GA. Distress over-tolerance and distress intolerance: A behavioral perspective. In: Zvolensky MJ, editor; Bernstein A, editor; Vujanovic AA, editor. In: Distress Tolerance. Guilford Press; New York, NY: 2010. [Google Scholar]
- 26.Moitra E. Herbert JD. Forman EM. Acceptance-based behavior therapy to promote HIV medication adherence. AIDS Care. 2011;23:1660–1667. doi: 10.1080/09540121.2011.579945. [DOI] [PubMed] [Google Scholar]