Abstract
The importance of nutritional components on growth and body composition outcomes has been demonstrated in multiple model organisms. Although zebrafish (Danio rerio) have an established role in research laboratories for its utility in understanding developmental biology and genetics, the influence of diet composition on basic growth outcomes is less well demonstrated. In the current study, four protein sources were tested in isolation using isonitrogenous diets or combined using a defined lab diet. Fish (n≈60/group) were group housed (n≤10 fish/1.8 L tank) and fed ad libitum three times daily for 12 weeks. Fish were assessed for effects on length, body weight, and body composition (lean and fat mass). Individuals fed wheat gluten protein were significantly shorter in length, with significantly lower body weight and lean mass in both male and female fish, although percent body fat was high compared with other diets. Casein-fed fish similarly had significantly reduced body length, body weight, and lean and fat mass in both male and female fish, with a low percent body fat compared with other diets (leanest). Fish protein hydrolysate-fed fish had significantly lower lean mass and a high percent body fat, whereas soy protein isolate diet performed similarly to a mixed-protein control diet for all measured outcomes. These results suggest that the protein source, with accompanying amino acid ratios or additional protein source differences, has a significant impact on growth and body composition outcomes in zebrafish when fed in a semipurified, defined diet background.
Introduction
Although zebrafish (Danio rerio) have been used extensively in a number of scientific disciplines (e.g., developmental biology, genetics, toxicology), their utility as a model of nutrition research has been limited, partly because of the lack of standardized, defined diets and husbandry conditions.1 This is particularly surprising given the extensive knowledge of developmental biology, the sequenced genome, available genetic models, and forward genetic screening capabilities, as well as the economy of scale allowing significantly larger sample sizes to be investigated for less cost than other common vertebrate models. Although there are multiple commercial diets that have been used in zebrafish studies, defined diets like those used in rodent research have only recently been reported.2,3 These studies have demonstrated that formulated diets of defined composition were sufficient for growth and survival promotion compared with commercial diets.
The influence of diet composition and quantity on growth, metabolism, disease, and longevity is well recognized among many species. A general theory for mammals has developed, which proposes “diets that promote growth and early maturation are inversely associated with health and longevity,” supported by diet composition studies and alterations in growth hormone signaling.4–7 Conversely, diets that delay growth or maturation, such as calorie restriction, reduce disease incidence, and increase longevity.8,9 However, observations from different fish species do not clearly support an inverse relationship between growth and longevity.10,11 Although it is commonly ascribed that caloric consumption is the main determinant of the growth, health, and longevity outcomes, it also appears that dietary composition (particularly the protein amount, source, and individual amino acids—e.g., methionine) may directly/indirectly contribute to health and longevity outcomes.12,13
Beyond basic diet macronutrient composition, there is an increasing awareness of the influence of essential dietary nutrients (e.g., amino acids) on growth and disease onset, metabolism, feeding behavior, and even longevity in a variety of animal models.12,14–19 While the essential amino acid requirements may be predicted based on other nutrition studies using teleost models,20–23 few nutrient requirement studies have been formally presented in the research literature for zebrafish.3,24,25 Nevertheless, although zebrafish nutrition and obesity research is still developing compared with rodent models,26 the information reported from decades of rodent nutrition research may be used to rapidly test dietary compositions for a variety of growth and eventual health-related outcomes.
Dietary macronutrient content including carbohydrate, fat, and protein is commonly reported and discussed as a relative percentage of total calories. Research in a number of animal models has shown that the source of dietary protein, independent of the relative caloric contribution, can have a significant impact on growth, reproduction, and disease/longevity.13 Protein sources are also reported to influence feed conversion efficiency, or a measure of how much weight is gained per calorie eaten, through alterations in energy uptake, utilization, metabolism, and/or food intake patterns.27–29 Certain protein sources, such as milk or egg, are described as growth promoting, whereas plant-based protein sources are sometimes described as growth limiting because of limitations of essential amino acids.30,31 This inference can sometimes be a technical distinction, in that even growth-limiting diets can permit weight gain, just at a lower rate than growth-promoting diets when provided in ad libitum rations. However, in the area of aging and disease, both diets and genetic alterations that are growth promoting often result in early-onset metabolic and age-related disease, along with increased mortality.9,32 A series of experiments were performed using four common protein sources (singularly or in combination) used in formulated diets to assess differences in growth including length, weight, and body composition to better understand the nutritional requirements of zebrafish within the laboratory research environment and determine the influence of various protein sources used in laboratory diets.
Materials and Methods
Zebrafish (AB strain) were obtained from the Aquatic Animal Research Core at University of Alabama at Birmingham (UAB). Embryos were collected randomly from a mass spawn of adult zebrafish (10 males and 10 females for each of the two cohorts, fed previously the mixed-protein diet described below and in Table 1). Embryos were transferred to Petri dishes (∼50/dish) and incubated for 5 days at 28.5°C. At 5 days postfertilization (dpf ), hatched larvae were transferred to 1.8 L tanks with ∼2 cm of static water and fed rotifers (Branchionus plicatilis) ad libitum enriched with Nannochloropsis (RotiGrow Omega, Reed Mariculture) three times daily until day 28. At 10 dpf water flow was initiated for all tanks as a slow drip. At day 28, fish were randomly distributed into 1.8 L tanks representing one of five diet treatments (n=10 individuals per tank, five tanks per diet treatment). A photograph of all fish in each tank was recorded with a Nikon DS FIL or Nikon D-70, and the length of each fish was determined by NIS Elements 3.1 image analysis from a digital image. Average lengths were evaluated by analysis of variance (ANOVA) to ensure no significant differences among the treatments at the beginning of the experiment. All zebrafish were maintained at 28°C and 1500 μS/cm conductivity in a recirculating system (Aquaneering, Inc.). Flow rates were adjusted to provide at least two water changes per hour within each tank. Municipal tap water was filtered through 5 μm sediment filter, followed by charcoal, reverse osmosis, and a cation/anion exchange resin (Kent Marine) before the addition of synthetic sea salts (Instant Ocean) to obtain final conductivity for the system water source. Sodium bicarbonate was used to maintain pH of the system water (adjusted daily to maintain ∼7.4 pH). At least 20% of system water was exchanged weekly. System water quality including total alkalinity (∼50 ppm CaCO2) and total hardness (∼200 ppm CaCO2) have been previously measured but were not systematically obtained over the entire course of the experiment. Tanks were maintained on the same conditioned water system throughout the experiment, but rotated across rack positions at weekly intervals to reduce environmental confounding from noise, light, vibration, or other unidentified sources. Tanks were siphoned weekly to remove any uneaten feed or debris. Total ammonia nitrogen, nitrite, and nitrate were measured colorimetrically (Mars Fish Care, Inc.), and all but nitrate remained below detectable limits.
Table 1.
Composition of Diets
| |
Amount included (g/100 g total) |
||||
|---|---|---|---|---|---|
| Ingredient | MIX | FPH | CAS | SOY | WG |
| Fish protein hydrolysate (82%)a,b | 18.20 | 59.00 | |||
| Casein (vita-free) (96%)a,c | 22.75 | 51.00 | |||
| Soy protein isolate (92%)a,d | 4.55 | 52.00 | |||
| Wheat gluten (80%)a,e | 9.10 | 60.00 | |||
| Dextrin | 18.25 | 13.85 | 21.85 | 20.85 | 12.85 |
| Soy lecithin | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Canthaxanthin | 2.31 | 2.31 | 2.31 | 2.31 | 2.31 |
| Ascorbylpalmitate | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Vitamin premix BML-2 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Mineral mix BTm | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Betaine | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Potassium phosphate monobasic | 1.15 | 1.15 | 1.15 | 1.15 | 1.15 |
| Alginate | 5.38 | 5.38 | 5.38 | 5.38 | 5.38 |
| Cholesterol | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Menhaden oil | 4.67 | 4.67 | 4.67 | 4.67 | 4.67 |
| Corn oil | 2.33 | 2.33 | 2.33 | 2.33 | 2.33 |
Protein content by percentage.
The Scoular Company, Sopropeche–C.P.S.P. 90.
MP Biomedicals, Cat no. 904798.
MP Biomedicals, Cat no. 905456.
Sigma Aldrich, Cat no. G5004.
MIX, mixed; FPH, fish protein hydrolysate; CAS, casein; SOY, soy protein isolate; WG, wheat gluten.
Diets
Diet ingredient information is provided in Table 1. Each diet was produced with a single, common base mix (all ingredients minus the protein) using chemically defined ingredients. To this base mix was added one of four protein sources or a combination of all four (control diet). The control diet contained a mixed-protein source including fish protein hydrolysate (FPH; Scoular Company), casein (CAS, vitamin-free; Affymetrix–USB), soy protein isolate (SOY; MP Biomedicals), and wheat gluten (WG; MP Biomedicals). Each experimental diet contained only a single-protein source with adjustments to the carbohydrate component (dextrin) to calorically compensate for nitrogen differences between individual protein sources. Diets were mixed in an orbital mixer (Kitchen Aid) and then extruded with a Kitchen Aid extruder (KPEXTA) and were air-dried to ∼10% moisture content before storage. Full diet analyses for proximate composition, amino acid composition, and mineral content were performed by MVTL Laboratories, Inc., and the results are provided in Table 2. For feeding, diets were ground to a powder (250–500 μm sieved) and measured aliquots (approx. ≥5% of body weight) were dispensed at ∼900, 1300, and 1700 hr during the light cycle in surplus quantities. Fish were observed to consume the diets at each level of the water column.
Table 2.
Protein Source Diets: Compositional Analysis
| MIX | FPH | CAS | SOY | WG | |
|---|---|---|---|---|---|
| Proximate analysis | |||||
| Moisture (%)a,b | 11.52, 11.33 | 10.97, 10.72 | 10.30, 10.18 | 10.08, 9.94 | 9.58, 9.45 |
| Fat (%)a,c | 12.34, 12.05 | 15.86, 15.65 | 10.47, 10.34 | 10.33, 10.19 | 11.10, 10.90 |
| Fiber (%)a | 1.60, 1.58 | 1.77, 1.67 | 1.41, 1.60 | 1.65, 1.67 | 1.73, 1.85 |
| Protein (%)a,d | 44.90, 44.40 | 45.30, 45.19 | 44.50, 43.97 | 43.90, 43.58 | 49.50, 49.09 |
| Ash (%)a | 6.84, 6.85 | 8.37, 8.34 | 7.89, 7.79 | 6.86, 6.90 | 5.95, 5.98 |
| Amino acidse | |||||
| Cysteine (%) | 0.400 | 0.400 | 0.190 | 0.530 | 1.010 |
| Methionine (%) | 1.080 | 1.160 | 1.320 | 0.560 | 0.730 |
| Lysine (%) | 2.930 | 3.200 | 3.610 | 2.700 | 0.660 |
| Alanine (%) | 1.894 | 2.915 | 1.427 | 1.932 | 1.249 |
| Arginine (%) | 2.090 | 2.748 | 1.642 | 3.187 | 1.547 |
| Aspartic Acid (%) | 3.284 | 3.849 | 3.322 | 4.996 | 1.509 |
| Glutamic Acid (%) | 9.631 | 5.626 | 10.120 | 8.388 | 18.060 |
| Glycine (%) | 2.072 | 3.984 | 0.876 | 1.848 | 1.622 |
| Isoleucine (%) | 2.023 | 1.709 | 2.394 | 2.132 | 1.763 |
| Leucine (%) | 3.631 | 2.866 | 4.354 | 3.500 | 3.293 |
| Serine (%) | 2.140 | 1.804 | 2.461 | 2.103 | 2.177 |
| Threonine (%) | 1.706 | 1.744 | 1.917 | 1.612 | 1.217 |
| Valine (%) | 2.424 | 2.019 | 3.016 | 2.196 | 1.868 |
| Histidine (%) | 1.120 | 0.940 | 1.337 | 1.110 | 0.975 |
| Phenylalanine (%) | 2.130 | 1.573 | 2.387 | 2.325 | 2.600 |
| Tyrosine (%) | 1.877 | 1.574 | 2.372 | 1.473 | 1.491 |
| Taurine (%) | 0.098 | 0.367 | 0.010 | 0.010 | 0.010 |
| Tryptophan (%) | 0.506 | 0.374 | 0.620 | 0.582 | 0.467 |
| Mineralse,f | |||||
| Calcium (%) | 0.81 | 0.86 | 0.80 | 0.85 | 0.80 |
| Copper (ppm) | 11.26 | 8.94 | 8.00 | 20.01 | 13.98 |
| Iron (ppm) | 123.60 | 125.30 | 79.21 | 139.50 | 99.34 |
| Magnesium (ppm) | 440.30 | 642.80 | 259.90 | 530.20 | 564.10 |
| Manganese (ppm) | 106.60 | 105.00 | 106.40 | 109.80 | 115.50 |
| Phosphorus (%) | 1.31 | 1.38 | 1.41 | 1.39 | 1.10 |
| Potassium (%) | 0.82 | 1.24 | 0.58 | 0.76 | 0.66 |
| Sodium (%) | 0.91 | 1.29 | 0.67 | 1.15 | 0.82 |
| Zinc (ppm) | 41.78 | 43.18 | 40.73 | 36.51 | 41.21 |
Duplicate measures.
Fat by ethyl ether extraction.
Protein=N×6.25.
Percent by weight.
Parts per million.
Growth measures
Similar to length measures at baseline for randomization, all fish were subsequently photographed in clear-bottom containers at several intervals (10, 13, and 16 weeks postfertilization). Digital images were used to measure individual fish body lengths (from the tip of the mouth to the base of the caudal fin) within a tank and averaged within the diet treatment against a reference length standard in all images. All lengths were recorded to the nearest 0.01 mm. Final body weight was measured at study completion on tricaine methanesulfonate (15 mg/L)-anesthetized fish before body composition assessment. Anesthetized fish were quickly blotted to remove surface moisture and weighed to the neared 0.0001 g.
Body composition assessment
Body composition measurement (lean and fat mass) was performed at study completion by quantitative magnetic resonance (QMR) using the EchoMRI 3-in-1 system and the tissue probe holder. Tricaine (MS-222; 300 mg/L) was used to rapidly immobilize the fish in a small volume of water (also containing MS-222; 300 mg/L) for the duration of the QMR measure. Anesthetic induction was performed on single fish (∼2–5 min) to monitor swimming motion, loss of equilibrium, cessation of opercular movement, and heart contractions.33 QMR scans were performed using the appropriate tissue sample holder (with MS-222 containing water) and high precision setting, requiring ∼3 min per scan. Body composition measures include lean mass (or fat free mass) and fat mass reported to the nearest 0.001 g. After the scans, fish were subsequently revived by submersion in fresh system water and gentle perfusion of the gills using a disposable pipette.
Euthanasia
At study completion, fish were euthanized by rapid submersion in ice-cold water with MS-222 (300 mg/L)33 and the carcass stored at −80°C until disposal.
Data analyses
Data are reported as means and standard error (SE) of the mean. Length (at each of the three measurement time points), final weight, fat, lean/fat free mass, and percent fat were separately compared by ANOVA using SAS v.9.1, adjusting for cohort and/or body weight as covariates (ANCOVA) when significance between groups or relationships were observed. Data and analyses were stratified by sex, to accommodate significant differences between sexes. Observed significant differences (p≤0.05) were further tested using Duncan's multiple range post hoc test for among-groups differences (p<0.05).
Results
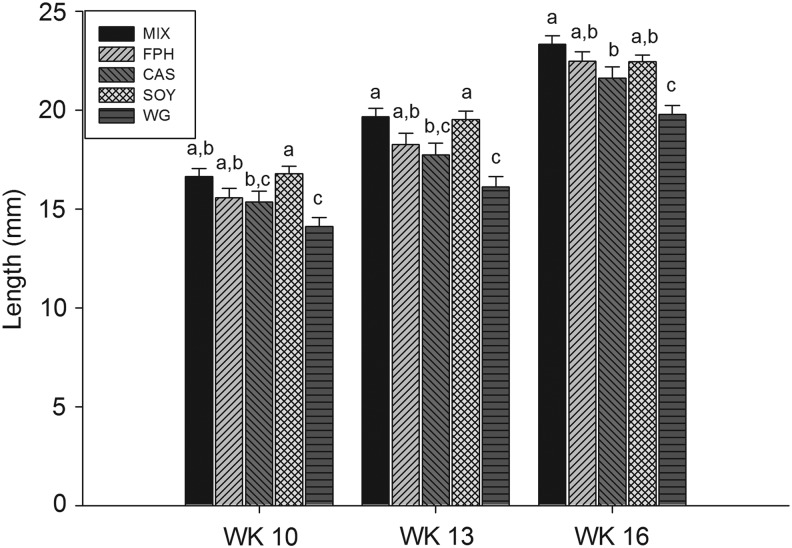
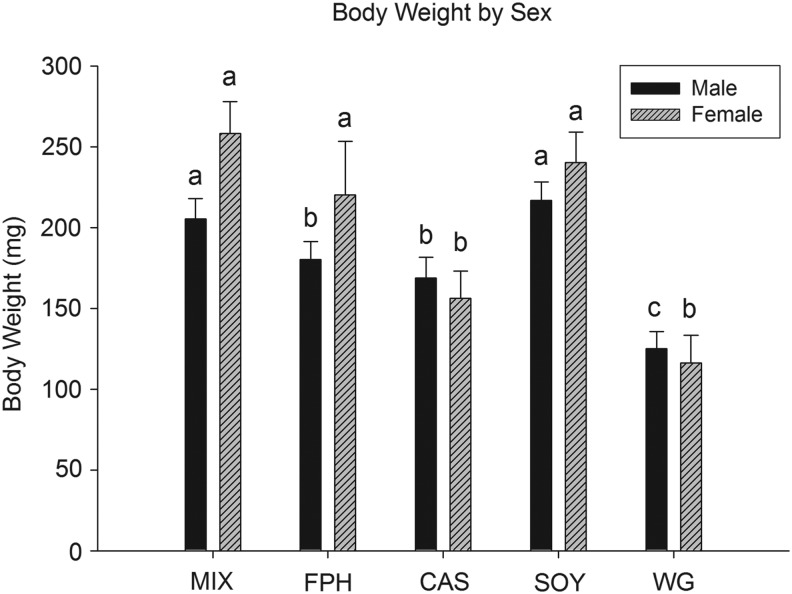
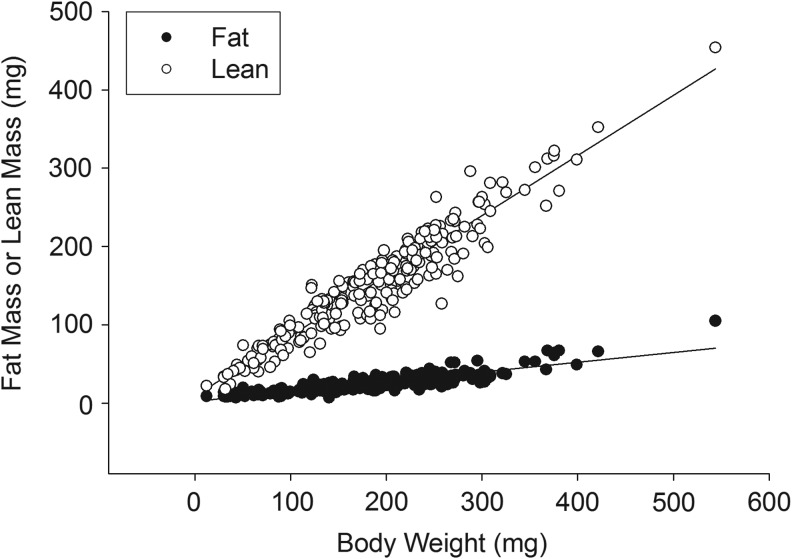
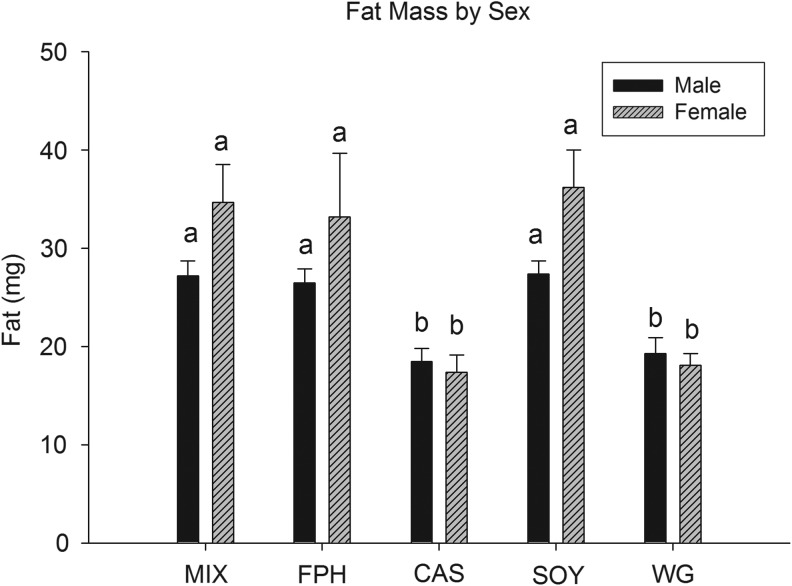
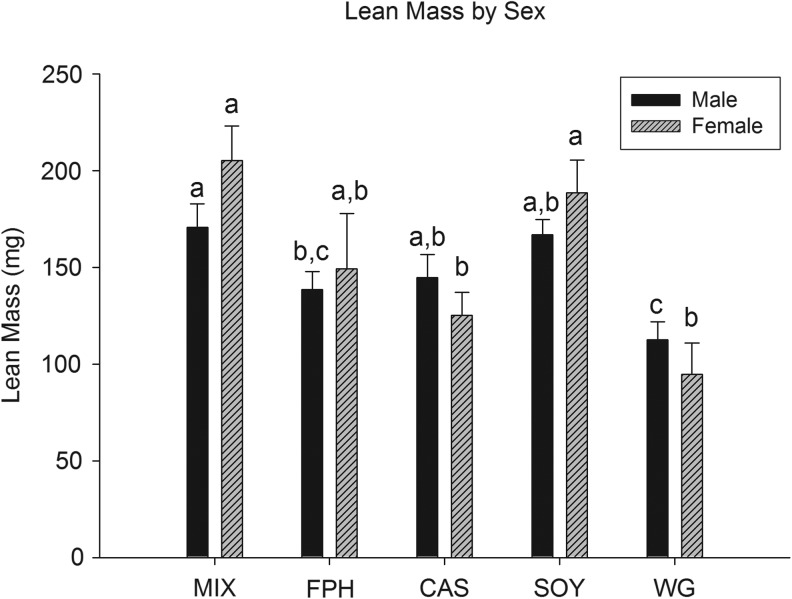
Fish consumed each of the five diets with no overt feeding differences observed during the course of the study. All diets initially floated and were consumed by fish at the surface of the water, but subsequently sank (given enough time) and were also consumed from the floor of the tanks. Despite the fact that all fish were fed ad libitum (in excess) three times per day, lengths were significantly different among protein sources at 10, 13, and 16 weeks (Table 3 and Fig. 1). Mean length was significantly smaller in the WG group compared with all others at each time point and study end in both cohorts (Table 3 and Fig. 1), with CAS fish having an intermediate length compared with the remaining groups (MIX, FPH, SOY; Table 3 and Fig. 1). Similarly, final body weight was lowest in the WG group (males and females), with small but significant differences present across the other groups when considering both sexes (MIX≈SOY>FPH>CAS>WG; Table 4 and Fig. 2). A significant, positive relationship was observed for body weight with both lean and fat mass measures for all fish (Fig. 3). Additionally, female fish as a whole were heavier (p<0.01), with a higher fat mass (p<0.01) than males (data not shown). There was a significant difference between groups for fat mass (p<0.0001) and lean mass (p<0.001), with CAS and WG fish having the lowest mean fat mass (Table 4 and Fig. 4), and WG fish having the lowest lean mass amounts in both sexes (Table 4 and Fig. 5), with these differences persisting after controlling for body weight (data not shown). CAS and FPH fish had a lower lean mass than the MIX controls (Table 4 and Fig. 5) after co-varying for body weight (data not shown). Similarly, the relative fat amount (% total fat) was significantly different between groups (p<0.001) with CAS-fed fish having the leanest (lowest% fat) body composition and the FPH and WG having the highest percent fat (Table 4, data not shown).
Table 3.
Length (mm) by Cohort and Group
| Cohort | Group | N | 10 weeks* | 13 weeks | 16 weeks |
|---|---|---|---|---|---|
| 1 | MIX | 26 | 16.94 (0.59)a | 19.57 (0.55)a | 22.83 (0.57)a |
| FPI | 26 | 15.46 (0.80)a,b | 17.72 (0.82)a,b | 21.31 (0.77)a,b | |
| CAS | 25 | 15.66 (0.81)a,b | 17.58 (0.83)a,b | 20.99 (0.84)a,b | |
| SOY | 25 | 16.46 (0.46)a | 18.20 (0.45)a | 20.65 (0.35)b | |
| WG | 25 | 14.27 (0.65)b | 15.99 (0.61)b | 18.80 (0.51)c | |
| 2 | MIX | 33 | 16.39 (0.60)a | 19.74 (0.65)a,b | 23.74 (0.62)a |
| FPI | 35 | 15.64 (0.62)a,b | 18.70 (0.76)a,b | 23.36 (0.61)a | |
| CAS | 35 | 15.12 (0.77)a,b | 17.87 (0.83)b,c | 22.11 (0.77)a,b | |
| SOY | 34 | 17.04 (0.58)a | 20.51 (0.63)a | 23.80 (0.38)a | |
| WG | 32 | 13.99 (0.69)b | 16.23 (0.79)c | 20.60 (0.70)b |
Values shown are mean (standard error of the mean). Different letters indicate significant differences between groups at p<0.05 (Duncan multiple range post hoc test).
Age denoted in weeks postfertilization.
FIG. 1.
Mean body length (mm) of zebrafish at 10, 13, and 16 weeks postfertilization fed either the mixed (MIX), fish protein hydrolysate (FPH), casein (CAS), soy protein isolate (SOY), or wheat gluten (WG) as the protein source in the diet. Different letters indicate between-group differences at p<0.05.
Table 4.
Final Body Weight and Composition by Cohort and Group
| Cohort | Group | Sex | N | Weight (mg) | Fat (mg) | Lean (mg) | % Fat |
|---|---|---|---|---|---|---|---|
| 1 | MIX | M | 13 | 204.4 (19.8)a | 26.7 (2.3)a | 164.2 (19.2)a | 14.86 (0.81) |
| F | 11 | 178.6 (24.2)a | 26.1 (3.4)a,b | 155.5 (17.4)a,b | 14.50 (0.83) | ||
| FPH | M | 13 | 163.7 (14.4)a,b | 25.2 (1.6)a | 127.6 (12.1)a,b,c | 17.25 (0.88) | |
| F | 10 | 145.9 (22.0)a,b | 23.8 (3.0)a,b | 109.0 (15.6)b,c | 19.02 (1.25) | ||
| CAS | M | 13 | 138.5 (20.0)b,c | 14.9 (1.5)b | 115.9 (16.2)b,c | 13.53 (1.80) | |
| F | 6 | 162.3 (25.5)a | 17.8 (2.4)b | 132.0 (17.6)a,b | 11.92 (0.38) | ||
| SOY | M | 14 | 168.8 (12.8)a,b | 23.6 (1.8)a | 138.4 (10.9)a,b | 14.65 (0.63) | |
| F | 10 | 212.0 (21.7)a | 32.7 (3.8)a | 176.2 (16.7)a | 15.55 (0.79) | ||
| WG | M | 18 | 107.3 (7.6)c | 17.0 (1.2)b | 93.6 (6.5)c | 15.61 (0.54) | |
| F | 6 | 79.4 (16.4)b | 17.0 (1.6)b | 70.0 (13.5)c | 21.06 (2.10) | ||
| 2 | MIX | M | 20 | 206.1 (16.9)a,b | 27.6 (2.1)a,b | 176.4 (15.6)a | 13.90 (0.44) |
| F | 14 | 320.9 (16.2)a | 45.1 (5.9)a,b | 266.2 (19.6)a | 14.16 (1.04) | ||
| FPH | M | 21 | 190.6 (15.4)b | 27.5 (2.2)a,b | 146.9 (13.5)a,b | 16.29 (0.61) | |
| F | 9 | 302.9 (54.4)a | 56.8 (18.0)a | 250.0 (76.8)a | 18.38 (0.14) | ||
| CAS | M | 22 | 186.7 (16.2)b | 21.3 (1.7)b | 166.8 (15.2)a,b | 11.65 (0.43) | |
| F | 5 | 149.0 (23.0)b | 16.8 (2.78)c | 115.3 (15.1)b | 12.59 (0.59) | ||
| SOY | M | 26 | 243.0 (13.3)a | 30.0 (1.5)a | 186.0 (9.0)a | 13.91 (0.32) | |
| F | 7 | 281.1 (28.2)a | 47.7 (8.6)a | 230.0 (45.4)a,b | 17.27 (0.27) | ||
| WG | M | 21 | 140.3 (18.4)c | 21.8 (2.9)b | 132.8 (17.1)b | 15.17 (1.15) | |
| F | 6 | 153.0 (22.5)b | 19.4 (1.8)b,c | 124.6 (26.9)b | 14.70 (1.57) |
Values shown are mean (standard error of the mean). Different letters indicate significant differences between groups within a given sex and cohort at p<0.05 (Duncan multiple range post hoc test).
FIG. 2.
Mean group body weight of zebrafish at 16 weeks postfertilization for males and females. Different letters indicate between-group differences within a given sex at p<0.05.
FIG. 3.
Relationship between body weight and fat mass or lean mass of zebrafish at 16 weeks postfertilization across all diet groups. Lean mass r2=0.8993, p<0.001; fat mass r2=0.7447, p<0.001.
FIG. 4.
Mean group total fat mass of zebrafish at 16 weeks postfertilization for males and females. Different letters indicate between-group differences within a given sex at p<0.05.
FIG. 5.
Mean group total lean mass of zebrafish at 16 weeks postfertilization for males and females. Different letters indicate between-group differences within a given sex at p<0.05.
Discussion
This study demonstrates a significant impact of dietary protein source, despite ad libitum feeding protocols and isonitrogenous diets, on growth outcomes, including length, body weight, and body composition, using the zebrafish model. Additionally, multiple single-protein source diets performed as well as a mixed-protein source diet for length and weight outcomes, with more complex body composition (lean and fat mass) responses observed among groups. WG protein was deficient for growth, producing fish in both sexes that were shorter in length, with significantly lower body weight and lean mass, although percent body fat was high compared with other diets. CAS-fed fish similarly had significantly lower body length, body weight, and lean and fat mass in both male and female fish, with the lowest percent body fat (leanest) compared with the other protein sources. FPH-fed fish, a presumably high-quality, replete protein source, had significantly lower lean mass and a high percent body fat, whereas SOY-fed fish resembled mixed-protein controls for all measured outcomes. Despite females being heavier and possessing a larger fat mass than males when all groups were combined, the significant differences between groups for growth outcomes were related to diet treatment and not sex-specific.
All diets were fed as a sole source of nutrients (no additional live feeds or commercial supplements) in a uniform physical state from 4 weeks postfertilization for the duration of the study. Additionally, the base formulation (excluding the protein sources) was kept consistent among groups, pointing to the significant impact dietary protein and potentially amino acid ratios may have on growth and body composition outcomes in zebrafish. The diets were formulated to maintain a similar nitrogen content (as presented in Table 2). However, because of the varying proportion of nitrogen present in the different dietary protein sources, an additional dietary caloric source had to be modified to approximate isocaloric levels between test diets. In this case, the carbohydrate content was manipulated to balance between diets because of its caloric density and the desire to measure body composition outcomes (lean and fat content), which may be expected to change with the increasing or decreasing dietary lipid content. Thus, we cannot formally exclude the possibility that manipulation of the dietary carbohydrate content also influences the measures growth outcomes,34 although previous findings would suggest that dietary carbohydrate does not affect growth outcomes at those levels.35 Diets with similar carbohydrate content and protein content but different protein sources (see Tables 1 and 2: SOY vs. CAS) still exhibited significant differences in multiple growth outcomes, most likely attributable to the protein source contribution to the individual diets.
While the individual, essential amino acid requirements of zebrafish have not been systematically tested, assuming similar requirements to other fish,20,23 it may not be surprising that the wheat-fed fish exhibited the lowest growth, provided the known limitations of lysine in wheat.23,36–38 Previous research with soy protein also might suggest that limitation of essential, sulfur-containing amino acids (methionine and cysteine) might be expected to limit growth outcomes,28,39,40 but the fish in this study responded well with similar growth outcomes to the MIX control diet. One explanation for this difference may reside in the high protein content of the diet (∼45%), which could offset any limitation of specific amino acids proportionally with a given protein source because of the high absolute mass amount present. Alternatively, dietary betaine, serving as a methyl donor, may promote production of methionine. Progressive reductions in the protein content of the diet (<45%) should be tested to determine minimal requirements for essential amino acids.
An additional limitation of this and the majority of nutrition studies is the diet complexity and proportional nature of nutrients. One example is the amino acid differences among diets. Since diets were formulated to be isonitrogenous, any elevation in a single amino acid must be compensated by a reduction in one or more amino acids to maintain the overall nitrogen balance (see Table 2). This complexity limits the ability to confidently ascribe the given growth outcomes to a single nutrient (amino acid) as the proportion of compensating changes in other nutrients may equally have attributed to the observed difference. Additionally, it is not yet known how feeding in excess of dietary needs three times a day may influence these and other outcomes. Accurate determination of food intake in an aquaculture system such as this is challenging, particularly on an individual fish basis. Given the observed variance among individuals within a single tank, it would be valuable to know whether food intake differences account for any of the variability. Similarly, with essential nutritional elements, it would be beneficial to know if limitation invokes a hypophagic response because of a perceived dietary inferiority or a hyperphagic response to increase overall nutrient intake to meet any given individual limiting requirement.
The diets performed well for growth measures, including length and overall body weight. Overall length, weight, and percent lipid were similar to the findings of Kaushik et al.,3 who report a length of 23 mm at 9 weeks of age with a total body lipid of ∼10%.3 Another recent work reports fish that appear to be significantly larger than those in the present study, with a final length of ∼33 mm and a body weight of 350 mg.24 Whether strain differences partially account for these growth differences has not been evaluated. Given the larger starting and final lengths,24 it would be of interest to determine if dietary composition differences produced similar growth responses in another zebrafish strain background or if earlier initiation of formulated feeds altered the growth parameters. Additionally, tank densities were different between the two studies, with the current study having three times the density of the previous work (e.g., current: ∼5.5 fish/L vs. ∼1.6 fish/L).24 Although the zebrafish model has garnered much recent attention for its potential use in lipid and obesity research,41–47 few studies have reported overall body composition outcomes. In the present work, the dietary protein source significantly impacted lean and fat mass outcomes. Across treatments the fish retained a relatively lean phenotype, with the CAS-fed fish being the leanest. Although the dietary protein amount has been shown to significantly influence food intake, body weight, and body composition outcomes (low protein–increased intake with greater body fat vs. high protein–decreased intake with greater body lean), the comparison of different protein sources has had mixed results regarding their effectiveness for desirable body composition alterations (fat reduction and lean accretion), particularly when provided in a purified, nonsupplemented form. Given the observed difference in body weight and composition, it would be of interest to know if physiological responses paralleled these growth outcomes, with alterations in endocrine profiles, metabolic responses, and reproductive output, which were not fully measured in this study. As the diets were isonitrogenous, the body composition response with changes in lean and fat mass between protein sources raises questions about the influence of amino acid ratios or other non-nutritive compounds that may accompany the protein sources for their molecular impact on both the host organism (zebrafish) as well as the microbiome inhabiting the gut.48 Future studies may assess nutrient-specific alteration in the microbioata with diet and body composition outcomes.
The data presented demonstrate a significant impact of the protein source on growth outcomes in zebrafish using a semipurified diet formulation. Finding which nutrient differences are responsible for the differential growth outcomes as well as determining minimal requirements for the zebrafish model will be important for diet standardization. Finally, these results suggest that body composition, both lean and fat mass, is significantly influenced by the protein source and should be carefully considered in future studies that may utilize the zebrafish as a model of diet-induced obesity or lipid metabolism.
Acknowledgments
We acknowledge Small Animal Phenotyping Core for assistance with body composition measures using QMR and the Nutrition Obesity Research Center Aquatic Animal Models Core for assistance with zebrafish diet manufacture, husbandry, and care, including Ms. Lacy Dennis for assistance with chemical carcass analyses.
This work was supported in part by a pilot/feasibility grant from the UAB Nutrition Obesity Research Center to D.L.S. (P30DK056336). This work was also supported by the UAB Animal Physiology Core (P60DK079626 and P30DK056336).
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The content and opinions expressed herein are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or any other organization with which the authors are affiliated.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- 2.Siccardi AJ., III Garris HW. Jones WT. Moseley DB. D'Abramo LR. Watts SA. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish. 2009;6:275–280. doi: 10.1089/zeb.2008.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushik S. Georga I. Koumoundouros G. Growth and body composition of zebrafish (Danio rerio) larvae fed a compound feed from first feeding onward: toward implications on nutrient requirements. Zebrafish. 2011;8:87–95. doi: 10.1089/zeb.2011.0696. [DOI] [PubMed] [Google Scholar]
- 4.Wang C. Weindruch R. Fernandez JR. Coffey CS. Patel P. Allison DB. Caloric restriction and body weight independently affect longevity in Wistar rats. Int J Obes Relat Metab Disord. 2004;28:357–362. doi: 10.1038/sj.ijo.0802518. [DOI] [PubMed] [Google Scholar]
- 5.Bartke A. Coschigano K. Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci. 2001;56:B340–B349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 7.Bartke A. Chandrashekar V. Bailey B. Zaczek D. Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR. Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Weindruch R. Walford RL. Springfield, IL: C. C. Thomas Publisher; 1988. The Retardation of Aging and Disease by Dietary Restriction. [Google Scholar]
- 10.Liu RK. Leung BE. Walford RL. Effect of temperature-transfer on growth of laboratory populations of a South American annual fish Cynolebias bellottii. Growth. 1975;39:337–343. [PubMed] [Google Scholar]
- 11.Liu RK. Walford RL. Observations on the lifespans of several species of annual fishes and of the world's smallest fishes. Exp Gerontol. 1970;5:241–246. doi: 10.1016/0531-5565(70)90044-6. [DOI] [PubMed] [Google Scholar]
- 12.Orentreich N. Matias JR. Defelice A. Zimmerman JA. Low methionine ingestion by rats extends life-span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 13.Nadon NL. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell. 2006;5:9–15. doi: 10.1111/j.1474-9726.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay F. Lavigne C. Jacques H. Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]
- 15.Plaisance EP. Henagan TM. Echlin H, et al. Role of beta-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R740–R750. doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richie JP. Leutzinger Y. Parthasarathy S. Malloy V. Orentreich N. Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman JA. Malloy V. Krajcik R. Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.Segall P. Long-term tryptophan restriction and aging in the rat. Aktuelle Gerontol. 1977;7:535–538. [PubMed] [Google Scholar]
- 19.Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T. Oohara I. Yamamoto T. Comparison of essential amino acid requirements with A/E ratio among fish species. Fisheries Sci. 1997;63:963–970. [Google Scholar]
- 21.Boisen S. Hvelplund T. Weisbjerg MR. Ideal amino acid profiles as a basis for feed protein evaluation. Livestock Prod Sci. 2000;64:239–251. [Google Scholar]
- 22.Schwarz FJ. Kirchgessner M. Deuringer U. Studies on the methionine requirement of carp (Cyprinus carpio L.) Aquaculture. 1998;161:121–129. [Google Scholar]
- 23.Mambrini M. Kaushik SJ. Indispensable amino acid requirements of fish: correspondence between quantitative data and amino acid profiles of tissue proteins. J Appl Ichthyol. 1995;11:240–247. [Google Scholar]
- 24.Gomez-Requeni P. Conceicao LEC. Jordal AEO. Ronnestad I. A reference growth curve for nutritional experiments in zebrafish (Danio rerio) and changes in whole body proteome during development. Fish Physiol Biochem. 2010;36:1199–1215. doi: 10.1007/s10695-010-9400-0. [DOI] [PubMed] [Google Scholar]
- 25.Goolish EM. Okutake K. Lesure S. Growth and survivorship of larval zebrafish Danio rerio on processed diets. North Am J Aquaculture. 1999;61:189–198. [Google Scholar]
- 26.Ulloa PE. Iturra P. Neira R. Araneda C. Zebrafish as a model organism for nutrition and growth: towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev Fish Biol Fisheries. 2011;21:649–666. [Google Scholar]
- 27.Gomez-Requeni P. Mingarro M. Calduch-Giner JA, et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata) Aquaculture. 2004;232:493–510. [Google Scholar]
- 28.Ai Q. Xie X. Effects of dietary soybean protein levels on energy budget of the southern catfish, Silurus meridionalis. Comp Biochem Physiol A Mol Integr Physiol. 2005;141:461–469. doi: 10.1016/j.cbpb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Sales J. The effect of fish meal replacement by soyabean products on fish growth: a meta-analysis. Br J Nutr. 2009;102:1709–1722. doi: 10.1017/S0007114509991279. [DOI] [PubMed] [Google Scholar]
- 30.Wingerd WH. Lactalbumin as a food ingredient. J Dairy Sci. 1970;54:1234–1236. [Google Scholar]
- 31.Sales J. The effect of fish meal replacement by soyabean products on fish growth: a meta-analysis. Br J Nutr. 2009;102:1709–1722. doi: 10.1017/S0007114509991279. [DOI] [PubMed] [Google Scholar]
- 32.Miller RA. Chrisp C. Atchley W. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol Ser A Biol Sci Med Sci. 2000;55:B455–B461. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JM. Bunte RM. Carty AJ. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio) J Am Assoc Lab Anim Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RP. Utilization of dietary carbohydrate by fish. Aquaculture. 1994;124:67–80. [Google Scholar]
- 35.Robison BD. Drew RE. Murdoch GK, et al. Sexual dimorphism in hepatic gene expression and the response to dietary carbohydrate manipulation in the zebrafish (Danio rerio) Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:141–154. doi: 10.1016/j.cbd.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Requeni P. de VM. Kousoulaki K. Jordal AE. Conceicao LE. Ronnestad I. Whole body proteome response to a dietary lysine imbalance in zebrafish Danio rerio. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6:178–186. doi: 10.1016/j.cbd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Abimorad EG. Favero GC. Castellani D. Garcia F. Carneiro DJ. Dietary supplementation of lysine and/or methionine on performance, nitrogen retention and excretion in pacu Piaractus mesopotamicus reared in cages. Aquaculture. 2009;295:266–270. [Google Scholar]
- 38.Liebert F. Current knowledge of protein and amino acid nutrition and metabolism in fish. J Anim Physiol Anim Nutr. 2009;93:787–793. doi: 10.1111/j.1439-0396.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 39.Ai QH. Xie XJ. Effects of replacement of fish meal by soybean meal and supplementation of methionine in fish meal/soybean meal-based diets on growth performance of the southern catfish Silurus meridionalis. J World Aquaculture Soc. 2005;36:498–507. [Google Scholar]
- 40.Cai YJ. Burtle GJ. Methionine requirement of channel catfish fed soybean meal-corn-based diets. J Anim Sci. 1996;74:514–521. doi: 10.2527/1996.743514x. [DOI] [PubMed] [Google Scholar]
- 41.Song Y. Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J. 2007;21:2042–2049. doi: 10.1096/fj.06-7503com. [DOI] [PubMed] [Google Scholar]
- 42.Oka T. Nishimura Y. Zang L, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10:21. doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadereit B. Kumar P. Wang WJ, et al. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig PM. Moon TW. Fasted zebrafish mimic genetic and physiological responses in mammals: a model for obesity and diabetes? Zebrafish. 2011;8:109–117. doi: 10.1089/zeb.2011.0702. [DOI] [PubMed] [Google Scholar]
- 45.Flynn EJ., III Trent CM. Rawls JF. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio) J Lipid Res. 2009;50:1641–1652. doi: 10.1194/jlr.M800590-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holtta-Vuori M. Salo VT. Nyberg L, et al. Zebrafish: gaining popularity in lipid research. Biochem J. 2010;429:235–242. doi: 10.1042/BJ20100293. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JL. Carten JD. Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawls JF. Mahowald MA. Ley RE. Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]