Abstract
In this article, we describe novel conditions for culture, expansion, and transdifferentiation of primary human dermal fibroblasts (hDFs) to induce expression of transcription factors (TFs) and hormones characteristic of the islets of Langerhans. We show that histones associated with the insulin gene are hyperacetylated and that insulin gene DNA is less methylated in islet cells compared to cells that do not express insulin. Using two compounds that alter the epigenetic signature of cells, romidepsin (Romi), a histone deacetylase inhibitor, and 5-Azacytidine (5-AzC), a chemical analogue of cytidine that cannot be methylated, we show that hDFs exhibit a distinctive regulation of expression of TFs involved in islet development as well as of induction of glucagon and insulin. Overexpression of Pdx1, a TF important for islet differentiation, and silencing of musculoaponeurotic fibrosarcoma oncogene homolog B, a TF that is expressed in mature glucagon-producing cells, result in induction of insulin to a higher level compared to Romi and 5-AzC alone. The cells obtained from this protocol exhibit glucose-stimulated insulin secretion and lower blood glucose levels of diabetic mice. These data show that fully differentiated nonislet-derived cells could be made to transdifferentiate to islet-like cells and that combining epigenetic modulation with TF modulation leads to enhanced insulin expression.
Introduction
In type 1 diabetes (T1D), there is a loss of insulin-producing β cells and patients are dependent on daily insulin injections for their survival [1]. Several protocols to transplant islets from cadaveric donors have been developed [2,3], however, their use is limited mainly because of a shortage of donors [4,5]. Hence, generation of islet-like β-cells from other cell types may be used in place of bona fide islets and could result in significant improvement over current therapeutic approaches for patients with diabetes.
There have been advances in generating insulin-producing cells from other adult cell types. Ferber et al. [6–11] reported successful transdifferentiation of hepatocytes and keratinocytes into insulin-producing cells by ectopic expression of the transcription factor (TF) pancreatic and duodenal homeobox 1 (Pdx1), which is important for pancreatic development and β cell maturation. By combining Pdx1 with the TF Nkx6.1, they were able to induce insulin expression to a greater extent [7]. Other protocols using nonadult cells, including human embryonic stem cells, somatic stem cells, induced pluripotent stem cells (iPSCs), or mouse stem cells, were developed [12–24]. Additional factors were included in these protocols to increase insulin synthesis and glucose-responsive insulin secretion such as GLP1/exendin-4, Wnt3A, noggin, KAAD-cyc, B27, nicotinamide, activin A, retinoic acid, and growth factors.
Epigenetic modifications are thought to account for specification of gene expression in different tissues. In islets, as in all tissues, histone acetylation is associated with transcription activation [25] and has previously been indicated to play a role in regulating insulin and glucagon gene expression [26]. DNA methylation usually is associated with repression of transcription [27].
We studied DNA methylation in islet cells and in islet cells that had dedifferentiated in culture and no longer produced insulin and glucagon. Compared to islets cells, we observed that the insulin gene promoter is hypermethylated in dedifferentiated islet cells. Together with the histone underacetylation [26], we hypothesize that these two epigenetic changes may account, at least partially, for the repression in insulin and glucagon gene transcription.
Primary human dermal fibroblasts (hDFs) were recently shown capable of undergoing differentiation into a wide range of cell types, including neurons, blood progenitors, and adipocytes [28–34]. This process has been termed transdifferentiation. In this article, we describe a 5-day protocol to transdifferentiate primary hDFs into insulin- and glucagon-producing cells. We induce the expression of insulin through increasing acetylation and decreasing methylation by two epigenetic modifying compounds—romidepsin (Romi), a histone deacetylase inhibitor (HDACi) and 5-Azacytidine (5-AzC), a cytidine analog that cannot be methylated. To our knowledge, only a single previous study reported [35] the use of a HDACi in an attempt to transdifferentiate adult cells, in this case, bone marrow cells to insulin-producing cells. We also studied the effect of silencing of musculoaponeurotic fibrosarcoma oncogene homolog B (MafB), a TF previously described to be specific for glucagon-producing α-cells in adults and inducing glucagon [36–38], in conjunction with overexpression of Pdx1, a pancreatic TF crucial for islet development, specific for adult insulin-producing cells [39,40] and an activator of insulin gene transcription. In combination with Romi and 5-AzC, we found that overexpression of Pdx1 resulted in higher insulin and glucagon transcription and silencing of MafB resulted in induction of insulin to higher levels as well as reduction of glucagon transcription.
Materials and Methods
Ethics statement
All animal work was conducted according to national and international guidelines.
Cells
Adult human primary dermal fibroblasts were obtained from ATCC. Human islets were obtained from cadaveric pancreases from the National Islet cell Resource Center Basic Science Islet Distribution Program. Islets were dispersed with 0.05% Trypsin-EDTA (Cellgro; Mediatech) for 5 min at 37°C, and then infected for 72 h with an adenovirus-containing green fluorescent protein (GFP) under the control of the rat insulin promoter. β-cells were sorted with the BD FACSAria II cell sorter. Dedifferentiated islets (islet-derived dedifferentiated cells that can be induced under serum deprivation to islet-like structures following proliferative expansion) were derived from fresh human islets as described previously [41]. Islet cells were grown in Dulbeco modified Eagle medium (DMEM) supplemented with 2% fetal calf serum, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM glutamine.
Viruses
Adenoviruses expressing mouse Pdx1, mouse MafA, and mouse Nkx6.1 were obtained from the Beta Cell Biology Consortium. Adenovirus-expressing rat NeuroD1 was obtained from Dr. Harry Heimberg [42].
A lentivirus encoding the -1600bp of the rat glucagon promoter fused to the GFP reporter gene was generated by cloning it into pLenti4/V5-DEST. shMafB lentivirus was purchased from Sigma Aldrich (Mission RNAi system).
Materials
Romidepsin was generously provided by Celgene Corporation and the National Cancer Institute, NIH. 5-AzC was purchased from Sigma Aldrich.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed with 100 μg of cell chromatin extracts. Assay was carried out using the Active Motifs chromatin shearing kit. Chromatin was incubated with acetylated histone H3 and H4 antibodies (Millipore) as well as rabbit IgG (Abcam). Binding was analyzed by real-time PCR with the following sets of PCR primers and 6FAM-labeled probes: hIns–275(F) TGTGAGCAGGGACAGGTCTG; hIn5–275(R) TCCTCAGGACCAGCGGG; hINS–275(P) 6FAM-CCACCGGGCCCCTGGTTAAGACTCTA; hGCG–207(F) CTT AAGTGATTTTCATGCGTGATTG; hGCG–207(R) TGGGA ATGGAGAGAGCAGCTT; hGCG–207(P) 6FAM-AAGTAGAAGGTGGATTTC; hPdx1: hPdx1-206 (F) ATTTCGTATGGGGAGATGTTCG, hPdx1-206(R) CTCCGCCGCCACCCCAATTAAC, hPdx1206(P) 6FAM-CTACGTACCTATACATAAACCGCCAA.
Insulin and glucagon measurements
Cells were incubated for 2 h at 37°C in the Krebs-Ringer bicarbonate buffer with Hepes (KRBH) containing 1.67 mM glucose. Indicated glucose or deoxyglucose concentrations were then added to the cells for 1 h at 37°C in KRBH. Glucagon in incubation buffers and cell extracts was measured by radioimmunoassay (Millipore), and insulin in incubation buffers and cell extracts was measured by enzyme-linked immunosorbent assay (Mercodia). Secretion was expressed as percentage of the total insulin content.
Immunofluorescence
Cells were fixed with fresh 4% paraformaldehyde in phosphate-buffered saline, and permeabilized with 0.15% Triton×100 in a blocking buffer containing 4% normal donkey serum. Cells were incubated with rabbit anti-human glucagon (Dako North America), rabbit anti-human c-peptide (Linco Research), rabbit anti- MafA (Bethyl Laboratories), rabbit anti-MafB (Bethyl Laboratories), or rabbit anti- Pdx1 (Abcam) antibodies in a blocking buffer for 1 h at 37°C, washed with the buffer, and then incubated with fluorescent secondary antibodies (Life Technologies) in the blocking buffer for 1 h at room temperature.
Coverslips were washed, mounted in Mowiol® (Sigma-Aldrich) with DAPI (Sigma-Aldrich), and images captured on a Zeiss META NLO confocal microscope.
Fluorescence in situ hybridization (FISH)
Cells were fixed in 4% formaldehyde in 2×SSC (1×SSC=0.15 M NaCI/0.015 M sodium citrate) for 15 min and followed by dehydration in ethanol.
Probes were purchased from Eurofins MWG Operon.
Ins:
[AminoC6+Alexa488]CCCGCCCAGCTCCACCTGCCCCACCTGCAGGTCCTCTGCCTCCCGGCG and
[AminoC6+Alexa488]GGTGTGAGCCGCACAGGTGTTGGTTCACAAAGGCTGCGGCTGGGTCAGG Gcg:
[AminoC6+Alexa546]GCCTGGGAAGCTGAGAATGATCTGGATTTCTCCTCTGTGTCTTGAAGG and
[AminoC6+Alexa546]GACGTTTGGCAATGTTATTCCTGTTCCTCTTGGTATTCATCAACCACTGC
Approximately 20 ng of each of the biotinylated probes was added to each slide together with 10 μg of salmon sperm DNA in a hybridization buffer (50% formamide/lx SSC/10% dextran sulfate). Hybridization was performed for 16–20 h at 37°C. Posthybridization washing consisted of two 5-min immersions in 50% formamide and 2×SSC, followed by 2×SSC and 0.l×SSC at 37°C. The slides were dipped briefly in water, allowed to air dry, and Mowiol mounting fluid containing DAPI added. Fluorescence localization was analyzed by confocal microscopy (Carl Zeiss Jena).
Fluorescence-activated cell sorting (FACS)
Flow cytometry analysis was performed using a BD FACSAria II cell sorter (Becton Dickinson).
Real-time RT-PCR
Quantitative PCR was performed on DNA and cDNAs. All reactions were conducted in 96-well plates in 25 ul. Each reaction contained cDNA from 100 ng total RNA, or DNA precipitated from 100 μg of chromatin extracts, for ChIP experiments, and appropriate amount of Universal PCR Master Mix (Applied Biosystems) and Primers/Probe mix. All primers and probes are from Applied Biosystems Assay-on-Demand. The cycle threshold value>40 was considered undetectable and calculated as a Ct of 41.
Bisulfite DNA sequencing
Genomic DNA was isolated from 106 cells by using Non-Organic DNA Extraction Kit (Millipore). Briefly, cells were detached and collected by centrifugation. Cell membranes were disrupted by incubation with a hypotonic buffer, and nuclei were collected and enzymatically deproteinated. Genomic DNA was purified by ethanol precipitation. One milligram of genomic DNA was treated using the CpGenome DNA Modification Kit (Millipore), according to the manufacturer's instructions, to produce the bisulfite-treated DNA (BS-DNA). Final DNA pellets were resuspended in 25 μL ddH2O.
The insulin promoter was amplified using 1 ul of BS-DNA, the following primers: forward TTGTTTTTAGTTGTGAGTAGGGATAG and reverse AACTTTATAATCTCAAAACCCATCTC (annealing temperature 55°C), and HotStarTaq Master Mix (Qiagen). A second round of PCR was performed to produce enough product, using 1 μL of the first round PCR product (in a 50 μL reaction), and the following primers: forw-BamHI AGGAGGATCC TTGTTTTTAGTTGTGAGTAGGGATAG and rev-XhoI AGGACTCGAG AACTTTATAATCTCAAAACCCATCTC (annealing temperature 55°C), which incorporate adhesive restriction sites. The PCR products were digested by BamHI and XhoI (New England BiolabsIpswich) at 37°C for 2 h, purified by the QiaQuick PCR Purification Kit (Qiagen), and ligated with the pShuttle-V5 vector (predigested and purified). The ligation was used to transform competent Escherichia coli cells (One Shot® OmniMAX™ 2 T1R; Invitrogen), and selected by Kana+ LB-agar plates overnight at 37°C. At least 20 colonies were picked up, and the inserts were amplified by PCR supplemented with 5% DMSO, with the following primers: pShuttleF TAAGAAGCTTGGTACCGAATTC and pShuttleR GCTCGCCGCAGCCGAACGACCG (annealing temperature 60°C). Clones with the correct size (about 450 bp) were analyzed by DNA sequencing with primers pShuttleF or pShuttleR (Eurofins MWG Operon).
Mice
About 1.5×106 of either hDFs that underwent the reprogramming protocol, or control hDFs were transplanted under the kidney capsule into NOD.Cg-Rag1tm1Mom Ins2Akita Il2rgtm1Wjl/SzJ (NRG-Akita) female mice (Jackson Laboratory) at 11 weeks of age. Blood glucose levels were measured using the Ascensia Contour glucometer (Bayer) on a biweekly basis a week before surgery and up to 3 weeks postsurgery. The C-peptide was measured at 3 weeks postsurgery using the Mercodia rat ultrasensitive kit. A total of three mice per group are described here.
Data analysis
Data are presented as mean±S.E (standard error) of at least three different experiments and analyzed by the Student's t test. A P value of less than 0.05 was considered to be statistically significant.
Results
Histone H3 and H4 were previously described to be acetylated in islets at the proximal promoter regions of the insulin, glucagon, and Pdx1 genes as well as exons 1 and 3 of the insulin gene [26,43–46]. This modification is often associated with activation of gene transcription [25]. As islet cells proliferate and adapt in culture, they stop expressing insulin and glucagon. In those dedifferentiated islet cells and in mesenchymal stem cells (MSCs), the insulin and glucagon were found to be less highly acetylated [26,47].
We examined whether other epigenetic modifications might account for the differences between islets and dedifferentiated islet cells. We studied DNA methylation at the insulin gene promoter and found it to be less highly methylated in islets compared to dedifferentiated islets (Table 1). Since gene methylation is often associated with repression [27], we hypothesized that the decreased methylation on the insulin gene in islet β cells compared to cells that do not express insulin would account for the differences in expression levels.
Table 1.
Methylation State of the Ins Promoter in β-cells and Dedifferentiated Islet Cells
| % Methylation | ||||||
|---|---|---|---|---|---|---|
| Promoter region | −234 | −206 | −180 | −135 | −102 | −69 |
| β-cells | 61 | 61 | 43 | 52 | 13 | 65 |
| Dedifferentiates islets | 96 | 46 | 38 | 88 | 38 | 92 |
At least 20 bisulfite sequencing of the insulin proximal promoter in islet β-cells and dedifferentiated islets. Values are the percentage of DNA methylation within the different positions of CpG sites.
Building upon the above results, we used two compounds, Romi, a HDACi, and 5-AzC, a cytidine analogue that cannot be methylated, to change the epigenetic modifications of cells in culture. In preliminary experiments with dedifferentiated islet cells, where neither glucagon or insulin transcripts are detectable, 30 nM Romi upregulated glucagon mRNA levels, but not insulin levels and 1 μM 5-AzC did not induce either gene. When the cells were treated with both Romi and 5-AzC, the expression of the insulin and glucagon genes was induced (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). Based on these findings, we were encouraged to use these agents in other adult cell types.
Implementing transplantation protocols using islets or dedifferentiated islets is difficult because of the shortage of cadaveric donors. hDFs can be easily obtained from patients, expanded many fold in culture, and used for autologous transplantation. We obtained similar findings in hDFs on the insulin and glucagon genes as we did in dedifferentiated islet cells. In hDFs, Romi alone was able to induce only glucagon transcription and 5-AzC alone did not induce either glucagon or insulin (data not shown). In contrast, Romi plus 5-AzC induced an increase in both glucagon and insulin genes (Fig. 1). These increases in mRNA levels remained stable over time if cells were first treated with a low dose of Romi (3 nM) for 48 h, and then exposed to 30 nM Romi and 1 μM 5-AzC every 48 h.
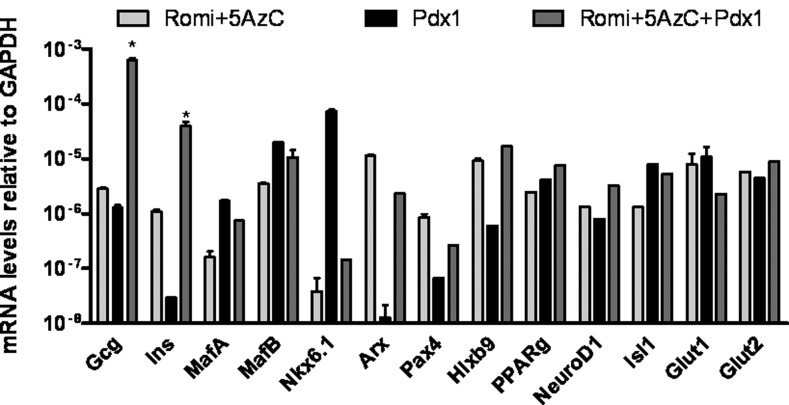
FIG. 1.
Transcriptional effects of Romi, 5-Azacytidine (5-AzC), and transcription factor (TF) on islet-specific hormones, TFs, and transporters. Real-time PCR analysis of gene expression in human dermal fibroblasts (hDFs) undergoing infection by adenoviral pancreatic and duodenal homeobox 1 (Pdx1) for 72 h and/or addition of 30 nM Romi and 1 μM 5- 5AzC for 48 h. Data are expressed relative to GAPDH mRNA. Values are the mean±S.E. from three separate experiments, each analyzed in duplicate. *, statistical significance with P<0.05 value using a Student's t-test.
To better understand the reprogramming of hDFs into islet-like cells, we studied the effects of pancreas-specific TFs on their transdifferentiation. The use of pancreatic TFs was previously described to induce insulin gene transcription or even convert glucagon into insulin-expressing cells [48–50]. We chose to study five pancreas-specific TFs, namely, Pdx1, MafA, Nkx6.1, NeuroD1, and Pax4 by expressing them via adenoviral infection in hDFs. With the exception of Pdx1, none of these TFs, either individually or in combination, resulted in induction of insulin gene transcription (the data for Pdx1 is shown in Fig. 1). Although expression of some of the other TFs following treatment with Romi and 5-AzC resulted in an increase in expression of insulin compared to Romi and 5-AzC alone, only Pdx1 plus Romi and 5-AzC caused a robust increase in insulin mRNA. Similar results were obtained with hDFs obtained from two other individuals (data not shown). Pancreas-specific TFs as well as both Glut1 and Glut2 glucose transporters specific for glucagon- and insulin-producing cells, respectively, were also upregulated by Romi and 5-AzC, or by the TF Pdx1, or a combination of Romi and 5-AzC with Pdx1. Except for PPARg, none of the islet-specific markers we measured was detectable in hDFs. PPARg was upregulated by ∼20-fold by Romi and 5-AzC. We validated that the increases observed for MafA, MafB, and Pdx1 mRNAs were associated with increases in protein levels (Supplementary Fig. S2).
We therefore established a protocol to transdifferentiate hDFs into islet-like cells producing insulin and glucagon. Primary hDFs were infected with Pdx1 adenoviruses (MOI=50). Seventy-two hours later, 30 nM Romi and 1 μM 5-AzC were added for 48 h. The analyses described below are for cells that underwent this reprogramming protocol after 5 days.
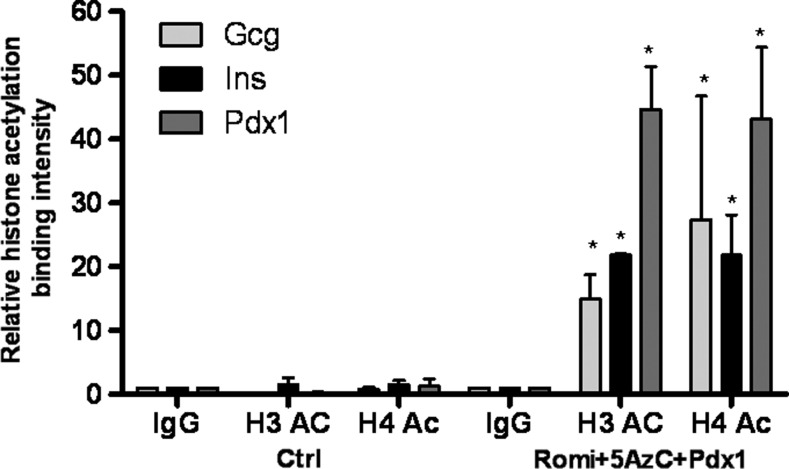
Comparable to what we observed in dedifferentiated islets, the insulin, glucagon, and Pdx1 promoter regions in hDFs are poorly acetylated (Fig. 2). Following addition of Romi and 5-AzC, histones H3 and H4 bound to the insulin, glucagon, and Pdx1 promoter regions are more highly acetylated as determined by ChIP (Fig. 2). Also, the insulin gene promoter is less methylated following the reprogramming protocol as estimated by bisulfite sequencing (Table 2). These two epigenetic modifications observed in hDFs following our reprogramming protocol may account for the changes in gene expression.
FIG. 2.
Following treatment of hDFs with Pdx1, Romi, and 5AzC, the Gcg, Ins, and Pdx1 promoter regions are more acetylated. Chromatin immunoprecipitation for acetylated histones H3 and H4 in hDFs undergoing the reprogramming protocol. Values are the mean±S.E. from three separate experiments, each analyzed in duplicate. *, statistical significance compared to hDF control levels with P<0.05 value using a Student's t-test.
Table 2.
Methylation State of the Ins Promoter in hDFs and hDFs After the Reprogramming Protocol
| % Methylation | ||||||
|---|---|---|---|---|---|---|
| Promoter region | −234 | −206 | −180 | −135 | −102 | −69 |
| hDFs | 100 | 100 | 91 | 89 | 58 | 91 |
| Pdx1+Romi+5AzC | 96 | 89 | 97 | 85 | 14 | 76 |
At least 20 bisulfite sequencing of the insulin proximal promoter in control hDFs and hDFs after the reprogramming protocols. Values are the percentage of DNA methylation within the different positions of CpG sites.
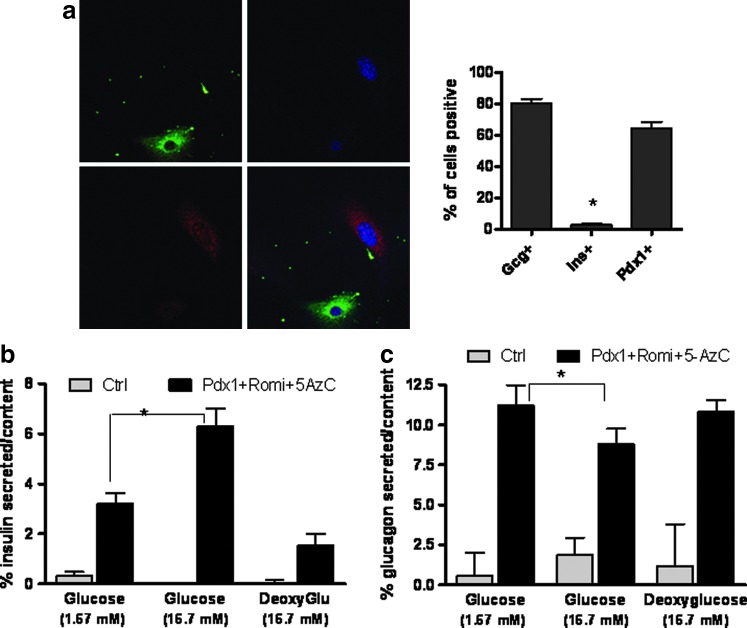
We confirmed the presence of c-peptide and glucagon by immunofluorescence staining. Over 80% of the cells stained positive for glucagon, while only around 2%–4% of the cells were positive for insulin. The TF Pdx1 was present in about 60% of the cells (Fig. 3a).
FIG. 3.
Glucagon and insulin are produced and secreted from hDFs undergoing the reprogramming protocol. (a) Left panel—immunolabeling of glucagon (red) and c-peptide (green). Right panel—quantification of cells stained positive for each antibody tested. *, statistical significance with P<0.05 value using a Student's t-test. (b) Enzyme-linked immunosorbent assay of the relative amount of insulin secreted versus the cellular insulin content in control hDFs and hDFs that have undergone the reprogramming protocol. (c) The ratio of glucagon secreted per cellular content of glucagon as measured by radioimmunoassay with different glucose concentrations in control cells and hDFs undergoing the reprogramming protocol. Data represent percent of hormones secreted versus the hormone content. Values are the mean±S.E. from three separate experiments. *, statistical significance with P<0.05 value using a Student's t-test.
To study whether the cells are secreting insulin in response to glucose, we measured insulin in the medium after incubation in low and high glucose concentrations (Fig. 3b). In a high glucose concentration (16.7 mM), cells that underwent the reprogramming protocol secrete twofold more insulin than these same cells in low glucose concentrations (1.67 mM). By contrast, high concentrations of deoxyglucose, a nonmetabolizable analogue of glucose, failed to induce insulin secretion from these cells. Glucagon secretion was significantly inhibited by high glucose concentrations, similar to islet cells. Deoxyglucose, however, did not reduce glucagon secretion (Fig. 3c). These data suggest coupling between insulin and glucagon storage within the cells and glucose sensing in the reprogrammed cells.
To better characterize the cells obtained from the reprogramming protocol, we stably infected primary hDFs with a lentivirus containing GFP under the control of the 1600 bp glucagon promoter. Following the reprogramming protocol, we sorted these cells by FACS (Fig. 4a). About 80% of the cells were GFP-positive. The GFP-positive cells were enriched with glucagon mRNA and did not express insulin mRNA (Fig. 4b). The GFP-negative population contained less glucagon mRNA, but was enriched for insulin mRNA. These results indicate that we obtained two cell populations: the majority of the cells express glucagon almost exclusively, while a small percentage of the cells express insulin.
FIG. 4.
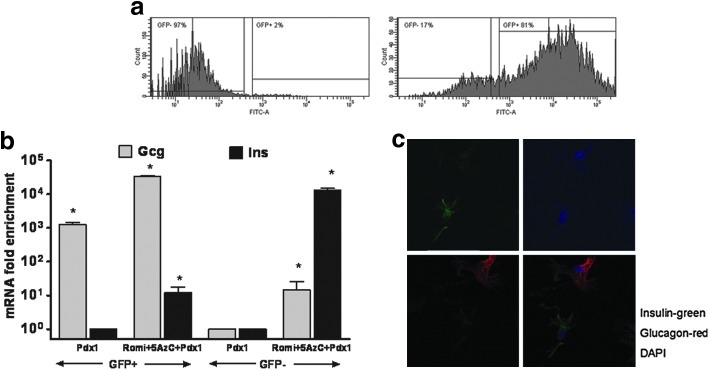
The cells originating from the reprogramming protocol consist of at least two populations, one that is enriched for glucagon and the other enriched for insulin. (a) Human primary dermal fibroblasts were stably infected with a lentivirus containing green fluorescent protein (GFP) under the control of the glucagon gene promoter. Left panel—control hDFs were infected with LacZ adenovirus, not Pdx1, and were not exposed to Romi and 5-AzC=. Right panel—cells undergoing the reprogramming protocol. Cells were sorted by FACS into cells expressing GFP and GFP-negative cells. (b) Real-time PCR analysis of glucagon and insulin in hDFs undergoing the protocol. Data are expressed relative to GAPDH mRNA. Values are the mean±S.E.M from three separate experiments, each analyzed in duplicate. *, statistical significance with P<0.05 value using a Student's t-test. (c) Fluorescent in situ hybridization with insulin (green) and glucagon (red) probes in cells under the reprogramming protocol.
To confirm that we obtained two distinct cell populations, we performed fluorescence in situ hybridization with probes for insulin and glucagon mRNAs. In concordance with the results obtained from the FACS sorting, there were no cells that coexpressed both glucagon and insulin, confirming the above results that there are two cell populations, one that expresses insulin and the other glucagon (Fig. 4c).
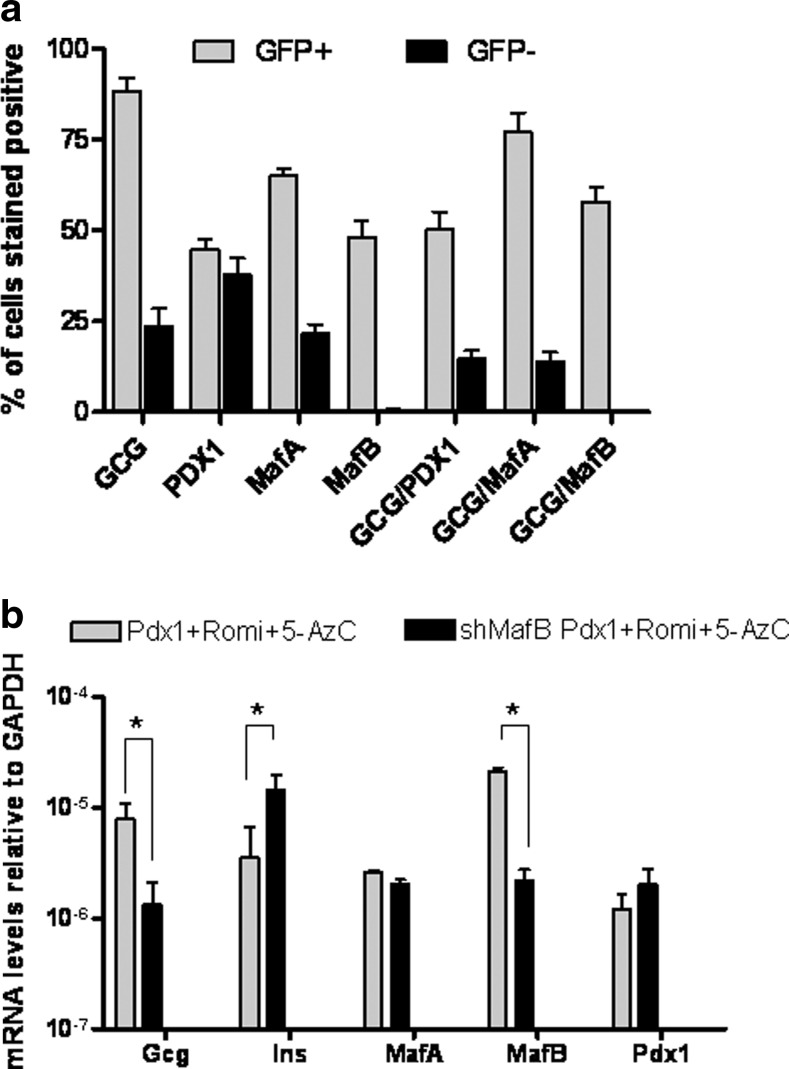
To further characterize the cell populations obtained, we performed immunostaining of the sorted cells. The GFP+ population was, as expected, enriched with glucagon-positive cells. The proportion of cells positive for Pdx1 was equally distributed between the GFP-positive and -negative populations. Interestingly, more GFP+ cells were also positive for MafA and MafB and all cells that stained positively for MafB were also positive for glucagon (Fig. 5a).
FIG. 5.
Musculoaponeurotic fibrosarcoma oncogene homolog B (MafB) and glucagon expression overlap and silencing of MafB results in more insulin and less glucagon expression. (a) Quantification from immunofluorescence staining of the sorted cells described in Figure 4a. Data shown as % of cells stained positive for each protein tested or a combination of glucagon and TF. (b) Quantitative real-time PCR of hDFs under the reprogramming protocol or silenced for MafB before the reprogramming protocol. Values are the mean±S.E. from three separate experiments. *, statistical significance with P<0.05 value using a Student's t-test.
We hypothesized that by decreasing the MafB-positive cells we would decrease the proportion of glucagon-positive cells and potentially increase the proportion of insulin-positive cells. For that purpose, we stably infected hDFs with a lentivirus containing MafB shRNA before pursuing the reprogramming protocol. The level of MafB mRNA was decreased by 10-fold, but there was no effect on Pdx1 or MafA mRNA levels (Fig. 5b). We found that silencing of MafB resulted in increased insulin and decreased glucagon mRNA levels thereby improving our reprogramming protocol.
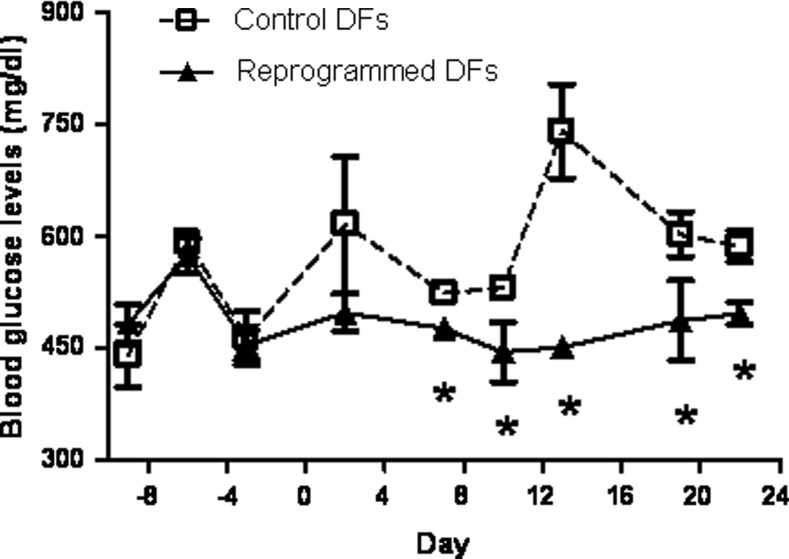
Finally, we transplanted NRG-Akita mice that are homozygous for the Rag1tm1Mom and Il2rgtm1Mom alleles and heterozygous for the Ins2Akita allele, which are both immunodeficient and develop spontaneous hyperglycemia [51,52], with 1.5×106 hDF/mouse that underwent the reprogramming protocol as well as control hDF cells. We were able to detect the human c-peptide in the serum of mice transplanted with the cells that underwent the reprogramming protocol at 3 weeks postsurgery (18.4±3.3 pmol/L), while no human c-peptide was detected in the control hDF transplanted mice. Blood glucose levels were also consistently lower in the mice transplanted with the reprogrammed fibroblasts: 477±24 mg/dl glucose (mean value for days 7 to 22) in mice transplanted with the reprogrammed hDFs versus 609±40 mg/dl glucose (Fig. 6) in mice transplanted with control hDFs (P<0.01). These results suggest that the reprogramming protocol described in this article generated cells that could potentially control glucose homeostasis in vivo.
FIG. 6.
Transplantation of reprogrammed hDFs heterozygous into Akita mice results in decreased blood glucose levels. Cg-Rag1tm1Mom Ins2Akita Il2rgtm1Wjl/SzJ (NRG-Akita) female mice were transplanted with either three separate populations of hDFs, which were reprogrammed separately and transplanted into three mice (1.5×106 hDFs were infected with Pdx1 adenoviruses and 72 h later, 30 nM Romi and 1 μM 5-AzC were added for 48 h), or with control DFs. Blood glucose levels were measured before and postsurgery (surgery day=0). Values are the mean±S.E. from three separate mice. *, statistical significance with P<0.05 value, using a Student's t-test.
Discussion
In this study, we showed that we can generate cells that secrete insulin in response to glucose from adult hDFs using two epigenetic modifying compounds. We further increased the efficiency of this process by adenoviral transduction of the Pdx1 TF in parallel to silencing the transcription of MafB.
We found that the majority of the cells that underwent the reprogramming protocol described in this article were glucagon-positive, while part of the remaining population was insulin-positive. Only a small number of cells coexpressed insulin and glucagon, similar to the adult endocrine pancreas. While it is possible that after 72 h in the reprogramming protocol the cells transition from a bi-hormonal state to a fully differentiated state [53], it is also possible that the single hormone-expressing cells we observed did not transition from a bi-hormonal stage, but were generated from a precursor cell that did not express any hormone. This is because it was previously described that other endocrine cells of the pancreas might originate from glucagon-expressing α-cells [53–55]. However, lineage-tagged transgenic mice showed that insulin and glucagon cells might originate independently in adults [56,57].
This study also demonstrates that human fibroblasts could be directly induced to become functional insulin- or glucagon-secreting cells without the need of going through an intermediate state to induce a pluripotent state by overexpressing pluripotent TFs. Pang et al. [58] as well as Szabo et al. [28] have previously shown that fibroblasts can be differentiated into neuronal or blood progenitor cells. Other recent studies also support the idea that it is unnecessary to go through a pluripotent state to obtain a functional cell that secrets insulin in response to glucose [6,9,35].
DNA methylation and histone modifications constitute major mechanisms that are responsible for epigenetic regulation of gene expression during development and differentiation [59–61]. The use of HDACis and DNA methylation inhibitors has previously been proposed to be of use in cell reprogramming [35,62–65]. Trichostatin A, a HDACi, and 5-AzC were demonstrated to induce β-cell differentiation from rat multipotent nestin-positive bone marrow stem cells [63]. Similarly, it was shown that sodium butyrate and trichostatin A promote ductal differentiation at the expense of the acinar fate. Following that, cells with exocrine function are converted to endocrine cells, capable of producing hormones such as insulin and somatostatin [62,66]. Our study suggests that compounds that modify histone acetylation and DNA methylation can also be used in adult human fibroblasts to give similar expression of pancreatic hormones.
In agreement with our finding that methylation on the insulin promoter is inversely correlated with insulin expression, recent studies proposed that insulin gene expression is regulated by DNA methylation [67,68]. Kuroda et al. showed that while the degree of DNA methylation is low in the insulin promoter of β-cells, the same promoter is highly methylated in other cell types [68].
The TF Pdx1 has been shown to be essential for the normal proliferation and differentiation of embryonic pancreatic precursors [39,40,69]. Pdx1 is expressed early in all pancreatic progenitor cells and is later restricted to adult β-cells plus a fraction of δ-cells. Lineage tracing studies have illustrated that Pdx1-expressing cells at E8.5 are multipotent progenitors, giving rise to all pancreatic lineages [70–72]. Several studies have previously shown that ectopic expression of Pdx1 can result in differentiation to a β-like cell phenotype. We show that overexpression of Pdx1 in hDFs results in expression of glucagon, but not insulin. We observed a similar effect of Pdx-1 overexpression in MSCs [26]. However, addition of Pdx1 before Romi and 5-AzC resulted in expression of both insulin and glucagon to a greater extent than Romi and 5-AzC alone.
The TF MafB is restricted to α-cells in adults. During pancreatic development, at E10.5, MafB is expressed in glucagon-positive cells and at E15.5, although all glucagon+ cells express MafB, a significant proportion of glucagon-negative cells express MafB. These early MafB+/Glucagon− cells express the panendocrine marker synaptophysin and some even express insulin [37,38], suggesting that MafB could play a role in the differentiation of β-cells. In addition, MafB knockout in mice was also shown to regulate the expression of genes specific for β-cell differentiation such as Pdx1, Nkx6.1, and GLUT2 and to be crucial for β-cell development [36,73]. In this study, we noted that following the reprogramming protocol, MafB expression is increased. By lowering the expression of MafB, we obtain higher levels of insulin mRNA and less glucagon mRNA, indicating that fine tuning of MafB expression improves the transdifferentiation of hDFs toward a β-cell phenotype.
This study used adult human cells to transdifferentiate them into islet-like cells. Of note, hDFs are readily obtainable from individuals and the number of cells can be expanded in culture before undergoing the reprogramming protocol. The combination of using epigenetic modulating factors together with overexpression and silencing of key genes important for endocrine pancreas development resulted in the production of functional insulin-secreting cells that can secrete insulin and decrease glycemic levels in diabetic mice.
The method described in this article provides cells that could be obtained directly from a diabetic patient, reprogrammed, and transplanted back to the patient, hence, bypassing the need of a cadaveric donor and a potential rejection by the immune system. The presence of both functional insulin- and glucagon-secreting cells might improve the patient's glycemic control as is the case for exogenous bihormonal treatment for diabetes [74,75].
Supplementary Material
Acknowledgments
This work is supported by the Intramural Research Program, NIDDK, NIH, Z01 DK011007 and by the SNSF PBGEP3-139875 award to LSK.
Author Disclosure Statement
M.C.G. and L.S.K. have a patent application for the Method of Reprogramming Differentiated Somatic Cells to Islet-Like Cells.
References
- 1.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 2.Emamaullee JA. Davis J. Pawlick R. Toso C. Merani S. Cai SX. Tseng B. Shapiro AM. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes. 2008;57:1556–1566. doi: 10.2337/db07-1452. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM. Lakey JR. Future trends in islet cell transplantation. Diabetes Technol Ther. 2000;2:449–452. doi: 10.1089/15209150050194314. [DOI] [PubMed] [Google Scholar]
- 4.Lakey JR. Mirbolooki M. Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM. Ricordi C. Hering BJ. Auchincloss H. Lindblad R. Robertson RP. Secchi A. Brendel MD. Berney T, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 6.Ferber S. Halkin A. Cohen H. Ber I. Einav Y. Goldberg I. Barshack I. Seijffers R. Kopolovic J. Kaiser N. Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 7.Gefen-Halevi S. Rachmut IH. Molakandov K. Berneman D. Mor E. Meivar-Levy I. Ferber S. NKX6.1 promotes PDX-1-induced liver to pancreatic beta-cells reprogramming. Cell Reprogram. 2010;12:655–664. doi: 10.1089/cell.2010.0030. [DOI] [PubMed] [Google Scholar]
- 8.Mauda-Havakuk M. Litichever N. Chernichovski E. Nakar O. Winkler E. Mazkereth R. Orenstein A. Bar-Meir E. Ravassard P. Meivar-Levy I. Ferber S. Ectopic PDX-1 expression directly reprograms human keratinocytes along pancreatic insulin-producing cells fate. PLoS One. 2011;6:e26298. doi: 10.1371/journal.pone.0026298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meivar-Levy I. Sapir T. Gefen-Halevi S. Aviv V. Barshack I. Onaca N. Mor E. Ferber S. Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein beta. Hepatology. 2007;46:898–905. doi: 10.1002/hep.21766. [DOI] [PubMed] [Google Scholar]
- 10.Shternhall-Ron K. Quintana FJ. Perl S. Meivar-Levy I. Barshack I. Cohen IR. Ferber S. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun. 2007;28:134–142. doi: 10.1016/j.jaut.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Zalzman M. Gupta S. Giri RK. Berkovich I. Sappal BS. Karnieli O. Zern MA. Fleischer N. Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amour KA. Bang AG. Eliazer S. Kelly OG. Agulnick AD. Smart NG. Moorman MA. Kroon E. Carpenter MK. Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 13.Kroon E. Martinson LA. Kadoya K. Bang AG. Kelly OG. Eliazer S. Young H. Richardson M. Smart NG, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 14.Segev H. Fishman B. Ziskind A. Shulman M. Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- 15.Chen S. Borowiak M. Fox JL. Maehr R. Osafune K. Davidow L. Lam K. Peng LF. Schreiber SL. Rubin LL. Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J. Au M. Lu K. Eshpeter A. Korbutt G. Fisk G. Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 17.Kunisada Y. Tsubooka-Yamazoe N. Shoji M. Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274–284. doi: 10.1016/j.scr.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Rezania A. Riedel MJ. Wideman RD. Karanu F. Ao Z. Warnock GL. Kieffer TJ. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim JH. Kim SE. Woo DH. Kim SK. Oh CH. McKay R. Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 20.Nostro MC. Sarangi F. Ogawa S. Holtzinger A. Corneo B. Li X. Micallef SJ. Park IH. Basford C, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D. Jiang W. Liu M. Sui X. Yin X. Chen S. Shi Y. Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 22.Hosoya M. Kunisada Y. Kurisaki A. Asashima M. Induction of differentiation of undifferentiated cells into pancreatic beta cells in vertebrates. Int J Dev Biol. 2012;56:313–323. doi: 10.1387/ijdb.123522mh. [DOI] [PubMed] [Google Scholar]
- 23.Thatava T. Nelson TJ. Edukulla R. Sakuma T. Ohmine S. Tonne JM. Yamada S. Kudva Y. Terzic A. Ikeda Y. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:283–293. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huangfu D. Osafune K. Maehr R. Guo W. Eijkelenboom A. Chen S. Muhlestein W. Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 25.Clayton AL. Hazzalin CA. Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Wilson LM. Wong SH. Yu N. Geras-Raaka E. Raaka BM. Gershengorn MC. Insulin but not glucagon gene is silenced in human pancreas-derived mesenchymal stem cells. Stem Cells. 2009;27:2703–2711. doi: 10.1002/stem.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda TB. Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 28.Szabo E. Rampalli S. Risueno RM. Schnerch A. Mitchell R. Fiebig-Comyn A. Levadoux-Martin M. Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 29.Bi D. Chen FG. Zhang WJ. Zhou GD. Cui L. Liu W. Cao Y. Differentiation of human multipotent dermal fibroblasts into islet-like cell clusters. BMC Cell Biol. 2010;11:46. doi: 10.1186/1471-2121-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon JH. Heo JS. Kim JS. Jun EK. Lee JH. Kim A. Kim J. Whang KY. Kang YK, et al. Reprogramming fibroblasts into induced pluripotent stem cells with Bmi1. Cell Res. 2011;21:1305–1315. doi: 10.1038/cr.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiya S. Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 32.Vierbuchen T. Ostermeier A. Pang ZP. Kokubu Y. Sudhof TC. Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuohelainen S. Pirinen E. Cerrada-Gimenez M. Keinanen TA. Uimari A. Pietila M. Khomutov AR. Janne J. Alhonen L. Spermidine is indispensable in differentiation of 3T3-L1 fibroblasts to adipocytes. J Cell Mol Med. 2010;14:1683–1692. doi: 10.1111/j.1582-4934.2009.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo AS. Sun AX. Li L. Shcheglovitov A. Portmann T. Li Y. Lee-Messer C. Dolmetsch RE. Tsien RW. Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thatava T. Ma B. Rohde M. Mayer H. Chromatin-remodeling factors allow differentiation of bone marrow cells into insulin-producing cells. Stem Cells. 2006;24:2858–2867. doi: 10.1634/stemcells.2006-0109. [DOI] [PubMed] [Google Scholar]
- 36.Artner I. Blanchi B. Raum JC. Guo M. Kaneko T. Cordes S. Sieweke M. Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artner I. Le Lay J. Hang Y. Elghazi L. Schisler JC. Henderson E. Sosa-Pineda B. Stein R. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura W. Kondo T. Salameh T. El Khattabi I. Dodge R. Bonner-Weir S. Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Offield MF. Jetton TL. Labosky PA. Ray M. Stein RW. Magnuson MA. Hogan BL. Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 40.Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 41.Gershengorn MC. Hardikar AA. Wei C. Geras-Raaka E. Marcus-Samuels B. Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 42.Heremans Y. Van De Casteele M. in't Veld P. Gradwohl G. Serup P. Madsen O. Pipeleers D. Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarti SK. Francis J. Ziesmann SM. Garmey JC. Mirmira RG. Covalent histone modifications underlie the developmental regulation of insulin gene transcription in pancreatic beta cells. J Biol Chem. 2003;278:23617–23623. doi: 10.1074/jbc.M303423200. [DOI] [PubMed] [Google Scholar]
- 44.Mosley AL. Ozcan S. Glucose regulates insulin gene transcription by hyperacetylation of histone h4. J Biol Chem. 2003;278:19660–19666. doi: 10.1074/jbc.M212375200. [DOI] [PubMed] [Google Scholar]
- 45.Mosley AL. Ozcan S. The pancreatic duodenal homeobox-1 protein (Pdx-1) interacts with histone deacetylases Hdac-1 and Hdac-2 on low levels of glucose. J Biol Chem. 2004;279:54241–54247. doi: 10.1074/jbc.M410379200. [DOI] [PubMed] [Google Scholar]
- 46.Francis J. Chakrabarti SK. Garmey JC. Mirmira RG. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. J Biol Chem. 2005;280:36244–36253. doi: 10.1074/jbc.M505741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davani B. Ikonomou L. Raaka BM. Geras-Raaka E. Morton RA. Marcus-Samuels B. Gershengorn MC. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215–3222. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- 48.Collombat P. Xu X. Ravassard P. Sosa-Pineda B. Dussaud S. Billestrup N. Madsen OD. Serup P. Heimberg H. Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aviv V. Meivar-Levy I. Rachmut IH. Rubinek T. Mor E. Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem. 2009;284:33509–33520. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meivar-Levy I. Ferber S. Regenerative medicine: using liver to generate pancreas for treating diabetes. Isr Med Assoc J. 2006;8:430–434. [PubMed] [Google Scholar]
- 51.Mathews CE. Langley SH. Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation. 2002;73:1333–1336. doi: 10.1097/00007890-200204270-00024. [DOI] [PubMed] [Google Scholar]
- 52.Brehm MA. Bortell R. Diiorio P. Leif J. Laning J. Cuthbert A. Yang C. Herlihy M. Burzenski L, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgammanull Ins2Akita mice. Diabetes. 2010;59:2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasadan K. Daume E. Preuett B. Spilde T. Bhatia A. Kobayashi H. Hembree M. Manna P. Gittes GK. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–3236. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- 54.Herrera PL. Huarte J. Sanvito F. Meda P. Orci L. Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- 55.Teitelman G. Alpert S. Polak JM. Martinez A. Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 56.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 57.Herrera PL. Orci L. Vassalli JD. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol Cell Endocrinol. 1998;140:45–50. doi: 10.1016/s0303-7207(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 58.Pang ZP. Yang N. Vierbuchen T. Ostermeier A. Fuentes DR. Yang TQ. Citri A. Sebastiano V. Marro S. Sudhof TC. Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kretsovali A. Hadjimichael C. Charmpilas N. Histone deacetylase inhibitors in cell pluripotency, differentiation, and reprogramming. Stem Cells Int. 2012;2012:184154. doi: 10.1155/2012/184154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li B. Carey M. Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Cedar H. Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 62.Haumaitre C. Lenoir O. Scharfmann R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol Cell Biol. 2008;28:6373–6383. doi: 10.1128/MCB.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milanesi A. Lee JW. Xu Q. Perin L. Yu JS. Differentiation of nestin-positive cells derived from bone marrow into pancreatic endocrine and ductal cells in vitro. J Endocrinol. 2011;209:193–201. doi: 10.1530/JOE-10-0344. [DOI] [PubMed] [Google Scholar]
- 64.Mali P. Chou BK. Yen J. Ye Z. Zou J. Dowey S. Brodsky RA. Ohm JE. Yu W, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28:713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenoir O. Flosseau K. Ma FX. Blondeau B. Mai A. Bassel-Duby R. Ravassard P. Olson EN. Haumaitre C. Scharfmann R. Specific control of pancreatic endocrine beta- and delta-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes. 2011;60:2861–2871. doi: 10.2337/db11-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haumaitre C. Lenoir O. Scharfmann R. Directing cell differentiation with small-molecule histone deacetylase inhibitors: the example of promoting pancreatic endocrine cells. Cell Cycle. 2009;8:536–544. doi: 10.4161/cc.8.4.7610. [DOI] [PubMed] [Google Scholar]
- 67.Mutskov V. Felsenfeld G. The human insulin gene is part of a large open chromatin domain specific for human islets. Proc Natl Acad Sci U S A. 2009;106:17419–17424. doi: 10.1073/pnas.0909288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroda A. Rauch TA. Todorov I. Ku HT. Al-Abdullah IH. Kandeel F. Mullen Y. Pfeifer GP. Ferreri K. Insulin gene expression is regulated by DNA methylation. PLoS One. 2009;4:e6953. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonsson J. Carlsson L. Edlund T. Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 70.Bonal C. Thorel F. Ait-Lounis A. Reith W. Trumpp A. Herrera PL. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology. 2009;136:309–319.e9. doi: 10.1053/j.gastro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Herrera PL. Defining the cell lineages of the islets of Langerhans using transgenic mice. Int J Dev Biol. 2002;46:97–103. [PubMed] [Google Scholar]
- 72.Herrera PL. Nepote V. Delacour A. Pancreatic cell lineage analyses in mice. Endocrine. 2002;19:267–278. doi: 10.1385/ENDO:19:3:267. [DOI] [PubMed] [Google Scholar]
- 73.Vanhoose AM. Samaras S. Artner I. Henderson E. Hang Y. Stein R. MafA and MafB regulate Pdx1 transcription through the Area II control region in pancreatic beta cells. J Biol Chem. 2008;283:22612–22619. doi: 10.1074/jbc.M802902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell SJ. Tan C. O'Keefe P. Ashraf S. Zaidi A. Fraser AG. Yousef ZR. Optimized temporary bi-ventricular pacing improves haemodynamic function after on-pump cardiac surgery in patients with severe left ventricular systolic dysfunction: a two-centre randomized control trial. Eur J Cardiothorac Surg. 2012;42:e146–e151. doi: 10.1093/ejcts/ezs492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobs PG. El Youssef J. Castle JR. Engle JM. Branigan DL. Johnson P. Massoud R. Kamath A. Ward WK. Development of a fully automated closed loop artificial pancreas control system with dual pump delivery of insulin and glucagon. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:397–400. doi: 10.1109/IEMBS.2011.6090127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.