Abstract
Melatonin is a neuroendocrine transducer of circadian/circannual rhythms able to synchronize organism's physiological activity. On the basis of our recent findings on appetite regulation by melatonin in the zebrafish brain, the aim of this study was to evaluate melatonin's role in peripheral circuitries regulating food intake, growth, and lipid metabolism. For this purpose, the effect of two melatonin doses (100 nM and 1 μM) administered for 10 days, via water, to adult zebrafish was evaluated at both physiological and molecular levels. The major signals controlling energy homeostasis were analyzed together. Additionally, the effect of melatonin doses on muscle metabolic resources was evaluated. The results obtained indicate that melatonin reduces food intake by stimulating molecules involved in appetite inhibition, such as leptin (LPT), in the liver and intestine and MC4R, a melanocortin system receptor, in the liver. Moreover, melatonin decreases hepatic insulin-like growth factor-I (IGF-I) gene expression, involved in growth process and other signals involved in lipid metabolism such as proliferator-activated receptors (PPARα, β, and γ) and sterol regulatory element-binding protein (SREBP). These results were correlated with lower levels of lipids in the muscles as evidenced by the macromolecular pools analyses. The findings obtained in this study could be of great interest for a better understanding of the molecular mechanisms as the basis of food intake control and, in turn, can be a useful tool for medical and aquaculture applications.
Introduction
In all vertebrates, appetite regulation is a complex phenomenon involving interactions between brain and peripheral signals to modulate the physiological response to nutrient ingestion. Peripheral control includes satiety and adiposity signals, while the central regulation is exerted by neuropeptides and neurotransmitters acting as orexigenic or anorexigenic factors.1 Altogether, these central and peripheral signals allow maintenance of energy balance and body weight stability by modulating energy expenditure and energy intake.2 Depending on daily living habits, vertebrates have adapted their behavior and their physiological functions, such as feeding, growth, reproduction, and osmoregulation.3,4
It is well known that appetite is under multifactorial control and that among different environmental synchronizers, the alternation of light and dark (circadian rhythm) is probably the main factor controlling the feeding rhythm. In this regard, considering that melatonin acts as a neuroendocrine transducer, able to synchronize the physiological functions,5 an increasing number of studies have focused on photoperiod and melatonin influence on food intake regulation in both mammals and fish.6–9 Melatonin is produced rhythmically with high levels at night and low levels during the day in both diurnal and nocturnal species,4 and its effect on food intake is often different (appetite stimulation, inhibition, or no effect) depending on the habits of each species.10–13 In particular, our recent study on the central regulation of appetite in zebrafish demonstrated that melatonin is able to modulate not only food intake but also the most central appetite signals.9
It has been demonstrated that melatonin, the hormonal mediator of biological rhythms, also acts at the peripheral level affecting adiposity, growth, and body weight, in both mammals and fish, although such effects are highly variable.7,14 In fish, a contrasting action of melatonin on growth has been observed: a significant weight increase was found in Atlantic salmon implanted with melatonin,15 while in both trout and goldfish, implants or injections reduced body weight and growth rate.16,17 On the other hand, it is well known that a central component of growth processes is the growth hormone (GH)–insulin-like growth factor-I (IGF-I) axis. Many of the growth-promoting actions of GH in fish are mediated by IGF-I,18,19 and Falcón et al.20 demonstrated a direct role of melatonin on GH secretion. In addition, recent studies have shown that melatonin influences carbohydrates and lipid metabolism in goldfish liver and muscle17; inconsistent results, instead, were found in mammals.21–23
In this regard, among signals that regulate feeding behavior and metabolism, leptin (LPT) plays a key role as a satiety molecule controlling body weight maintenance. In both mammals and fish, LPT is secreted by a variety of tissues such as adipocytes, stomach, muscle, placenta, and brain,24–27 and its anorexigenic action is exerted by stimulating melanocyte-stimulating hormone and subsequently MC4R, the key receptor in appetite regulation of the melanocortin system.28,29 While in mammals MC4R is exclusively expressed in the central nervous system, in fish it is also peripherally expressed, indicating a role for pro-opiomelanocortin (POMC) molecules on peripheral regulation of energy homeostasis.30 Moreover, in mammals it is now clear that LPT and POMC pathways exert an integrated control of appetite and fat metabolism31; in fact, MC4R genetic disruption in mice directly promoted lipid uptake and fat accumulation in white adipose tissue.32 A similar relationship between the two systems is easily conceivable also in fish considering that several studies clearly indicate an involvement of melanocortin receptors in the regulation of fish energy metabolism.33
Focusing on molecules involved in lipid metabolism, proliferator-activated receptors (PPARs) and sterol regulatory element-binding proteins (SREBPs) are the key signals of the hepatic fat pathway. PPARs are members of the nuclear receptor family that regulates the expression of genes that control fatty acid synthesis, storage, and catabolism. Three subtypes of PPARs, termed α, β (δ), and γ, have been identified in mammals' liver as well as in fish. The overall features including the primary structure and tissue distribution are similar to those of their mammalian counterparts,34,35 resulting a good tool in fish metabolism studies. Concerning SREBP, this sterol-sensitive transcription factor is synthesized as a membrane-bound precursor protein that in the liver is cleaved to its mature form when cellular sterols are depleted. Knight et al.36 elucidate a clear link between SREBP and PPARα in the liver, where the latter controls SREBP expression and activity. These authors demonstrated that the hepatic gene expression of both signals is more pronounced during the light phase of the diurnal cycle, confirming the importance of photoperiod in metabolism control.
Unlike mammals, in fish, only one study is available on SREBP activity. Migliarini and Carnevali37 analyzed SREBP gene expression in zebrafish lipid metabolism in relation to the endocannabinoid system, but a possible relationship between this lipid regulator and melatonin has not yet been analyzed.
Considering the limited and contrasting data available on the control of energy balance by melatonin and on the bases of previous results obtained in our laboratory on the central control of melatonin in zebrafish appetite,9 in this study we aimed to elucidate the melatonin effects on peripheral organs involved in appetite, growth, and lipid metabolism in Danio rerio. Major peripheral signals involved in energy homeostasis control and growth such as LPT, MC4R, PPARs, SREBP, and IGF-1 were analyzed with a concomitantly evaluation of metabolic resources distribution.
Materials and Methods
Animals and maintenance
The experiments were carried out in adult male zebrafish aged 10 months (D. rerio, AB wild-type strain) obtained from our facilities. Fish were spawned from the same broodstock.
Fish were maintained in 100 L aquaria, with a constant flow of filtered fresh water. Photoperiod was 14 light:10 dark (lights on at 07:00; ZT 0, zeitgeber time) and water temperature was 27±1°C. Fish were fed daily with a 2% body weight ration of floating pellets (Sera Vipagran). Animals were acclimated to these conditions for at least 15 days before the assay, showing normal feeding and activity patterns during this acclimation period. Care and the use of the animals were in accordance with the Guidelines on the Handling and Training of Laboratory Animals by the Universities Federation for Animal Welfare and with the Italian animal welfare legislation (D.L. 116/92).
Experimental design
Melatonin treatment
Two groups of zebrafish (n=10 fish per group), in triplicate, maintained with the photoperiod previously described, were exposed via water to two different doses of melatonin (Sigma Aldrich), 100 nM and 1 μM, following previous studies.9,38 Briefly, for stock solution, melatonin was directly dissolved to 100 μM concentration in water. The actual levels of melatonin were confirmed by high-performance liquid chromatography with fluorescence detection using serial dilutions, in three independent experiments. The treatment or control solution (water) was administered directly into the fish tank. The final concentration of melatonin in the tanks was 100 nM and 1 μM, respectively.
Treatment was 10 days long, melatonin doses were added daily at 11:00 a.m. (ZT 4), and the concentrations were maintained constant throughout the experiment by renewing water every 24 h in each tank. This avoided a melatonin accumulation into the water as previously described.9
A control group (n=10), in triplicate, was kept at the same rearing conditions but without melatonin administration. For the control group and for each group exposed to two melatonin doses, the treatment was performed in three different tanks (in triplicate).
Food intake analysis
During the experiment, the animals received preweighed food in excess (5% body weight) every day, 30 min after melatonin treatment. Specifically, food intake (FI) was measured at the end of the 10 days' treatment. FI was thus monitored for 5 h after the last melatonin administration. FI was calculated as follows: FI=Wi – (Wf×F), where Wi=initial dry food weight, Wf=remaining dry food weight, and F=correction factor. F was previously calculated in the absence of fish to determine the effect of water dissolution on food pellets during the feeding time, and represents the reduction in food weight after food remains 5 h into the aquaria (F=0.856±0.0054) following previous studies.9,39
At the end of the FI experiment (ZT 9), zebrafish were anesthetized (0.1 mg/L MS222; Sigma) and livers, muscles, and intestines were dissected and stored at −80°C until analysis. Considering RNA sensitivity, the sampling of liver, intestine, and muscle from each fish was performed as fast as possible and in the presence of dry ice. The food was analyzed by filtering the water in the tanks on a 30 μm sieve, after manually removing feces with a Pasteur pipette; the remaining food was then concentrated in a 5 mL volume and dried at 60°C over night.
RNA extraction and cDNA synthesis
Total RNA was extracted from liver and intestine samples with RNeasy Mini Kit (Qiagen). Final RNA concentrations were determined by optical density measurement at 260 nm, and the RNA integrity was verified by ethidium bromide staining of 28S and 18S ribosomal RNA bands on 1% agarose gel. First-strand cDNA synthesis was performed as already described by Carnevali and Maradonna.40
Real-time polymerase chain reaction
A relative quantification of cDNA was made using β-ACT9,41 and ARP42 as normalizers in each sample in order to standardize the results by eliminating variation in mRNA and cDNA quantity and quality.43 Reference genes were chosen because the mRNA levels were close to those of our target genes and the levels did not vary between experimental treatments. No amplification product was observed in negative control and no primer–dimer formation was observed in the control templates. The data obtained were analyzed using the iQ5 optical system software version 2.0 (Bio-Rad) including Genex Macro iQ5 Conversion and genex Macro iQ5 files. Data were normalized using genex Macro iQ5 file. The calculations in this spreadsheet are derived from the algorithms outlined by Vandesompele et al.44 and from the geNorm manual and associated calculations.
Primers were designed using polymerase chain reaction (PCR) designer software PRIMER3, starting from zebrafish sequences available in GenBank and used at a final concentration of 10 pmol/μL. Primers sequences are shown in Table 1.
Table 1.
List of Primers Used in This Study
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Leptin | 5′-AGCTCTCCGCTCAACCTGTA-3′ | 5′-CAGCGGGAATCTCTGGATAA-3′ |
| MC4R | 5′-ATCTCCACGGAGGTCTTCCT-3 | 5′-CGAAGCATTGGAGACACTCA-3′ |
| SREBP | 5′-GCTGTTACCCTCTGCTGAAG-3′ | 5′-TGAAACCGCTGCCTTGAC-3′ |
| PPARα | 5′-TCCACATGAACAAAGCCAAA-3′ | 5′-AGCGTACTGGCAGAAAAGG-3′ |
| PPARβ | 5′-TGGAGTACGAGCGATGTGAG-3′ | 5′-TAGTCCAGCCACCAGCTTCT-3′ |
| PPARγ | 5′-CTGCCGCATACACAAGAAGA-3′ | 5′-TCACGTCACTGGAGAACTCG-3′ |
| β-actin | 5′-GGTACCCATCTCCTGCTCCAA-3′ | 5′-GAGCGTGGCTACTCCTTCACC-3′ |
PCRs were performed with the SYBR green method in an iQ5 Multicolor Real-Time PCR Detection System (BioRad). Triplicate PCRs were carried out for each sample analyzed. The reactions were set on a 96-well plate by mixing, for each sample, 1 μL of diluted (1/10) cDNA, 5 μL of 2× concentrated SYBR Green PCR Master Mix (BioRad), containing SYBR Green as fluorescent intercalating agent, 0.3 μM forward primer, and 0.3 μM of reverse primer. The thermal profile, for all reactions, was 3 min at 95°C and then 45 cycles of 10 s at 95°C, 20 s at 60°C, and 20 s at 72°C. The fluorescence was monitored at the end of each cycle. Additional dissociation curve analysis was performed and showed in all cases one single peak. Data obtained were treated by iQ5 optical system software version 2.0.
Fourier transform infrared spectroscopy
With the purpose to evaluate melatonin effects on macromolecular pools, distribution Fourier transform infrared (FT-IR) spectroscopy was carried on zebrafish muscle tissue.
Sample preparation
Muscle tissues sampled from zebrafish exposed to melatonin 100 nM and 1 μM and from the control group were sonicated in a milliQ water buffer, divided in 50 μL rates, deposed on Si supports, dried at 60°C for 12 h, and spectroscopically analyzed using Spectrum GX1 Perkin Elmer spectrometer.
Data processing for infrared spectroscopy
On samples' spectra, interpolated in the range 1800–880 cm−1 and two points baseline linear fitted, three bands were selected at 1739, 1706 (νC=O of triglycerides), and 1630 (amide I) cm−1, on which heights were calculated (Spectrum 5.3 Perkin Elmer software package). For each specimen, 5 samples were analyzed, for a total of 15.
Statistical analysis
The data obtained were examined by one-way ANOVA followed by the Tukey post-test, using a statistical software package, Graph Pad Prism5 (Graph Pad Software Inc.), with significance set at p<0.05.
Results
Food intake analysis
The two different doses (100 nM and 1 μM) of the hormone, administered via water, significantly reduced food intake with respect to the control group. The strongest decrease in food intake was observed with the higher dose (about twofold) as shown in Table 2.
Table 2.
Food Intake Control by Melatonin Treatment (100 nM and 1 μM) in Zebrafish Adults
Data are expressed as mean food intake (mg) per body weight (g). Results are expressed as mean±standard error of the mean.
Significant differences evaluated by one-way analysis of variance with the Tukey post-test (p<0.05).
Melatonin effects on hepatic signals involved in appetite control and energy balance
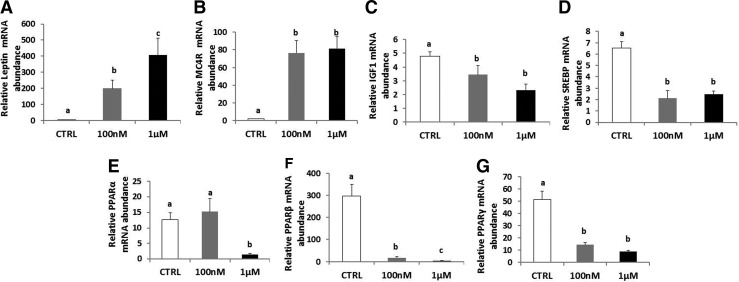
Real-time PCR analysis clearly demonstrated a key role of melatonin in regulating signals involved in food intake, growth, and lipid metabolism in the liver (Fig. 1A–G). Figure 1A shows changes in LPT gene expression. Both melatonin doses caused a great (200- and 400-fold) increase in mRNA levels with respect to the control, with the strongest effect being observed with the higher dose (1 μM).
FIG. 1.
(A–G) The figure represents the expression of the different genes (LPT, MC4R, IGF-1, SREBP, and PPARs) analyzed by real-time polymerase chain reaction in the zebrafish liver after exposure to melatonin (100 nM and 1 μM). Results are expressed as mean±standard error of the mean (SEM). Different letters indicate significant differences on the basis of the Tukey post-test (p<0.05). CTRL, control.
LPT, as well as MC4R, the other anorexigenic signal tested, was affected by melatonin treatment: a strong increase of MC4R mRNA levels was observed for both doses tested with respect to the control (Fig. 1B); the increase was not dose related.
Regarding growth, as shown in Figure 1C, melatonin induced a significant decrease in IGF-1 mRNA levels, for both doses tested, with a downward trend with respect to the control.
Moving to lipid metabolism, some hepatic key genes were analyzed. First, SREBP gene expression was significantly reduced compared with the control by both melatonin doses; the effect was not dose related (Fig. 1D). Second, a similar trend was observed for PPARα, β, and γ mRNA levels (Fig. 1E–G). Figure 1E shows the changes of PPARs' gene expression by melatonin in the liver. Only the higher dose (1 μM) induced a significant decrease in PPARα mRNA levels, whereas the lower dose did not induce any change compared with the control. PPARβ and PPARγ gene expression levels were significantly decreased by both melatonin doses, and PPARβ mRNA levels showed a dose-related reduction (Fig. 1F, G).
Melatonin effect on intestine signals involved in appetite control
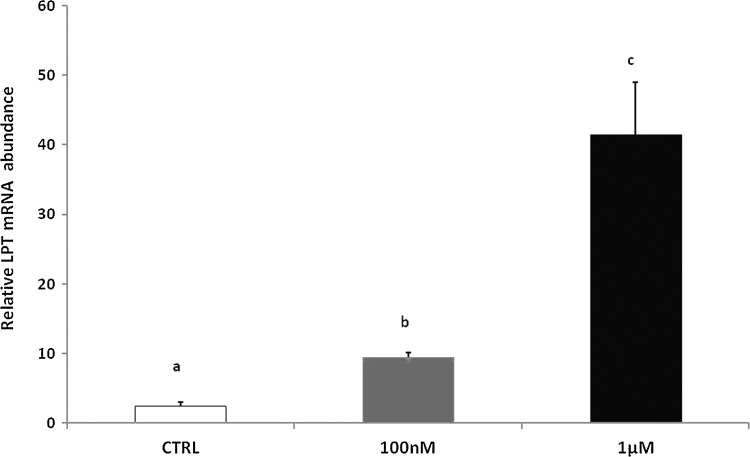
As already described in the liver, in the intestine the two melatonin doses (100 nM and 1 μM) showed a modulation of LPT mRNA levels that was dose related; in fact, both doses significantly increased LPT gene expression with the strongest effect being observable with 1 μM melatonin (Fig. 2).
FIG. 2.
LPT gene expression in the zebrafish intestine after exposure to melatonin (100 nM and 1 μM). Results are expressed as mean±SEM. Different letters indicate significant differences on the basis of the Tukey post-test (p<0.05).
Melatonin effect on the macromolecular pools in the muscle
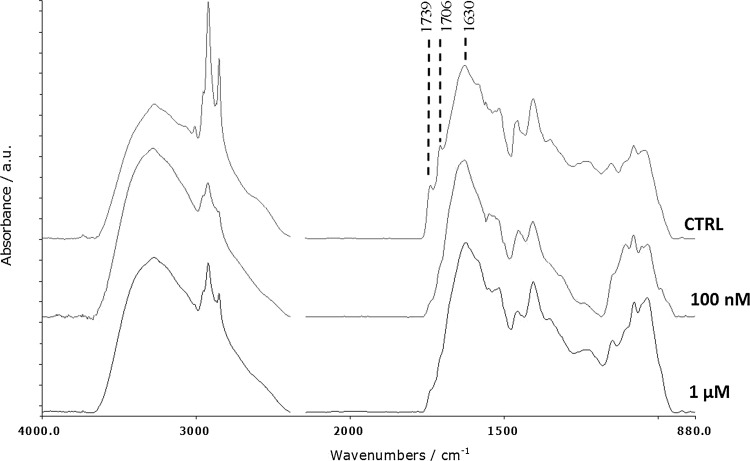
To understand melatonin implication on metabolic resources, distribution FT-IR analyses were conducted on muscle tissue. In this regard, three bands were taken into account at 1739, 1706 (νC=O of ester functional groups), and 1630 (amide I) cm−1: the first and the second, highly sensitive to hydration and hydrogen bonding, are attributable to ester groups in triglycerides, while the latter is strongly correlated with the protein pattern.45 The results showed a significant decrease in the lipid component for both melatonin doses compared with the control, while protein pools were not significantly changed (Fig. 3 and Table 3).
FIG. 3.
Representative spectra of the macromolecular pools of control, 100 nM and 1 μM muscle tissue in the region 4000–880 cm−1.
Table 3.
Relative Heights of Meaningful Infrared Bands Absorption
| Infrared bands | 1630 | 1706 | 1739 | 1630/(1706+1739) |
|---|---|---|---|---|
| Control | 0.0939 | 0.0586 | 0.0364 | 0.99 |
| 100 nM | 0.0802 | 0.0217 | 0.0016 | 3.44 |
| 1 μM | 0.1053 | 0.0213 | 0.0077 | 3.63 |
Discussion
It is well known that a specific neurocircuitry, which is mainly located in hypothalamic and brain stem areas, continuously monitors peripheral signals reflecting energy status and initiates appropriate behavioral and metabolic responses to fluctuations in nutrient availability.46 Since the finding of melatonin production in the gastrointestinal tract of several vertebrate species,47 including fish,48 a growing number of studies have been involved in the relationship between this hormone and food intake processes.9 Melatonin contributes to synchronizing behaviors and neuroendocrine regulations with the daily and annual variations of the photoperiod, contributing to the maintenance of energy balance in vertebrates.4 Melatonin is also considered an anorexigenic factor in diurnal and nocturnal fish species8,17 though several authors suggested that the effects could be attributed to a sedative action of the molecule.49
Considering the increasing interest on melatonin influence on food intake and metabolism, on the basis of our recent results obtained in the zebrafish brain by melatonin, this study highlights the possible relationship between this hormone and peripheral circuitries controlling food intake, growth, and lipid metabolism in D. rerio.
In the present study, melatonin administration induces a reduction of food intake and accordingly, in peripheral districts, determines a stimulation of satiety and anorexigenic signals such as LPT in the liver and intestine and MC4R in the liver.
Induction of LPT and MC4R mRNA levels by melatonin may suggest that this hormone is able to modulate appetite by inducing anorexigenic signal production in peripheral tissues. The stimulation of MC4R gene expression by melatonin in the liver concomitant with food intake reduction suggests that in zebrafish, this receptor is associated with energy homeostasis control in peripheral tissues as already observed in barfin flounder.30 In addition, these results showed an increase in MC4R and LPT expression levels in the liver as previously observed in the brain,9 supporting their involvement in food intake regulation. As demonstrated by several studies, melatonin involvement in appetite/metabolism regulation may be related not to an action per se but to the onset of different mechanisms. In particular, the anorexigenic action of this hormone could be mediated by a delay in the gastric emptying rate as seen in rats and goldfish,50,51 or by its stimulatory action on fat mobilization.17,22 In addition, more recently, Zhdanova52 focused on sleep regulation in zebrafish, highlighting that melatonin action on food intake reduction can be due to an alteration of the circadian rhythm and to a sleep promotion and not to a direct effect of the hormone. It is clear that added studies are requested to discriminating among these different factors.
Regarding melatonin influence on metabolism, signals involved in growth and lipid metabolism were considered together with metabolic resources distribution in the muscle. The results showed an involvement of melatonin in all circuits analyzed, suggesting a role for this hormone as a key player in the system that regulates growth, metabolism, and thus energy balance.
Several studies conducted in mammals have shown that increasing the photoperiod results in increased appetite, growth, GH production, and IGF-1 levels.53 Similar changes in GH–IGF-1 axis have been reported in many fish species, such as salmon,54,55 medaka,56 and sea bream.57 In particular, Falcón and colleagues20 demonstrated, for the first time in fish, that melatonin modulates GH secretion, acting directly on trout pituitary cells. Nevertheless, Taylor et al.58 analyzed the effect of melatonin implants in rainbow trout, finding that supraphysiological doses of melatonin inhibit the growth rate apparently without a direct action on the IGF system. In contrast, our results showed a reduction in the IGF-1 gene expression level after melatonin treatment, but it is not clear whether melatonin acts on IGF-1 directly or via activation of anorexigenic pathways, suggesting that melatonin may be the intermediary in the process that conveys photoperiodic information to the somatotropic axis.
In addition, this study highlights the role of melatonin on lipid metabolism and metabolic resources distribution: all signals investigated in the liver, such as SREBP and PPARs, were negatively modulated by melatonin administration, and in muscle a reduction of lipid macromolecular pool was evident. This decrease could be a consequence of food intake reduction: during the fasting period, the lipid component is commonly dismantled to recover energy for the whole body, as seen in mammals.59
Moreover, the results recently obtained in mammals by Knight et al.36 further highlighted the relationship between PPARs and SREBP. Not only did these authors show that the inclusion of a PPARα activator in the diet of normal mice can stimulate the hepatic expression of genes involved in fatty acid oxidation and in de novo lipid synthesis (SREBP), but also they demonstrated a circadian trend in the expression of PPAR and SREBP. Thus, the findings obtained in the present study support the hypothesis that melatonin could act as a hormonal mediator controlling the circadian expression of PPARs and SREBP also in fish. Anyway, additional studies are needed to elucidate the entire pathway.
In conclusion, the present study indicates that melatonin is involved in peripheral circuitries regulating appetite as well as metabolism. Nevertheless, preliminary data in intestine and liver do not show the presence of the six melatonin receptors so far identified in zebrafish, suggesting a brain-mediated action of melatonin in the peripheral districts, as also demonstrated by Conde-Sieira and collaborators60 in rainbow trout glucosensing mechanisms.
The findings obtained in the present study could be of great interest for a better understanding of the molecular mechanisms at the basis of food intake control and in turn can be a useful tool for aquaculture applications.
Acknowledgment
This study was supported by COFIN 2008, awarded to Prof. Oliana Carnevali.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Valassi E. Scacchi M. Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Lin X. Volkoff H. Narnaware Y. Bernier NJ. Peyon P. Peter RE. Brain regulation of feeding behavior and food intake in fish. Comp Biochem Physiol A Mol Integr Physiol. 2000;126:415–434. doi: 10.1016/s1095-6433(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 4.Falcón J. Besseau L. Sauzet S. Bœuf G. Melatonin effect on the hypothalamo-pituitary axis in fish. Trends Endocrinol Metab. 2007;18:81–88. doi: 10.1016/j.tem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Azpeleta C. Martínez-Alvarez RM. Delgado MJ. Isorna E. De Pedro N. Melatonin reduces locomotor activity and circulating cortisol in goldfish. Horm Behav. 2010;57:323–329. doi: 10.1016/j.yhbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bubenik GA. Pang SF. The role of serotonin and melatonin in gastrointestinal physiology: ontogeny, regulation of food intake and mutual serotonin-melatonin feedback. J Pineal Res. 1994;16:91–99. doi: 10.1111/j.1600-079x.1994.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Pinillos ML. De Pedro N. Alonso-Gómez AL. Alonso-Bedate M. Delgado MJ. Food intake inhibition by melatonin in goldfish (Carassius auratus) Physiol Behav. 2001;72:629–634. doi: 10.1016/s0031-9384(00)00399-1. [DOI] [PubMed] [Google Scholar]
- 8.Rubio VC. Sánchez-Vázquez FJ. Madrid JA. Oral administration of melatonin reduces food intake and modifies macronutrient selection in European sea bass (Dicentrarchus labrax, L.) J Pineal Res. 2004;37:42–47. doi: 10.1111/j.1600-079X.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- 9.Piccinetti CC. Migliarini B. Olivotto I. Coletti G. Amici A. Carnevali O. Appetite regulation: the central role of melatonin in Danio rerio. Horm Behav. 2010;58:780–785. doi: 10.1016/j.yhbeh.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Bermúdez FF. Forbes JM. Injidi MH. Involvement of melatonin and thyroid hormone in the control of sleep, food intake and energy metabolism in the domestic fowl. J Physiol. 1983;337:19–27. doi: 10.1113/jphysiol.1983.sp014608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubenik GA. Pang SF. Hacker RR. Smith PS. Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of food. J Pineal Res. 1996;21:251–256. doi: 10.1111/j.1600-079x.1996.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 12.Shaji AV. Kulkarni SK. Evidence of GABAergic modulation in melatonin-induced short term memory deficits and food consumption. Methods Find Exp Clin Pharmacol. 1998;20:311–319. doi: 10.1358/mf.1998.20.4.485685. [DOI] [PubMed] [Google Scholar]
- 13.Wolden-Hanson T. Mitton DR. McCants RL. Yellon S M. Wilkinson CW. Matsumoto AM. Rasmussen DD. Daily melatonin administration to middle-aged male rats suppresses body weight, intra-abdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–497. doi: 10.1210/endo.141.2.7311. [DOI] [PubMed] [Google Scholar]
- 14.Huether G. Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. In: Pierpaoli W, editor; Regelson W, editor; Fabris N, editor. In The Aging Clock: The Pineal Gland and Other Pacemakers in the Progression of Aging and Carcinogenesis. The New York Academy of Sciences; New York: 1994. pp. 146–158. [DOI] [PubMed] [Google Scholar]
- 15.Porter MJR. Randall CF. Bromage NR. Thorpe JE. The role of melatonin and the pineal gland on the development and smoltification of Atlantic salmon (Salmo salar) parr. Aquaculture. 1998;168:139–155. [Google Scholar]
- 16.Taylor JF. North BP. Porter MJR. Bromage NR. Migaud H. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Onchorhyncus mykiss. Gen Comp Endocrinol. 2006;256:216–234. [Google Scholar]
- 17.De Pedro N. Martínez-álvarez RM. Delgado J. Melatonin reduces body weight in goldfish (Carassius auratus): effects on metabolic resources and some feeding regulators. J Pineal Res. 2008;45:32–39. doi: 10.1111/j.1600-079X.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 18.Duan C. The insulin-like growth factor system and its biological actions in fish. Am Zool. 1997;37:491–503. [Google Scholar]
- 19.Duan C. Plisetskaya EM. Dickhoff WW. Expression of insulin-like growth factor-I in normally and abnormally developing coho salmon (Oncorhynchus kisutch) Endocrinology. 1995;136:446–452. doi: 10.1210/endo.136.2.7835275. [DOI] [PubMed] [Google Scholar]
- 20.Falcón J. Besseau L. Fazzari D. Attia J. Gaildrat P. Beauchaud M. Boeuf G. Melatonin modulates secretion of growth hormone and prolactin by trout pituitary glands and cells in culture. Endocrinology. 2003;144:4648–4658. doi: 10.1210/en.2003-0707. [DOI] [PubMed] [Google Scholar]
- 21.Mustonen AM. Nieminen P. Hyvärinene H. Melatonin and the wintering strategy of the tundra vole, Microtus oeconomus. Zool Sci. 2002;19:683–687. doi: 10.2108/zsj.19.683. [DOI] [PubMed] [Google Scholar]
- 22.Nieminen P. Käkelä R. Mustonen AM. Hyvärinen H. Asikainen J. Exogenous melatonin affects lipids and enzyme activities in mink (Mustela vison) liver. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128:203–211. doi: 10.1016/s1532-0456(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 23.Bojková B. Marková M. Ahlersová E. Ahlers I. Adámeková E. Kubatka P. Kassayová M. Metabolic effects of prolonged melatonin administration and short-term fasting in laboratory rats. Acta Vet Brno. 2006;75:2–32. [Google Scholar]
- 24.Ahima RS. Prabakaran D. Mantzoros C. Qu D. Lowell B. Maratos-Flier E. Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 25.Ahima RS. Saper CB. Flier JS. Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 26.Volkoff H. Eykelbosh AJ. Peter RE. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003;972:90–109. doi: 10.1016/s0006-8993(03)02507-1. [DOI] [PubMed] [Google Scholar]
- 27.Morash B. Li A. Murphy PR. Wilkinson M. Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW. Seeley RJ. Woods SC. Weigle DS. Campfield LA. Burn P. Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 29.Seeley RJ. Yagaloff KA. Fisher SL. Burn P. Thiele TE. van Dijk G, et al. Melanocortin receptors in leptin effects. Nature. 1997;390:349–351. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y. Tsuchiya K. Yamanome T. Schiöth HB. Kawauchi H. Takahashi A. Food deprivation increases the expression of melanocortin-4 receptor in the liver of barfin flounder, Verasper moseri. Gen Comp Endocrinol. 2008;155:280–287. doi: 10.1016/j.ygcen.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Forbes S. Bui S. Robinson BR. Hochgeschwender U. Brennan MB. Integrated control of appetite and fat metabolism by the leptin-proopiomelanocortin pathway. Proc Natl Acad Sci U S A. 2001;98:4233–4237. doi: 10.1073/pnas.071054298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueiras R. Wiedmer P. Perez-Tilve D. Veyrat-Durebex C. Keogh JM. Sutton GM, et al. The central melanocortin system directly control peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yada T. Moriyama S. Suzuki Y. Azuma T. Takahashi A. Hirose S, et al. Relationships between obesity and metabolic hormones in the “cobalt” variant of rainbow trout. Gen Comp Endocrinol. 2002;128:36–43. doi: 10.1016/s0016-6480(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 34.Ibabe A. Bilbao E. Cajaraville MP. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) depending on gender and developmental stage. Histochem Cell Biol. 2005;123:75–87. doi: 10.1007/s00418-004-0737-2. [DOI] [PubMed] [Google Scholar]
- 35.Oku H. Umino T. Molecular characterization of peroxisome proliferator-activated receptors (PPARs) and their gene expression in the differentiating adipocytes of red sea bream Pagrus major. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:268–277. doi: 10.1016/j.cbpb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Knight BL. Hebbachi A. Hauton D. Brown AM. Wiggins D. Patel DD, et al. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem J. 2005;389:413–421. doi: 10.1042/BJ20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migliarini B. Carnevali O. Anandamide modulates growth and lipid metabolism in the zebrafish Danio rerio. Mol Cell Endocrinol. 2008;286:12–16. doi: 10.1016/j.mce.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Zhdanova IV. Yu L. Lopez-Patiño M. Shang E. Kishi S. Guelin E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res Bull. 2008;75:433–441. doi: 10.1016/j.brainresbull.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Patiño MA. Guijarro AI. Isorna E. Delgado MJ. Alonso-Bedate M. De Pedro N. Neuropeptide Y has a stimulatory action on feeding behavior in goldfish (Carassius auratus) Eur J Pharmacol. 1999;377:147–153. doi: 10.1016/s0014-2999(99)00408-2. [DOI] [PubMed] [Google Scholar]
- 40.Carnevali O. Maradonna F. Exposure to xenobiotic compounds: looking for new biomarkers. Gen Comp Endocrinol. 2003;131:203–209. doi: 10.1016/s0016-6480(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 41.Reyes-Becerril M. López-Medina T. Ascencio-Valle F. Esteban MÁ. Immune response of gilthead sea bream (Sparus aurata) following experimental infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2011;31:564–570. doi: 10.1016/j.fsi.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Gioacchini G. Giorgini E. Merrifield LD. Hardiman G. Borini A. Vaccari L. Carnevali O. Probiotics can induce follicle maturational competence: the Danio rerio case. Biol Reprod. 2012;86:1–11. doi: 10.1095/biolreprod.111.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bustin SA. Benes V. Garson JA. Hellemans J. Huggett J. Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 44.Vandesompele J. De Preter K. Pattyn F. Poppe B. Van Roy N. De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1–research0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis RNAH. McElhaney RN. Vibrational spectroscopy of lipids. In: Chalmers JM, editor; Griffiths PR, editor. Handbook of Vibrational Spectroscopy. Vol. 5. Wiley; Chichester: 2002. pp. 34–64. [Google Scholar]
- 46.Seeley RJ. Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- 47.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 48.Lepage O. Larson ET. Mayer I. Winberg S. Tryptophan affects both gastrointestinal melatonin production and interrenal activity in stressed and nonstressed rainbow trout. J Pineal Res. 2005;38:264–271. doi: 10.1111/j.1600-079X.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhdanova IV. Wang SY. Leclair OU. Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 50.Kasimay O. Cakir B. Devseren E. Yegen BC. Exogenous melatonin delays gastric emptying rate in rats: role of CCK2 and 5-HT3 receptors. J Physiol Pharmacol. 2005;56:543–553. [PubMed] [Google Scholar]
- 51.Velarde E. Alonso-Gómez AL. De Pedro N, et al. Caracterización de la actividad miométrica del intestino de Carassius auratus para el estudio del efecto in vitro de la melatonina en la actividad gastrointestinal. www.civa2006.org. 2006. pp. 249–258.www.civa2006.org
- 52.Zhdanova IV. Sleep and its regulation in zebrafish. Rev Neurosci. 2011;22:27–36. doi: 10.1515/RNS.2011.005. [DOI] [PubMed] [Google Scholar]
- 53.Webster JR. Corson ID. Littlejohn RP. Martin SK. Suttie JM. The roles of photoperiod and nutrition in the seasonal increases in growth and insulin-like growth factor-I secretion in male red deer. Anim Sci. 2001;73:305–311. [Google Scholar]
- 54.Bjornsson BT. The biology of salmon growth hormone: from daylight to dominance. Fish Physiol Biochem. 1997;17:9–24. [Google Scholar]
- 55.Beckman BR. Shimizu M. Gadberry BA. Cooper KA. Response of the somatotropic axis of juvenile coho salmon to alterations in plane of nutrition with an analysis of the relationships among growth rate and circulating IGF-I and 41 kDa IGFBP. Gen Comp Endocrinol. 2004;135:334–344. doi: 10.1016/j.ygcen.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Davis CR. Okihiro MS. Hinton DE. Effects of husbandry practices, gender, and normal physiological variation on growth and reproduction of Japanese medaka, Oryzias latipes. Aquat Toxicol. 2002;60:185–201. doi: 10.1016/s0166-445x(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 57.Kissil GW. Lupatsch I. Elizur A. Zohar Y. Long photoperiod delayed spawning and increased somatic growth in gilthead sea bream (Sparus aurata) Aquaculture. 2001;200:363–379. [Google Scholar]
- 58.Taylor JF. Migaud H. Porter MJR. Bromage NR. Photoperiod influences growth rate and plasma insulin-like growth factor-1 in juvenile rainbow trout, Oncorhynchus mykiss. Gen Comp End. 2005;142:169–185. doi: 10.1016/j.ygcen.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Kersten S. Seydoux J. Peters JM. Gonzalez FJ. Desvergne B. Wahli W. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conde-Sieira M. Librán-Pérez M. López Patiño MA. Soengas JL. Míguez JM. Melatonin treatment alters glucosensing capacity and mRNA expression levels of peptides related to food intake control in rainbow trout hypothalamus. Gen Comp Endocrinol. 2012;178:131–138. doi: 10.1016/j.ygcen.2012.04.011. [DOI] [PubMed] [Google Scholar]