Abstract
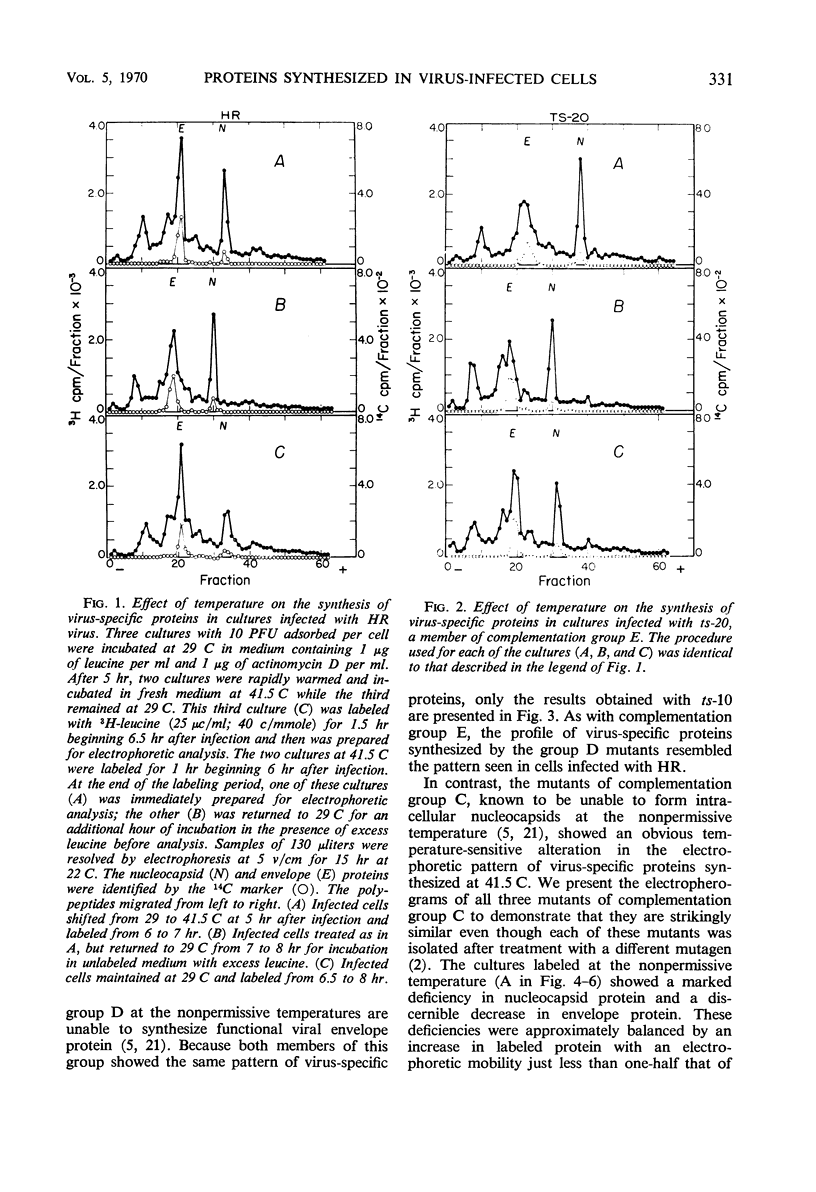
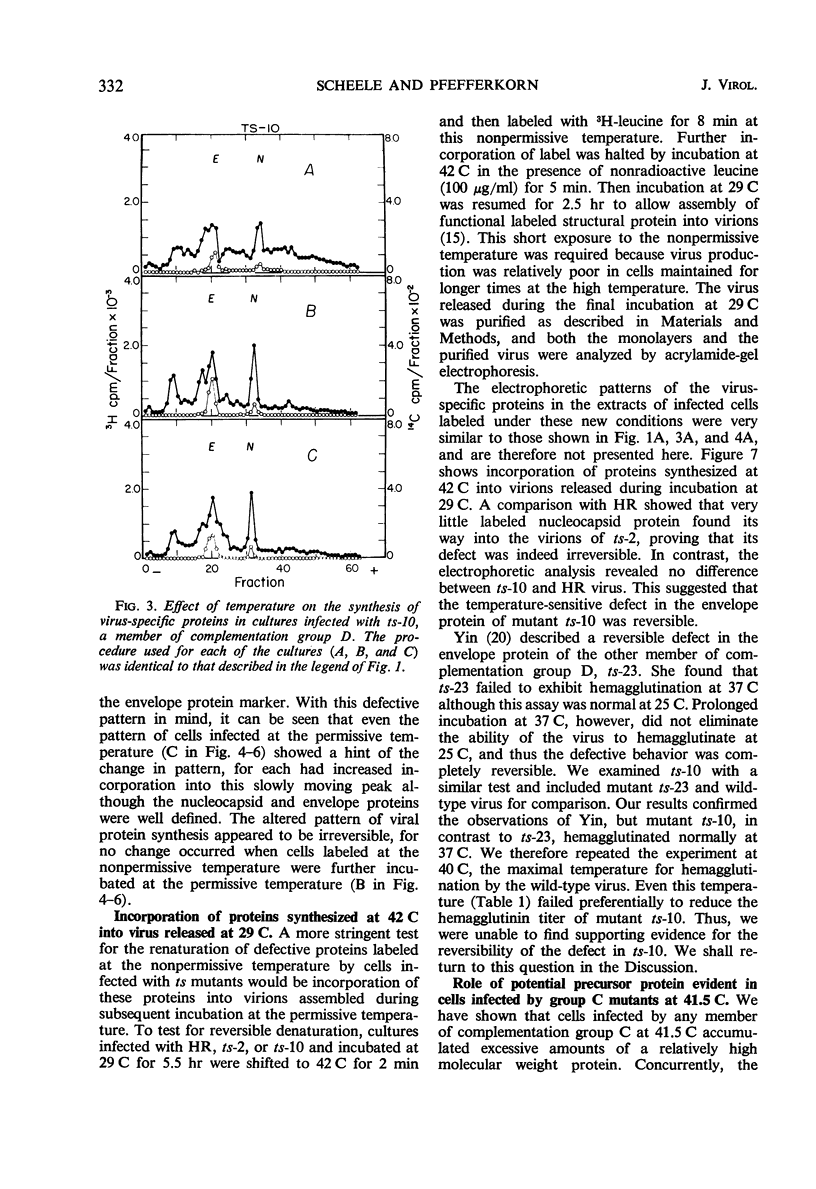
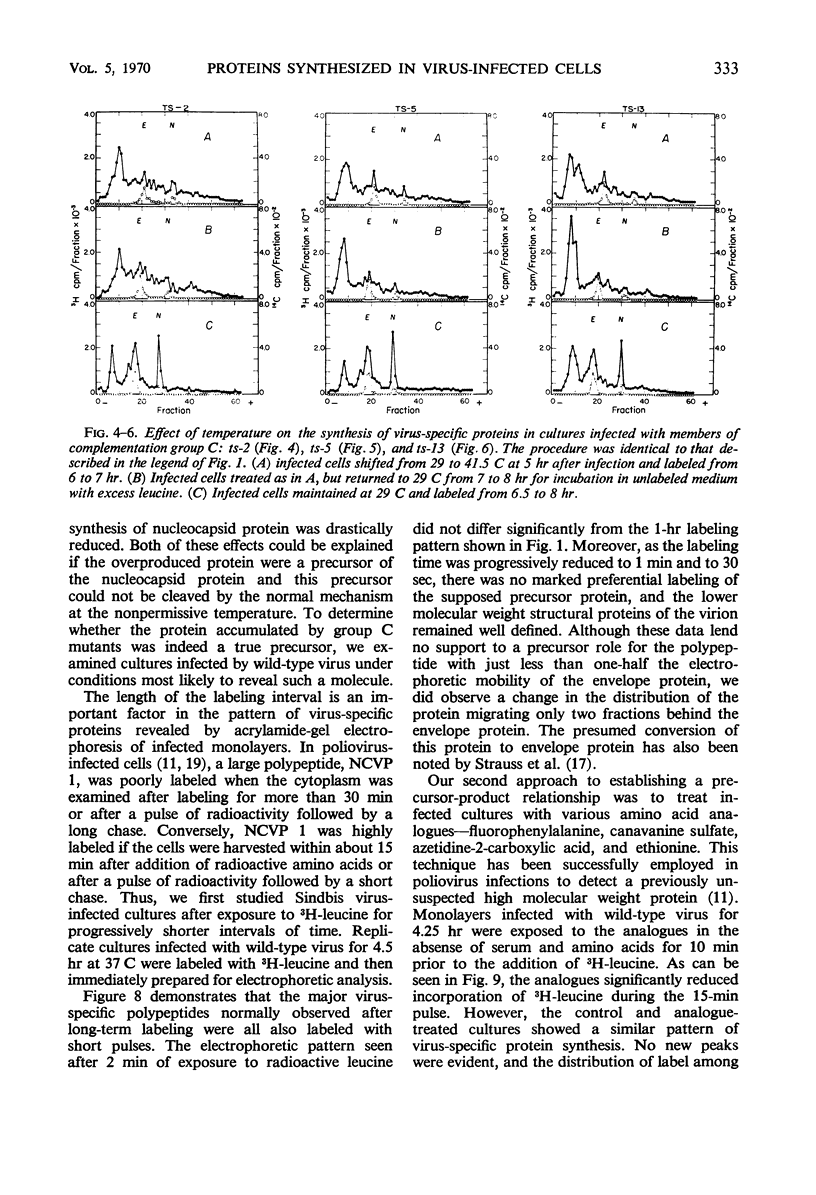
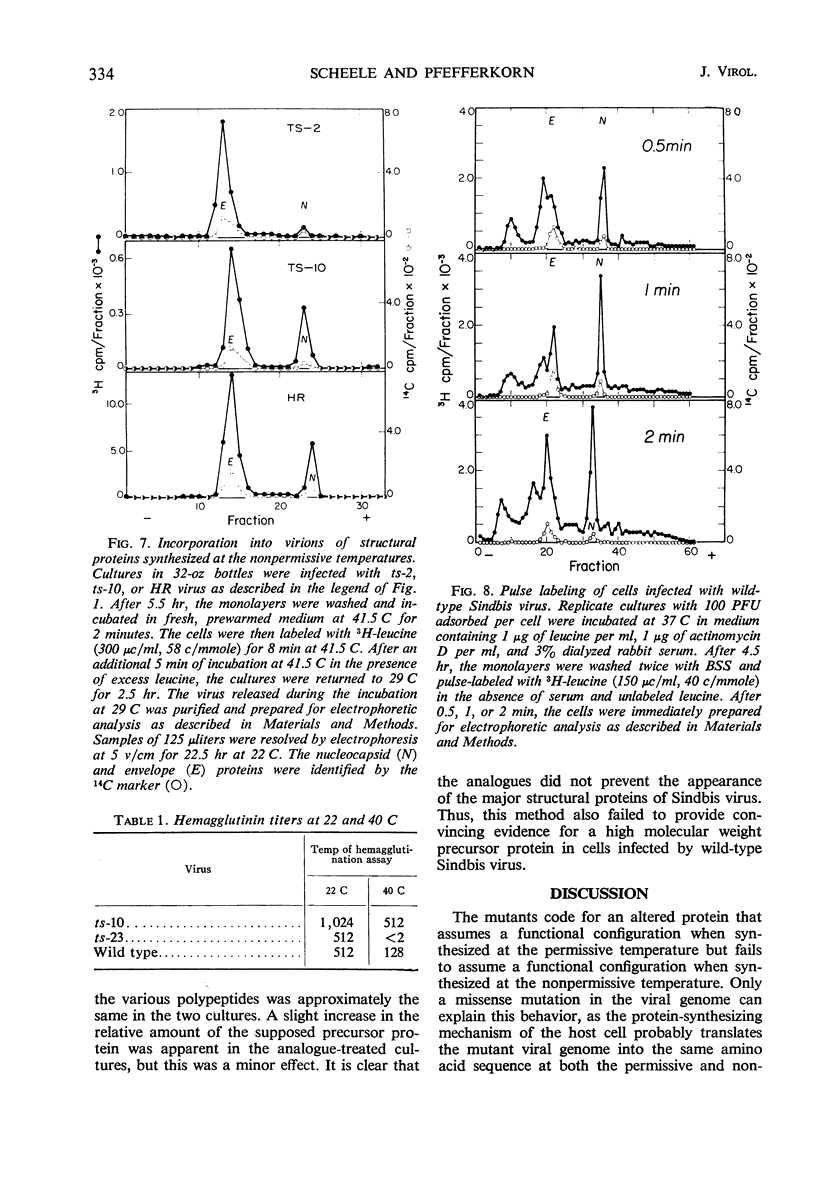
All Sindbis virus temperature-sensitive mutants defective in “late” functions were systematically surveyed by acrylamide-gel electrophoresis for similarities and differences in the intracellular pattern of virus-specific proteins synthesized at the permissive and nonpermissive temperatures. Only cells infected with mutants of complementation group C showed an altered pattern. At the nonpermissive temperature, these mutants failed to induce the synthesis of a polypeptide corresponding to the nucleocapsid protein and instead overproduced a protein of higher molecular weight than either viral structural protein. This defect was shown to be irreversible by the finding that 3H-leucine incorporated at 41.5 C specifically failed to appear in the nucleocapsid of virions subsequently released at 29 C. Attempts to demonstrate a precursor protein in wild-type infections were inconclusive.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckwith J. R. Regulation of the lac operon. Recent studies on the regulation of lactose metabolism in Escherichia coli support the operon model. Science. 1967 May 5;156(3775):597–604. doi: 10.1126/science.156.3775.597. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Temperature-sensitive mutants of Sindbis virus: biochemical correlates of complementation. J Virol. 1967 Oct;1(5):956–962. doi: 10.1128/jvi.1.5.956-962.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Properties of 42S and 26S Sindbis viral ribonucleic acid species. J Virol. 1969 Oct;4(4):429–438. doi: 10.1128/jvi.4.4.429-438.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Primary gene products of an arbovirus. Biochem Biophys Res Commun. 1969 Oct 8;37(2):369–373. doi: 10.1016/0006-291x(69)90744-x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Kinetics of incorporation of structural proteins into Sindbis virions. J Virol. 1969 Apr;3(4):369–375. doi: 10.1128/jvi.3.4.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lockart R. Z., Jr Maturation defects in temperature-sensitive mutants of Sindbis virus. J Virol. 1968 Jul;2(7):728–737. doi: 10.1128/jvi.2.7.728-737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H. Temperature-sensitive behavior of hemagglutinin in a temperature-sensitive mutant virion of Sindbis. J Virol. 1969 Oct;4(4):547–548. doi: 10.1128/jvi.4.4.547-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



