Abstract
Objective. This trial aims to look for the protein biomarker of “toxin syndrome” of CHD patients. Methods. We have performed two trials in this paper. The first trial was a randomized controlled trial (RCT) of the plasma proteome in unstable angina (UA) patients by Maldi-Tof Mass. The second trial was a nested case-control study in 1503 stable CHD patients with one-year followup for acute cardiovascular events (ACEs). Results. In the RCT study, 12 protein spots were found to be the differential protein for the significant differences between the difference of before and after treatment in group A and group B; 2 of them (3207.37 Da and 4279.95 Da) was considered to be unique to “toxin syndrome” for being differential proteins of group B but not group A. These 2 spots were identified as Isoform 1 of Fibrinogen alpha chain precursor (FGA, 3207.37 Da) and Isoform 2 of inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4, 4279.95 Da), respectively. In the nested case-control study, the result of Western blot demonstrated that protein expression of ITIH4 in the group with followup ACEs was significantly lower than the matched group without followup ACEs (P = 0.027). Conclusion. ITIH4 might be a new potential biomarker of CHD “toxin syndrome” in TCM, indicating the potential role in early identifying high-risk CHD patients in stable period.
1. Introduction
Syndrome differentiation is a unique diagnostic method of traditional Chinese medicine (TCM) [1, 2]. “Blood stasis syndrome” (BSS) is considered as a major and key syndrome in the process of coronary heart disease (CHD) in TCM [3, 4], and activating blood circulation and dissolving stasis has been a mainstream treatment for CHD. However, some stable CHD patients develop acute cardiovascular events (ACEs), while others do not, why? Based on this question, we proposed a hypothesis of “blood stasis and toxin” considering blood stasis was a constant pathogenesis in CHD, while “toxin” was the trigger in transforming to ACEs [5].
The original meaning of “toxin” is a kind of poisonous herb but it has been considered as a pathogenic factor in a narrow sense and pathogenesis, medicine, and syndrome in a broad sense. It is often seen in the fields of epidemic febrile diseases and surgical diseases (such as carbuncle, abscess, hard furuncle, and sore). Zhang et al. [6] presented a theory of “artery carbuncle” according to previous studies that arteriosclerosis plaque has the characteristics such as redness, swelling, and being hot on the local scale, just like the traditional “toxin syndrome.” Heat-clearing and detoxifying treatment has been widely used in CHD, especially acute coronary syndrome patients [7–9]. Previous studies showed that Rhizoma Coptidis, Cyrtomium Rhizome, compound simiaoyongan decoction, and Huanglian Jiedu decoction could improve clinical symptoms by multiple mechanisms such as anti-inflammatory action, lipid regulation, and AS plaque reduction [10–19]. Furthermore, a lot of researches indicated that drugs for activating blood circulation and detoxifying had a better effect on relieving angina than drugs for activating blood circulation only; it might be related to the effect of anti- inflammatory action [20–27].
Changes in macroscopic manifestation certainly have the corresponding microscopic biological basis. Inflammation has been proved to be a biomarker for CHD/ACS, and the proteome supported us with a new technology for studying it further. The proteome is a subject studying all the proteins in a cell, a kind of tissue, or an organism in specific conditions or at specific times and has been one of the most potential and effective approaches for decoding and revealing the biological foundation and essence of syndromes. Different syndromes consequentially have relevant differential protein expressions; meanwhile, one syndrome also has different protein expressions after treatment of different medicines. Therefore, the protein's characteristics of a specific syndrome can be reflected by the effectiveness of prescriptions corresponding to syndromes.
Berberine extracted from Rhizoma Coptidis, a representative herb of clearing heat and detoxifying, could inhibit the expressions of inflammatory factors such as thromboxane A2 and prostaglandin I2 after the injury of blood vessels [10]. Xiongshao capsule, consisting of active ingredients ( Chuanxiongol and paeoniflorin), has shown beneficial effect in atherosclerosis or CHD in clinical and experimental studies [28–34]. Therefore, it was served as a representative Chinese medicine for activating blood circulation.
The aim of this study was to look for the protein biomarker of “toxin syndrome” of CHD patients, which is anticipated to help early identification of high-risk CHD patients in stable period.
2. Design and Ethics Statement
There are two parts in this paper (Figure 1). The first one was a randomized controlled trial (RCT) with 2 study groups conducted at 2 cooperating hospitals (Anzhen Hospital and Tongren Hospital) to look for biomarkers for “toxin syndrome” of TCM. The other was a nested case-control study with a follow-up for ACEs conducted at 5 cooperating hospitals (China Academy of Chinese Medical Sciences Xiyuan Hospital, China-Japan friendship Hospital, Anzhen Hospital, Tongren Hospital, and Fujian Integrative Medicine Clinic) to verify the biomarker found in RCT. The trials were carried out according to the Declaration of Helsinki, and the protocols were approved by the institutional review boards and ethics committees at each center. All the patients provided written informed consent.
Figure 1.
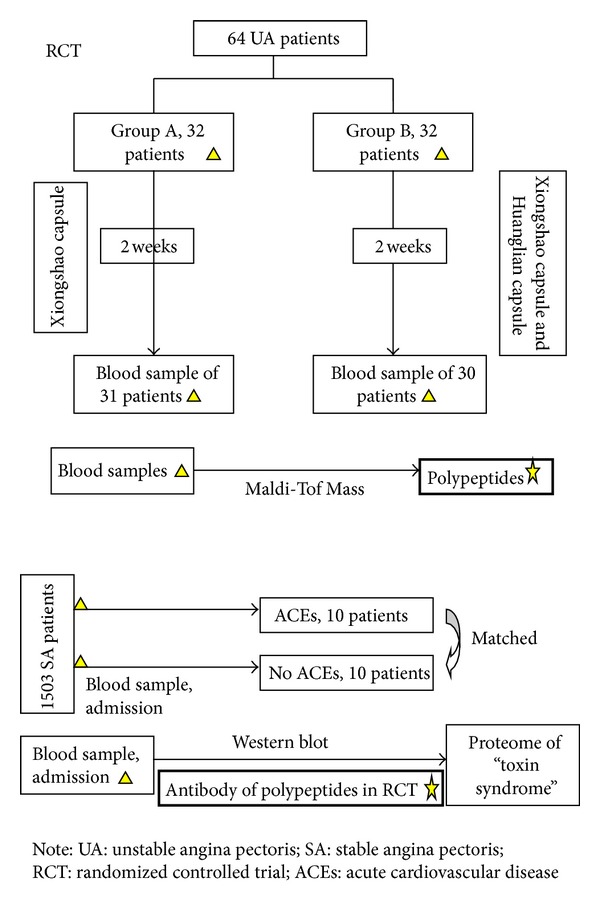
Flow chart of the study.
3. Materials and Methods
3.1. Randomized Controlled Trial
3.1.1. Patients
Fasting serum samples were obtained from 64 patients with UA (ICD-10 : I20.0/20.1/20.9) [35, 36] aged between 40 and 75 years old. All of the patients who were admitted into the 2 cooperating hospitals were enrolled in the study. The inclusion criteria were successful PCI in 48 hours after the first severe angina and BSS of TCM (including Qi-stagnation-blood-stasis syndrome and Qi-deficiency-blood-stasis syndrome) [37–39]. Patients were excluded if they met any of the following criteria: presence of (1) stable angina or acute myocardial infarction; (2) inflammation, fever, trauma, bure, or surgery in one recent month; (3) active tuberculosis or rheumatic autoimmune disease; (4) known renal insufficiency and serum creatinine >2.5 mg/dL in male and >2.0 mg/dL in female; (5) known hepatic insufficiency and alanine transaminase (ALT) > three times value of the normal level; (6) severe heart failure (EF < 35%); (7) complication by severe primary disease such as hematologic systems and psychological abnormalities; (8) malignancies; (9) organ transplantation; (10) participants of other clinical trials; (11) taking other Chinese patent drugs; (12) pregnancy or breast-feeding. Patients were removed from analysis if they could not estimate the efficacy for they did not take any medicine or did not participate reexamination as proposal.
3.1.2. Groups and Drugs
64 eligible patients were randomly assigned in a 1:1 ratio to group of activating blood circulation (Xiongshao capsule, Z20053499, Hospital preparation approved by Beijing drug administration, group A) or group of activating blood circulation and detoxification (Huanglian capsule, Z19983042, Hubei Xianglian Pharmaceutical Co.,Ltd and Xiongshao capsule, group B). Randomization table was performed centrally with the use of SAS software and reserved by a specific person who did not participate this clinic research. Randomized number was obtained by telephone if any patient was eligible.
In the group of activating blood circulation, Xiongshao capsule was taken as 500 mg (2 capsules) 3 times per day for 2 weeks. In the group of activating blood circulation and detoxification, Huanglian capsule was taken as 500 mg (2 capsules) 3 times per day, and Xiongshao capsule was taken as 500 mg (2 capsules) 3 times per day for 2 weeks.
All patients received western standardized medication including antiplatelet drugs (aspirin and/or clopidogrel hydrogen sulfate), anticoagulant drugs (heparin or low molecular weight heparin), anti-ischemic drugs (nitrates, β-blocker, calcium channel blocker, and angiotensin-converting enzyme inhibitors), and statins.
3.1.3. Data Collection
At the beginning of the trial, all patients filled out a standardized questionnaire containing general information, past history, risk stratification of UA [40], angina score, primary symptom score of TCM [41], BSS score [42], medical treatment, and PCI surgery. In addition, to obtain the serum, at the beginning and the end of the trial, 2 ml of blood from each patient with an empty stomach was drawn into common coagulation-promoting tubes, centrifuged at 3000 r for 10 min at room temperature to remove insoluble materials, cells, and debris, and supernatants were kept at −80°C until use.
3.1.4. Reagents and Instruments
The WCX magnetic bead kit (Bruker Daltonics Tech, Beijing, China), alpha-cyano-4-hydroxycinnamic acid (HCCA), MALDI-TOF MS (type: microflex, Bruker Daltonics Biosciences, Bremen, Germany), 100% ethanol (chromatographic grade), and 100% acetone (chromatographic grade) were freshly prepared (sigma).
3.1.5. WCX Fractionation and MALDI-TOF MS Analysis
The suspension in the WCX magnetic bead kit was mixed by shaking. After eluting and beating, the magnetic beads were separated from the protein, and the eluted peptide samples were transferred to a 0.5 mL clean sample tube for further MS analysis. Five microliters of HCCA substrate solution (0.4 g/L, dissolved in acetone and ethanol) and 0.8–1.2 µL of elution were mixed. Then, 0.8–1.2 µL of this mixture was applied to a metal target plate and dried at room temperature. Finally, the prepared sample was analyzed by MALDI-TOF MS. A range of 1000–10,000 Da peptide molecular weights was collected, and 400 shots of laser energy were used. Peptide mass fingerprints were obtained by accumulating 50 single MS signal scans.
3.1.6. Peptide Sequence
Experiment for 4280.13 m/z peptide identification was performed using a nano-liquid chromatography-electrospray ionization-tandem mass spectrometry (nano-LC/ESI-mass spectrometry/mass spectrometry) system consisting of an Acquity UPLC system (Waters) and an LTQ Orbitrap XL mass spectrometer (Thermo Fisher) equipped with a nano-ESI source. The peptide solutions were loaded to a C18 trap column (nano-Acquity) (180 μm × 20 mm × 5 μm (symmetry)). The flow rate was 15 μL/min. Then the desalted peptides were analyzed by C18 analytical column (nano-Acquity) (75 μm × 150 mm × 3.5 μm (symmetry)) at a flow rate of 400 nL/min. The mobile phases A (5% acetonitrile, 0.1% formic acid) and B (95% acetonitrile, 0.1% formic acid) were used for analytical columns. The gradient elution profile was as follows: 5%B–50%B–80%B-80%B–50%B–5%B in 100 min. The MS instrument was operated in a data-dependent model. The range of full scan was 400–2000 m/z with a mass resolution of 100,000 (m/z 400). The eight most intense monoisotope ions were the precursors for collision induced dissociation. Mass spectrometry was limited to two consecutive scans per precursor ion followed by 60 s of dynamic exclusion.
3.1.7. Statistical Analysis
ClinProTools (ClinProt software version 2.1, Bruker Daltonics) was used to subtract baseline, normalize spectra (using total ion current), and determine peak m/z values and intensities in the mass range of 1000 to 10,000 Da. The signal-to-noise (S/N) ratio should be higher than five. To align the spectra, a mass shift of no more than 0.1% was determined. The peak area was used as quantitative standardization. Student's t-test was used for analysis of normally distributed continuous data, while Wilcoxon test for nonnormally distributed continuous data. Chi-square test was used for categorical data analysis. A P value <0.05 was considered significant.
3.2. Nested Case-Control Study
3.2.1. Patients
1503 patients with stable CHD (old myocardial infarction or at least one significant (>50%) stenosis that was documented on a recent coronary angiogram and WHO [35]) younger than 80 years old were enrolled from 5 cooperating hospitals. Stable CHD was defined as no symptoms or stable exertional angina or patients in stable condition after ACS for at least 1 month. Patients were excluded if they met any of the following criteria: presence of (1) inflammation, fever, trauma, bure, or surgery in one recent month; (2) active tuberculosis or rheumatic autoimmune disease; (3) severe heart failure (EF < 35%); (4) complication by severe valvular heart disease, or myocardiopathy; (5) complication by severe chronic obstructive pulmonary disease (COPD), pulmonary heart disease or respiratory failure; (6) known renal insufficiency and serum creatinine >2.5 mg/dL in male and >2.0 mg/dL in female; (7) known hepatic insufficiency and alanine transaminase (ALT) > three times value of the normal level; (8) complication by severe primary disease such as hematologic systems; (9) severe psychological abnormalities; (10) malignancies; (11) viscera transplantation; (11) life expectancy less than 3 years. Patients were removed from analysis if a mistaken inclusion or lack of necessary record for analysis or failure to follow up for ACEs because of missing contact information took place.
3.2.2. Data Collection
In all patients, follow-up was scheduled at 0.5 and 1 year after inclusion of the trial. At every visit of the trial, information was obtained from each patient by use of a standardized questionnaire, the information regarding general information, past history, and the secondary cardiovascular events in follow-up. Physicians collecting information were unaware of the purpose of the study. Secondary cardiovascular events were defined as death from heart disease, nonfatal myocardial infarction (MI), or ischemic cerebrovascular events (stroke or transient ischemic attack). All the cardiovascular events were estimated by consulting medical records. In addition, the serum also was collected at every visit, and the method of blood collection, centrifugation, and storage was the same as that of RCT.
Twenty three patients were confirmed as ACEs during one-year follow-up, and 10 patients were selected for their well preserved serum sample. Another 10 patients with no follow-up ACEs were matched in a 1 : 1 ratio by sex, age (±5 years), hypertension history, diabetes history, and myocardial infarction history. All the sera at the admission of these 20 patients were adopted for verifying the differential protein of “toxin syndrome” obtained from RCT by Western blot method.
3.2.3. Western Blot
To detect the inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4) obtained from RCT (see results section), blood serum stored in −80°C refrigerator was assayed using Western blot as described before [43]. Additionally, ITIH4 antibody (1 : 2500, Sigma, USA) was used for detection of ITIH4. The horseradish peroxidase (HRP) conjugated anti-mouse IgG (0.1 mL/cm2, Santa Cruz Biotechnology, UAS) was used as the secondary antibody, and signals were visualized using the enhanced chemiluminescence system (ECL, Pierce, USA).
3.3. Statistical Analysis
Statistical analysis was performed by a statistician in a blind fashion. Statistical analysis was performed with SPSS15.0 software. All tests were two tailed, and a statistical probability of <0.05 was considered significant. Normality test and homogeneity test of variances were conducted. Frequency table, percentage or constituent ratio for describing enumeration data; for describing measurement data. χ 2 test or Fisher exact test if necessary was used for comparison of enumeration data, t-test was used for comparison of measurement data (corrected t test was used if variant heterogeneity), and Wilcoxon tests were used for abnormal distribution.
4. Results
4.1. Patients' Characteristics in RCT
64 participants with UA were enrolled in 5 centers and were randomized into two groups: 32 to receive Xiongshao capsule (group A) and 32 to receive Xiongshao capsule and Huanglian capsule (group B). During the course of the study, one patient was excluded in group A due to incomplete follow-up, while two patients were excluded in group B with 1 incomplete follow-up, and 1 noncompliance with medications. They were removed from statistics as the stated protocol. Thus finally, the population in analysis consisted of 61 patients, with 31 patients in group A and 30 patients in group B. The baseline characteristics of the UA patients were summarized in Table 1. The two groups were well matched with regard to baseline clinical and angiographic characteristics (P > 0.05).
Table 1.
Baseline information of two groups in RCT.
| Groups | Group A | Group B |
|---|---|---|
| Age | ||
| Minimum value (years) | 48 | 42 |
| Maximum value (years) | 74 | 75 |
| Mean value (years) | 61.94 ± 8.41 | 61.24 ± 9.86 |
| Sex | ||
| Male (proportion) | 22 (71%) | 25 (86.2%) |
| Female (proportion) | 9 (29%) | 5 (13.8%) |
| Angina score | 14.42 ± 4.86 | 14.89 ± 4.63 |
| Primary symptom score of TCM | 17.97 ± 6.74 | 18.94 ± 5.64 |
| BSS score | 10.89 ± 4.62 | 10.59 ± 3.38 |
| Past history | ||
| Hypertension (N) | 18 | 16 |
| Diabetes (N) | 7 | 9 |
| Dislipidemia (N) | 11 | 12 |
| Stroke (N) | 2 | 4 |
| Peripheral vascular atherosclerosis (N) | 5 | 3 |
| Old myocardial infarction (N) | 4 | 1 |
| Western medicine | ||
| Aspirin (N) | 31 | 30 |
| Clopidogrel hydrogen sulfate (N) | 31 | 30 |
| Nitrates (N) | 21 | 16 |
| β-blocker (N) | 28 | 30 |
| ACEI/ARB (N) | 19 | 17 |
| CCB (N) | 3 | 9 |
| Low molecular weight heparin (N) | 16 | 19 |
| Statins (N) | 30 | 29 |
| UA risk stratification | ||
| Low risk (N) | 0 | 0 |
| Mediate risk (N) | 26 | 24 |
| High risk (N) | 5 | 6 |
| Number of stenosed coronary vessel | ||
| 1 vessel (N) | 8 | 12 |
| 2 vessels (N) | 12 | 7 |
| 3 vessels (N) | 11 | 11 |
| Lesions nature | ||
| De novo (N) | 28 | 26 |
| Restenosis (N) | 3 | 4 |
| Stent type | ||
| Sirolimus-eluting stent (N) | 21 | 21 |
| paclitaxel-eluting stent (N) | 8 | 6 |
| Mixed drug-eluting stents (N) | 2 | 3 |
| Total length of stents | 22.94 ± 7.23 | 21.67 ± 9.69 |
Note: group A patients have taken the Xiongshao capsule; group B patients have taken the Xiongshao capsule and Huanglian capsule.
4.2. Sample Processing in RCT
During the course of the protein analysis, five blood samples were excluded from group A due to bad peptide mass spectrometry; thus, the population in differential protein analysis consisted of 56 patients, with 26 patients in group A and 30 patients in group B. Acquisition mass range 500–10000 Da (low-to-medium molecular mass range) would be studied in bioinformatics analysis.
4.3. MALDI-TOF Mass Spectrometry Analysis of Peptides in Serum of RCT
Statistical analysis of the data revealed that the expression of 24 spots was altered after treatment as compared with that at admission in group A (7 of them upregulated and 17 downregulated, Table 2, Figure 2). The expression of 15 spots was altered after treatment as compared with that at admission in group B (8 of them upregulated and 17 downregulated, Table 3, Figure 3), and 4 of the 15 spots were the same as group A. Twelve protein spots were found (Table 4) to be the differential protein for the significant differences between the difference of before-after treatment in group A and group B; 2 of them (3207.37 Da and 4279.95 Da) were considered to be unique to “toxin syndrome” for being differential proteins of group B but not group A. These 2 spots were identified by mass spectrometry (Figures 4 and 5).
Table 2.
Comparison of before and after treatment in group A ().
| Mass (Da) | Ave ± StdDev (A-Q) | Ave ± StdDev (A-H) | P |
|---|---|---|---|
| 1076.12 | 3.77 ± 2.35 | 2.54 ± 1.24 | 0.016099 |
| 1136.37 | 7.25 ± 3.12 | 5.42 ± 2.08 | 0.008065 |
| 1205.62 | 9 ± 3.32 | 6.92 ± 4.43 | 0.018784 |
| 1329.42 | 14.44 ± 7.31 | 10.26 ± 4.92 | 0.000268 |
| 1348.81 | 10.41 ± 4.08 | 7.09 ± 2.89 | 0.000806 |
| 1464.89 | 24.93 ± 13.64 | 13.52 ± 6.44 | 0.000293 |
| 1519.06 | 18.49 ± 8.17 | 11.39 ± 5.92 | 0.000111 |
| 1544.61 | 30.66 ± 17.33 | 19.41 ± 16.3 | 0.011662 |
| 1616.74 | 33.35 ± 22.17 | 18.7 ± 9.09 | 0.002143 |
| 2209.31 | 37.24 ± 18.84 | 25.45 ± 17.84 | 0.01935 |
| 2279.51 | 50.15 ± 17.26 | 40.99 ± 14.08 | 0.018232 |
| 2644.01 | 30.08 ± 20.39 | 22.42 ± 14.8 | 0.000347 |
| 2660.01 | 288.15 ± 231.67 | 206.03 ± 161.86 | 0.003587 |
| 2862.02 | 77.55 ± 78.74 | 45.15 ± 29.33 | 0.00951 |
| 3261.70 | 125.1 ± 63.45 | 159.89 ± 62.44 | 0.043161 |
| 3277.49 | 45.57 ± 23.64 | 59.7 ± 29.1 | 0.015557 |
| 4053.87 | 71.26 ± 38.16 | 95.63 ± 53.55 | 0.047052 |
| 4710.25 | 15.53 ± 5.5 | 12.84 ± 4.04 | 0.028146 |
| 4936.10 | 17.25 ± 15.28 | 9.77 ± 2.99 | 0.015955 |
| 4964.02 | 147.4 ± 184.34 | 59.95 ± 34.25 | 0.019728 |
| 5807.76 | 52.08 ± 23.63 | 70.92 ± 24.68 | 0.010841 |
| 5822.51 | 22.33 ± 13.95 | 31.77 ± 10.34 | 0.007368 |
| 5904.69 | 881.7 ± 598.83 | 1266.04 ± 415.59 | 0.00471 |
| 6049.22 | 27.36 ± 10.77 | 32.41 ± 10.1 | 0.023583 |
Note: paired sample t test was used, 2-tailed, and P < 0.05 was considered significant; Ave: peak area/intensity average; StdDev: standard deviation of the peak area/intensity average; A-Q: before treatment in group A; A-H: after treatment in group A.
Figure 2.
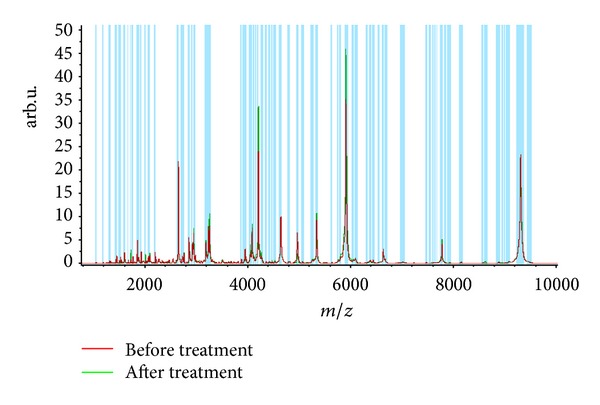
Peptide mass spectrometry before and after treatment in group A.
Table 3.
Comparison of before and after treatment in group B ().
| Mass (Da) | Ave ± StdDev (B_Q) | Ave ± StdDev (B_H) | P |
|---|---|---|---|
| 1616.74 | 32.83 ± 18.26 | 23.95 ± 12.74 | 0.033415 |
| 2209.31 | 29.04 ± 14.35 | 21.23 ± 10.27 | 0.022373 |
| 2881.04 | 49.8 ± 18.98 | 38.19 ± 10.15 | 0.010917 |
| 3207.37 | 56.15 ± 17.06 | 47.84 ± 12.01 | 0.023899 |
| 4053.87 | 60.23 ± 22.82 | 83.04 ± 42.14 | 0.010323 |
| 4266.31 | 35.94 ± 19.99 | 45.73 ± 19.4 | 0.020676 |
| 4279.95 | 18.9 ± 8.79 | 27.23 ± 23.79 | 0.025866 |
| 4817.85 | 20.34 ± 17.41 | 11.15 ± 7.93 | 0.012491 |
| 4936.10 | 21.91 ± 27.22 | 10.29 ± 3.94 | 0.02628 |
| 5066.25 | 25.87 ± 7.61 | 32.1 ± 11.2 | 0.019568 |
| 5248.63 | 21.01 ± 5.89 | 25.23 ± 8.04 | 0.025751 |
| 6378.01 | 47.98 ± 47.92 | 28.92 ± 10.34 | 0.045958 |
| 7833.86 | 10.85 ± 2.21 | 12.97 ± 4.82 | 0.040051 |
| 9064.40 | 30.36 ± 8.86 | 35.83 ± 10.12 | 0.012614 |
| 9290.26 | 907.98 ± 442.14 | 1126.48 ± 455.76 | 0.023601 |
Note: paired sample t-test was used, 2-tailed, and P < 0.05 was considered significant; Ave: peak area/intensity average; StdDev: standard deviation of the peak area/intensity average; B-Q: before treatment in group B; B-H: after treatment in group B; overstriking mass: unique to group B.
Figure 3.
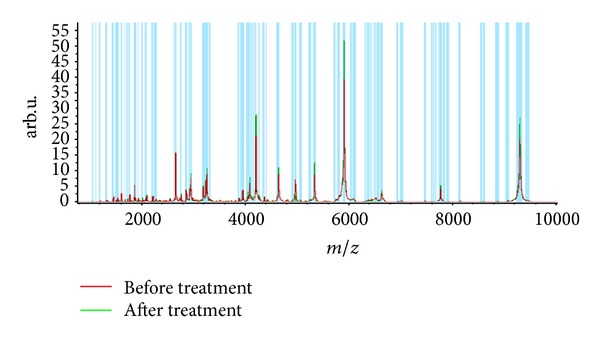
Peptide mass spectrometry before and after treatment in group B.
Table 4.
Comparison of difference between before and after treatment in the two groups.
| Mass (Da) | Ave ± StdDev (A_H − A_Q) | Ave ± StdDev (B_H − B_Q) | P |
|---|---|---|---|
| 1329.42 | −4.18 ± 5.02 | 0.4 ± 6.86 | 0.005918 |
| 1519.06 | −7.1 ± 7.9 | −2.47 ± 9.08 | 0.046435 |
| 2660.01 | −82.12 ± 130.27 | 3.85 ± 184.48 | 0.047102 |
| 2881.04 | 4.86 ± 16.64 | −11.62 ± 23.4 | 0.003442 |
| 3207.37 | 6.56 ± 21.34 | −8.31 ± 19.09 | 0.008696 |
| 3277.49 | 14.13 ± 27.75 | −9.87 ± 33.72 | 0.005087 |
| 3972.15 | −8.69 ± 22.59 | 3.47 ± 15.5 | 0.02561 |
| 4279.95 | −2.49 ± 14.27 | 8.32 ± 19.41 | 0.020196 |
| 5066.25 | −0.43 ± 10.97 | 6.23 ± 13.8 | 0.049446 |
| 5807.76 | 18.84 ± 34.89 | −2.2 ± 36.2 | 0.031283 |
| 5822.51 | 9.44 ± 16.49 | −7.75 ± 32.03 | 0.013558 |
| 6088.68 | 8 ± 28.11 | −8.21 ± 25.58 | 0.029233 |
Note: paired sample t-test was used, 2-tailed, and P < 0.05 was considered significant; Ave: peak area/intensity average; StdDev: standard deviation of the peak area/intensity average; “A_H − A_Q”: subtraction between after and before treatment in group A; “B_H − B_Q”: subtraction between after and before treatment in group B; overstriking mass: unique to “Toxin.”
Figure 4.
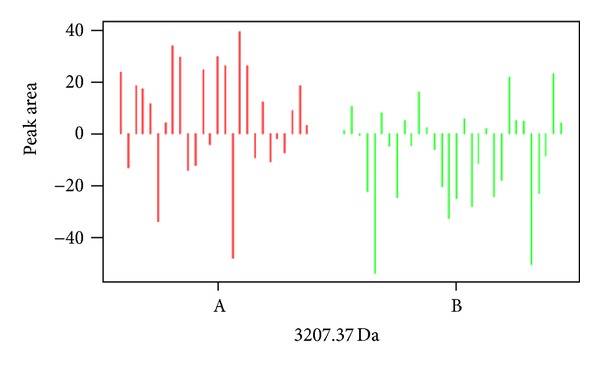
Difference of 3207 Da protein peak between group A and group B.
Figure 5.
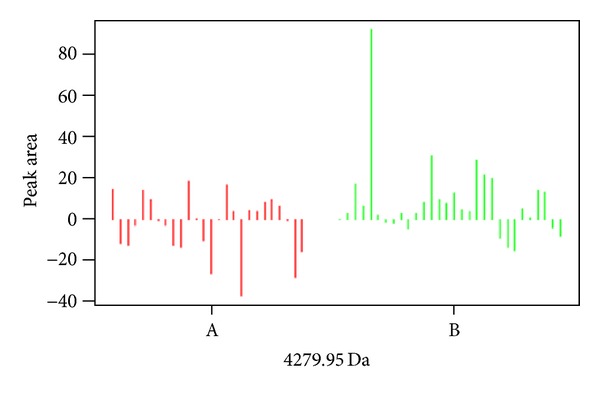
Difference of 4279.95 Da protein peak between group A and group B.
4.4. Identification of Protein Fragments by Proteome Analysis in RCT
Isoform 2 of inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4) and Isoform 1 of Fibrinogen alpha chain precursor (FGA) were identified in different spots by proteome analysis which can be served as biomarkers of “toxin syndrome” in CHD patients (Table 5).
Table 5.
Peptides identification unique to “Toxin”.
| Mass | IPI | Gene_Symbol | Amino acid sequence |
|---|---|---|---|
| 4280.13 Da | IPI00218192.3 | ITIH4 Isoform 2 of inter-alpha-trypsin inhibitor heavy chain H4 | R.NVHSAGAAGSRMNFRPGVLSSRQLGLPGPPDVPDHAAYHPF.R |
|
| |||
| 3206.42 Da | IPI00021885.1 | FGA Isoform 1 of Fibrinogen alpha chain precursor | K.SSSYSKQFTSSTSYNRGDSTFESKSYKM*.A |
4.5. Western Blot
A large multicenter nested casecontrol study was conducted for verifying the unique protein biomarker to “toxin syndrome” of TCM obtained from RCT. The admission blood samples of 20 patients were collected for Western blot (10 patients, resp. in the ACEs group and the matched group). We assay the serum protein concentrations and based on the readings load the same amount of protein. In the posttranslational process, the protein ITIH4 was modified and cleaved by plasma kallikrein to yield 100 kDa and 35 kDa fragments. Statistics indicated that protein expression of ITIH4 in the ACEs group was significantly lower than that in the matched group (P = 0.027) (Table 6, Figure 6). Therefore, the results of nested case-control study further demonstrated the biomarker identified in the RCT, which indicated that the reduced ITIH4 might be a unique protein biomarker/bioinformation of “toxin syndrome” in CHD patients.
Table 6.
ITIH4 expression between ACEs group and matched group.
| Groups | Patients | MOD | P |
|---|---|---|---|
| ACEs group | 10 | 8.41 ± 4.04 | 0.027 |
| Matched group | 10 | 11.57 ± 5.34 |
Note: paired sample t-test was used, 2-tailed, and P < 0.05 was considered significant; MOD: mean optical density.
Figure 6.
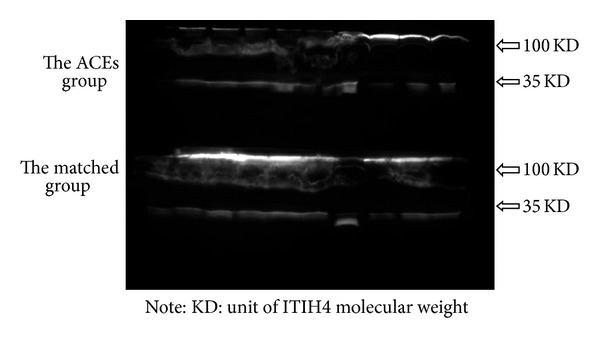
ITIH4 expression between the ACEs group and the Matched group.
5. Discussion
As the development of systems biology and the advancement of the human genome project increased, more and more attention has been paid on the importance of proteome. In this study, we identified 2 peptides (FGA and ITIH4) related to CHD “toxin syndrome” by “taking special drugs to ascertain syndromes” in RCT using MOLDI-TOF MS. Since fibrinogen has been proven to be a risk factor for ACEs in many previous studies [44–46], we only verified another differential protein, ITIH4, by Western blot in a subsequent nested case-control study. Finally, ITIH4 was ascertained to be a new biomarker for CHD “toxin syndrome,” which can also be served as a new risk predictor for ACEs in stable CHD patients.
BSS is one of the basic syndromes in CHD, and “toxin syndrome” is the key in pathogenesis of disease progression. BSS and “toxin syndrome” can coexist or transform to each other, which make up the whole pathological process of CHD [5]. From the macroscopic point of view, Xu et al. [47, 48] enrolled 254 stable CHD patients, collected the clinical information and ACEs in follow-up, and thus concluded a series of clinical manifestations for “toxin syndrome” in stable CHD patients including pain in substernal, headache, uneven or irregular pulse, frequent pharyngalgia, and increased high-sensitivity C-reactive protein (hs-CRP). Other scholars [49] collected clinical information and then summarized a differentiation standard for “toxin-stasis syndrome” of ACS in Chinese medicine. From the microcosmic point of view, “inflammatory reaction” is always a research focus for CHD “toxin syndrome.” Wen et al. [23] investigated the effect on plaque stabilization among herbs regulating blood circulation (Salvia Miltiorrhiza, Radix Paeoniae Rubra), activating blood circulation (Szechuan Lovage Rhizome, Panax Notoginseng), and breaking blood stasis (Peach Seed, Rhubarb Root Parched in Wine) from inflammation, pathomorphology, cellular composition, and so on; indicated Rhubarb root parched in wine was the best for its effect of breaking blood stasis and detoxification. Zhou et al. [26] proposed the hypothesis of “activating blood circulation and detoxification—inflammatory reaction inhibition—plaque stabilization,” then compared the effect on plaque stabilization among herbs activating blood circulation (Panax Notoginsenosides), herbs for detoxification (Goldthread Rhizome extract) and herbs activating blood circulation and detoxification (Rhubarb alcohol extract and Polygonum Cuspidatum extract). The results indicated the superior effect of Rhubarb alcohol extract and Polygonum Cuspidatum extract on stabilizing vulnerable plaque, showing the “class effect” of herbs activating blood circulation and detoxification in inhibiting inflammatory reaction and stabilizing plaque.
The function of ITIH4 in European Molecular Biology Laboratory-The European Bioinformatics Institute (EMBL-EBI) showed that ITIH4 is a type II acute-phase protein (APP) involved in inflammatory responses to trauma. And it may also play a role in liver development and regeneration [50]. Inter-alpha-trypsin inhibitor (ITI) family proteins are all composed by light chain (bikunin) and at least 6 heavy chains. Lots of researches have proved that bikunin could inhibit the activity of protease, but little studies have paid attention to heavy chains of ITI. In 2000, Japanese scholar Choi-Miura et al. found that inter-alpha-trypsin inhibitor family heavy chain-related protein (IHRP) could inhibit the aggregation and phagocytosis of actins in polymorphonuclear leukocyte, implying that IHRP might be a new APP involved in inflammatory responses [51]. In 2004, Fujita et al. showed that genetic locus mutation of ITIH4 might be one of possible factors for dyslipidemia [52]. Then, Piñeiro et al. proved that ITIH4 was a new APP isolated from cattle during experimental infection [53]. Recently, Kashyap et al. found that ITIH4 showed high expression in normal subjects but no expression or little expression in patients with acute ischemic stroke (AIS), and this protein could return to normal level in blood serum gradually as the patients were getting better. The scholars considered that ITIH4 was a novel biomarker in inflammatory responses for AIS due to its close relationship with S-100 β-β, neuron specific enolase (NSE), interleukin-2 (IL-2), and interleukin-10 (IL-10) expression [54].
Results in this study showed that the group of activating blood circulation and detoxification could significantly increase ITIH4 expression and decrease FGA expression compared with the group of activating blood circulation and indicated that ITIH4 and FGA might be potential protein biomarkers for “toxin syndrome” of CHD. ITIH4 was further demonstrated in a nested case-control study, indicating its potential role as a new prewarning biomarker in stable CHD patients.
Before recommending the conclusion of this study to clinical practice, we have to consider the following weaknesses. We cannot ascertain whether the tendency of these two polypeptides is always the same in the process of “toxin syndrome” development. Therefore, a large prospective cohort study collecting information in more time points is necessary.
6. Conclusion
ITIH4 might be a new potential biomarker of CHD “toxin syndrome” in TCM, indicating the potential role as a prewarning biomarker in stable CHD patients.
Conflict of Interests
All authors declare that they have no conflict of interests.
Authors' Contribution
Keji Chen and Dazhuo Shi conceived and designed the trials and performed interpretation of the results; Hao Xu and Qinghua Shang carried out the project and drafted the paper; Hao Chen and Jianpeng Du collected the information of all volunteers; and Geng Li and Jianyan Wen performed the experiments in this trial. All authors read and discussed the paper, and all gave approval for the publication.
Acknowledgments
The current work was partially supported by the National Key Basic Research Program of China (no. 2006CB504803) and the Twelve Five-Year Plan of China (nos. 2013BAI02B01 and 2013BAI13B01). Hao Xu and Qinghua Shang are cofirst authors.
References
- 1.Ferreira AS, Lopes AJ. Chinese medicine pattern differentiation and its implications for clinical practice. Chinese Journal of Integrative Medicine. 2011;17(11):818–823. doi: 10.1007/s11655-011-0892-y. [DOI] [PubMed] [Google Scholar]
- 2.Mei MF. A systematic analysis of the theory and practice of syndrome differentiation. Chinese Journal of Integrative Medicine. 2011;17(11):803–810. doi: 10.1007/s11655-011-0890-0. [DOI] [PubMed] [Google Scholar]
- 3.Li O, Xu H. The occurrence of cardiovascular events of coronary heart disease inpatients and study on chinese medicine syndrome distribution laws. Chinese Journal of Integrated Traditional and Western Medicine. 2012;32(5):603–606. [PubMed] [Google Scholar]
- 4.Gao ZY, Zhang JC, Xu H, et al. Analysis of relationships among syndrome, therapeutic treatment, and Chinese herbal medicine in patients with coronary artery disease based on complex networks. Chinese Journal of Integrated Traditional and Western Medicine. 2010;8(3):238–243. doi: 10.3736/jcim20100307. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Shi DZ, Yin HJ, Zhang JC, Chen KJ. Blood-stasis and toxin causing catastrophe hypothesis and acute cardiovascular events: proposal of the hypothesis and its clinical significance. Chinese Journal of Integrated Traditional and Western Medicine. 2008;28(10):934–938. [PubMed] [Google Scholar]
- 6.Zhang Z, Yang GL, Zhang HY, Chen M, Chen Y, Luo ZB. A hypothesis of atherosclerosis plaque from the surgical carbuncle theory. Liaoning Journal of Traditional Chinese Medicine. 2008;35(2):201–202. [Google Scholar]
- 7.Wang L, Wei LB, Liu XF, Ding SW, Peng M. Toxin theory and acute coronary syndrome. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2005;3(12):1080–1081. [Google Scholar]
- 8.Du WX, Liu CY, Zhang HX, Zhang M, Song HW. Acute myocardial infarction and TCM theory. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2006;4(5):434–436. [Google Scholar]
- 9.Wu W, Peng R. Progress of heat & toxin pathogenesis in coronary heart disease. Journal of New Chinese Medicine. 2007;39(6):3–4. [Google Scholar]
- 10.Duan H, Zhang HK, Liu JJ, Nie WJ, Ma Y, Gao YQ. Effect of berberine on IL-6 of carotid artery after ballon injury in rabbits. Journal of Medical Forum. 2006;27(10):6–9. [Google Scholar]
- 11.Wu M, Wang J. Advance on study in anti-atherosclerosis mechanism of berberine. China Journal of Chinese Materia Medica. 2008;33(18):2013–2016. [PubMed] [Google Scholar]
- 12.Lei ZQ, Chen SY, Gao XM. Traditional Chinese Pharmacology. 1st edition. Shanghai Science and Technology Press; 1995. [Google Scholar]
- 13.He SW, Zhao RH, Wu HJ, Guo AH. Effect of wild purslane on atherosclerosis formation in rabbit. Chinese Journal of Preventive Medicine. 1997;31(2):p. 91. [Google Scholar]
- 14.Tan HB. Study of gynostemma pentaphylla on atherosclerosis in rabbit. Chinese Journal of Gerontology. 2007;27(6):519–521. [Google Scholar]
- 15.Xie LD, Chen LX, Wu SX, Nie B. Overview of clinical and basic research of Simiao Yong’an decoction in the treatment of coronary heart disease. Global Traditional Chinese Medicine. 2012;5(8):629–633. [Google Scholar]
- 16.Zhu N, Cao XM, Cai YX, Wu Y, Wang BB. Effect of huanglian jiedu decoction on CRP and TNF-αin coronary heart disease. Journal of Emergency in Traditional Chinese Medicine. 2012;21(4):542–543. [Google Scholar]
- 17.Li GQ, Huang P, Cheng HM, et al. Effect of huanglian jiedu decoction on T cell expression regulated by CD4+ CD25+ in rats with atherosclerosis. Guangdong Medical Journal. 2010;31(3):329–331. [Google Scholar]
- 18.Guo ZY, Huang P, Li GQ. Effect of huanglian jiedu decoction on the expression of MCP-1/CCR2 mRNA in atherosclerosis rats. Traditional Chinese Drug Research and Clinical Pharmacology. 2010;21(6):583–586. [Google Scholar]
- 19.Zheng F, Zhou MX, Xu H, Chen KJ. Effects of herbs with function of activating blood circulation and detoxication on serum infl ammatory markers and blood lipids in stable patients with coronary heart disease. China Journal of Traditional Chinese Medicine and Pharmacy. 2009;24(9):1153–11576. [Google Scholar]
- 20.Lu XH, Ding SW. Clinical effect and mechanism on treating unstable angina pectoris by huanglian jiedu capsule. Journal of Shandong University of Traditional Chinese Medicine. 2005;29(6):457–460. [Google Scholar]
- 21.Yu R, He SX, Ye XL, Wang XC. The effect of therapeutic method of blood-activating and detoxifying of TCM on serum level of sCD40L in the patients with acute coronary syndrome. Journal of Emergency in Traditional Chinese Medicine. 2008;17(11):1500–1501. [Google Scholar]
- 22.Yu R, Hu XS, He SX, Ye XL, Zhang XX. The effect of huoxuejiedu decoction on serum level of MMP-1,MMP-9,TIMP-1 in the patients with acute coronary syndrome. Journal of Emergency in Traditional Chinese Medicine. 2008;17(10):1337–1346. [Google Scholar]
- 23.Wen C, Xu H, Chen QF, Li P, Sheng X. Effects of herbs of activation blood on atherosclerotic plaque morphology in ApoE gene-deficient mice. Chinese Journal of Pathophysiology. 2005;21(5):864–867. [Google Scholar]
- 24.Wen C, Xu H, Huang QF, Chen KJ. Effect of drugs for promoting blood circulation on blood lipids and inflammatory reaction of atherosclerotic plaques in ApoE gene deficiency mice. Chinese Journal of Integrated Traditional and Western Medicine. 2005;25(4):345–349. [PubMed] [Google Scholar]
- 25.Zhou MX, Xu H, Chen KJ, Pan L, Wen C, Liu JG. Effects of some active ingredients of Chinese drugs for activating blood circulation and detoxicating on blood lipids and atherosclerotic plaque inflammatory reaction in ApoE-gene knockout mice. Chinese Journal of Integrated Traditional and Western Medicine. 2008;28(2):126–130. [PubMed] [Google Scholar]
- 26.Zhou MX, Xu H, Chen KJ, Pan L, Wen C, Guo YR. Effects of several herbal extractives with the effect of promoting blood flow and detoxication on atherosclerotic plaque stability in aorta of apoE-gene knockout mice. Chinese Journal of Pathophysiology. 2008;24(11):2097–2102. [Google Scholar]
- 27.Zhang J-C, Chen K-J, Zheng G-J, et al. Regulatory effect of Chinese herbal compound for detoxifying and activating blood circulation on expression of NF-kappaB and MMP-9 in aorta of apolipoprotein E gene knocked-out mice. Chinese Journal of Integrated Traditional and Western Medicine. 2007;21(1):40–44. [PubMed] [Google Scholar]
- 28.Chen K-J, Shi D-Z, Xu H, et al. XS0601 reduces the incidence of restenosis: a prospective study of 335 patients undergoing percutaneous coronary intervention in China. Chinese Medical Journal. 2006;119(1):6–13. [PubMed] [Google Scholar]
- 29.Xu H, Chen KJ, Shi DZ, Ma XC, lv SZ, Mao JM. Clinical study of Xiongshao capsule in preventing resterosis after coronary interventional treatment. Chinese Journal of Integrative Medicine. 2002;8(3):162–166. [Google Scholar]
- 30.Shang Q-H, Xu H, Lu X-Y, Wen C, Shi D-Z, Chen K-J. A multi-center randomized double-blind placebo-controlled trial of Xiongshao capsule in preventing restenosis after percutaneous coronary intervention: a subgroup analysis of senile patients. Chinese Journal of Integrative Medicine. 2011;17(9):669–674. doi: 10.1007/s11655-011-0843-7. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Shi DZ, Chen KJ, et al. Effect of Xiongshao capsule on vascular remodeling in porcine coronary balloon injury model. Chinese Journal of Integrative Medicine. 2000;6(4):278–282. [PubMed] [Google Scholar]
- 32.Li L-Z, Liu J-G, Ma L-B, et al. Effect of Xiongshao capsule on lipid metabolism and platelet aggregation in experimental atherosclerosis rabbits. Chinese Journal of Integrated Traditional and Western Medicine. 2008;28(12):1100–1103. [PubMed] [Google Scholar]
- 33.Zhang DW, Zhang L, Liu JG, Wang CL, Shi DZ, Chen KJ. Effects of Xiongshao capsule combined with ischemic postconditioning on monocyte chemoattractant protein-1 and tumor necrosis factor-α in rat myocardium with ischemic reperfusion injury. Chinese Journal of Integrated Traditional and Western Medicine. 2010;30(12):1279–1283. [PubMed] [Google Scholar]
- 34.Xu FQ, Xu H, Liu JG, Chen KJ. Effects of Xiongshao capsule on the proliferation of vascular smooth muscle cells in rabbits with atherosclerosis. Chinese Journal of Integrated Traditional and Western Medicine. 2008;28(10):912–916. [PubMed] [Google Scholar]
- 35.Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on Standardization of Clinical NomenclatureS. Nomenclature and criteria for diagnosis of ischemic heart disease. Circulation. 1979;59(3):607–609. doi: 10.1161/01.cir.59.3.607. [DOI] [PubMed] [Google Scholar]
- 36.Chinese Society of Cardiology. Guideline for diagnosis and treatment of patients with unstable angina and non- ST-segment elevation myocardial infarction. Chinese Journal of Cardiology. 2007;35(4):295–304. [PubMed] [Google Scholar]
- 37.Subcommittee of Cardiovascular Diseases of China Society of Integrated Traditional Chinese and Western Medicine. Criteria for TCM syndrome differentiation of patients with coronary heart disease. Chinese Journal of Integrated Traditional and Western Medicine. 1991;11(5):257–258. [Google Scholar]
- 38.Professional Committee of Activating Blood Circulation in Chinese Association of the Integration of Traditional and Western Medicine. Criteria for diagnosis of blood stasis syndrome. Chinese Journal of Integrated Traditional and Western Medicine. 1987;7(3):p. 129. [Google Scholar]
- 39.Fu CG, Gao ZY, Wang PL, et al. Study on the diagnostic criteria for coronary heart disease patients of blood stasis syndrome. Chinese Journal of Integrated Traditional and Western Medicine. 2012;32(9):1285–1286. [PubMed] [Google Scholar]
- 40.Chinese Society of Cardiology. Guildline on diagnosis and treatment of unstable angina pectoris and non-ST elecated myocardial infarcion. Chinese Journal of Cardiology. 2007;35(4):295–304. [Google Scholar]
- 41.Zheng YY. Chinese Herbal Medicine Clinical Research Guilding Principles(for Trial Implementation) Chinese Medicine and Technology Publishing House; 2002. [Google Scholar]
- 42.Chen KJ. Study on Activating Blood Circulation and Cllinical Application. Peking Union Medical College Press; 1993. [Google Scholar]
- 43.Gong Y, Wang X, Liu J, et al. NSPc1, a mainly nuclear localized protein of novel PcG family members, has a transcription repression activity related to its PKC phosphorylation site at S183. FEBS Letters. 2005;579(1):115–121. doi: 10.1016/j.febslet.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 44.Tataru MC, Schulte H, Von EA, Heinrich J, Assmann G, Koehler E. Plasma fibrinogen in relation to the severity of arteriosclerosis in patients with stable angina pectoris after myocardial infarction. Coronary Artery Disease. 2001;12(3):157–165. doi: 10.1097/00019501-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Naito M. Effects of fibrinogen, fibrin and their degradation products on the behavior of vascular smooth muscle cells. Nippon Ronen Igakkai Zasshi. 2000;37(6):458–463. doi: 10.3143/geriatrics.37.458. [DOI] [PubMed] [Google Scholar]
- 46.Ma HL, Lu X, Yang HJ, et al. Fibrinogen is the one of risk factors of coronary heart disease. Chinese Journal of Thrombosis and Hemostasis. 2008;14(1):8–11. [Google Scholar]
- 47.Xu H, Qu D, Zheng F, Shi DZ, Chen KJ. Clinical manifestations of “Blood-stasis and Toxin” in patients with stable coronary heart disease. Chinese Journal of Integrated Traditional and Western Medicine. 2010;30(2):125–129. [PubMed] [Google Scholar]
- 48.Feng Y, Xu H, Qu D, Zheng F, Shi DZ, Chen KJ. Study on the tongue manifestations for the blood-stasis and toxin syndrome in the stable patients of coronary heart disease. Chinese Journal of Integrative Medicine. 2011;17(5):333–338. doi: 10.1007/s11655-011-0615-4. [DOI] [PubMed] [Google Scholar]
- 49.Chen H. The clinical research on differentiation standard for toxin-stasis syndrome of ACS in Chinese medicine [Doctoral dissertation] China Academy of Chinese Medical Sciences; 2009. [Google Scholar]
- 50. http://www.uniprot.org/uniprot/Q14624.
- 51.Choi-Miura NH, Takahashi K, Yoda M, et al. The novel acute phase protein, IHRP, inhibits actin polymerization and phagocytosis of polymorphonuclear cells. Inflammation Research. 2000;49(6):305–310. doi: 10.1007/PL00000211. [DOI] [PubMed] [Google Scholar]
- 52.Fujita Y, Ezura Y, Emi M, et al. Hypercholesterolemia associated with splice-junction variation of inter-α-trypsin inhibitor heavy chain 4 (ITIH4) gene. Journal of Human Genetics. 2004;49(1):24–28. doi: 10.1007/s10038-003-0101-8. [DOI] [PubMed] [Google Scholar]
- 53.Piñeiro M, Andrés M, Iturralde M, et al. ITIH4 (inter-alpha-trypsin inhibitor heavy chain 4) is a new acute-phase protein isolated from cattle during experimental infection. Infection and Immunity. 2004;72(7):3777–3782. doi: 10.1128/IAI.72.7.3777-3782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashyap RS, Nayak AR, Deshpande PS, et al. Inter-α-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clinica Chimica Acta. 2009;402(1-2):160–163. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]


