Abstract
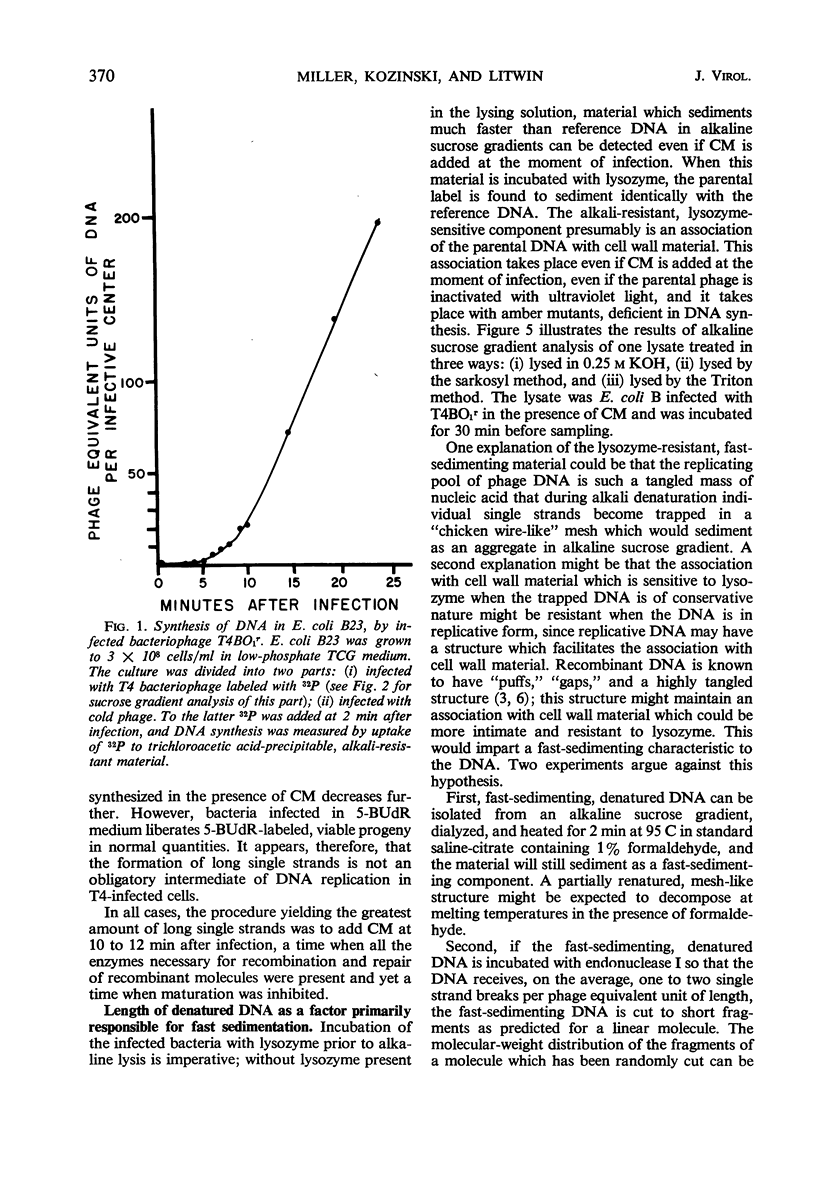
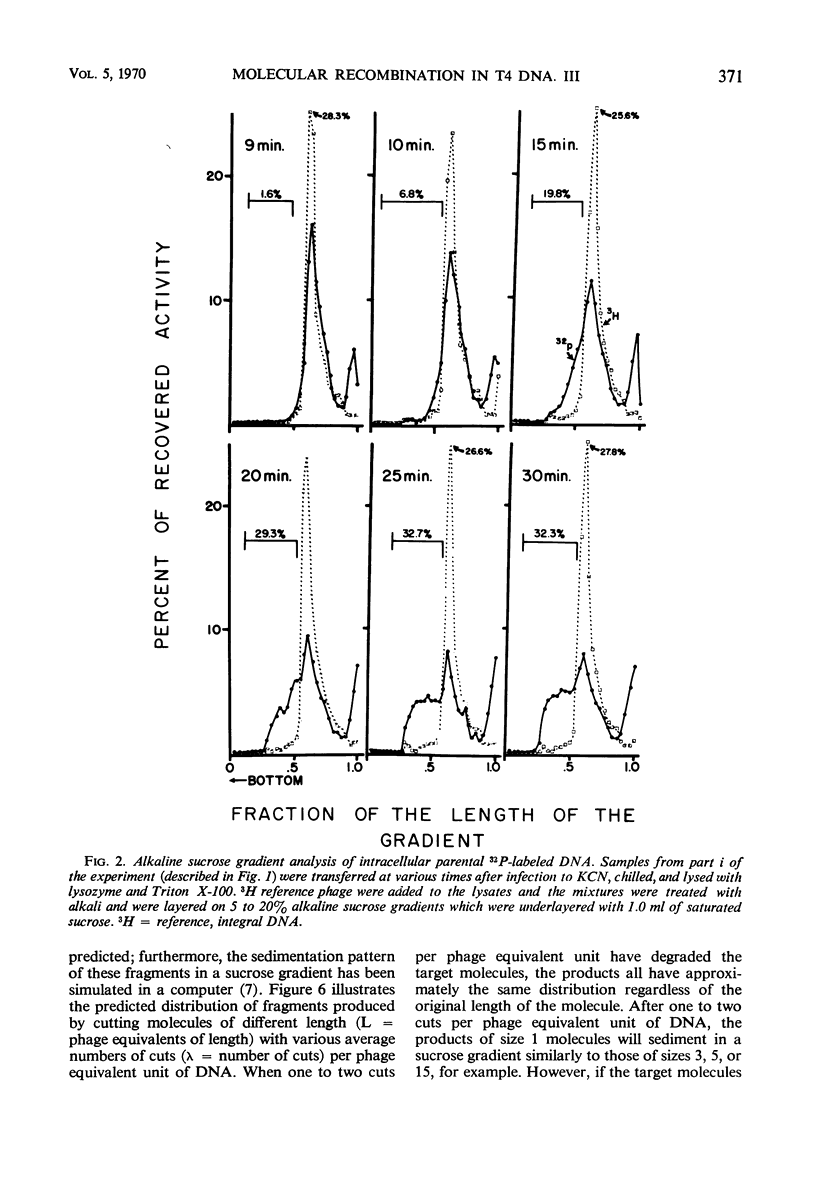
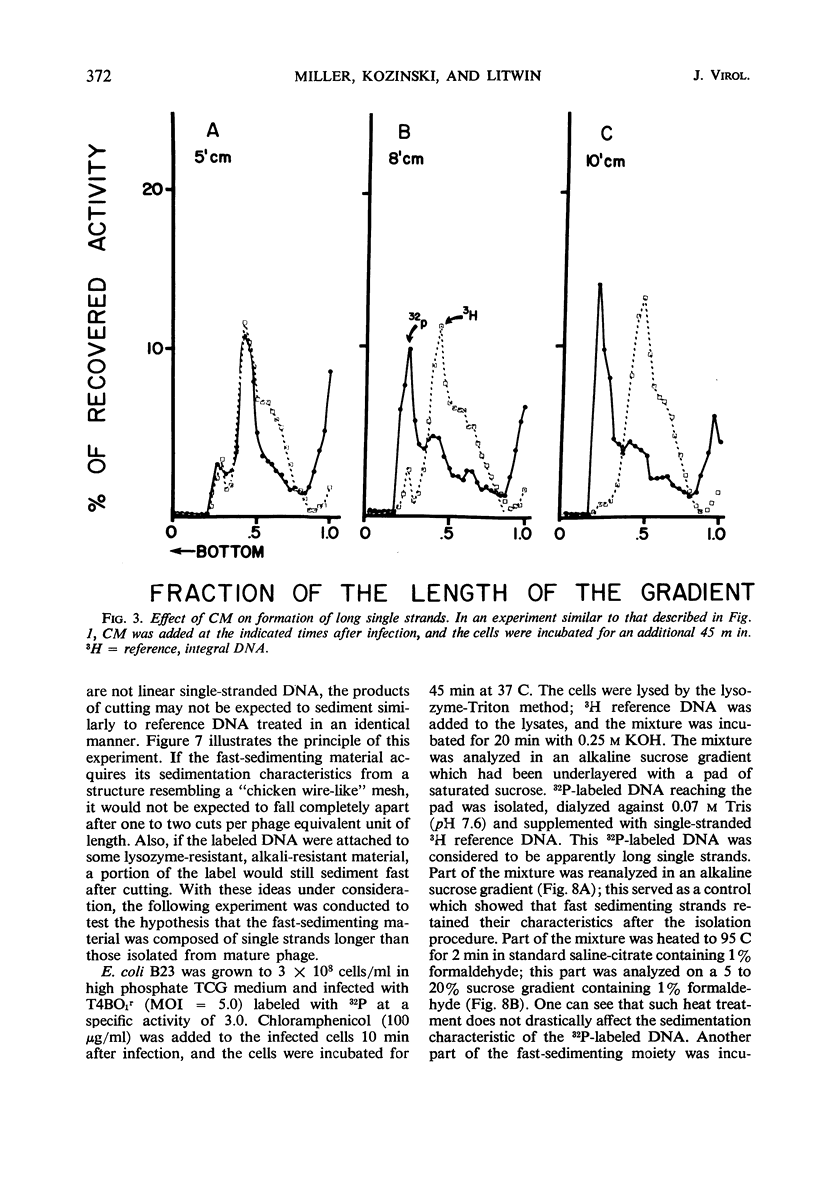
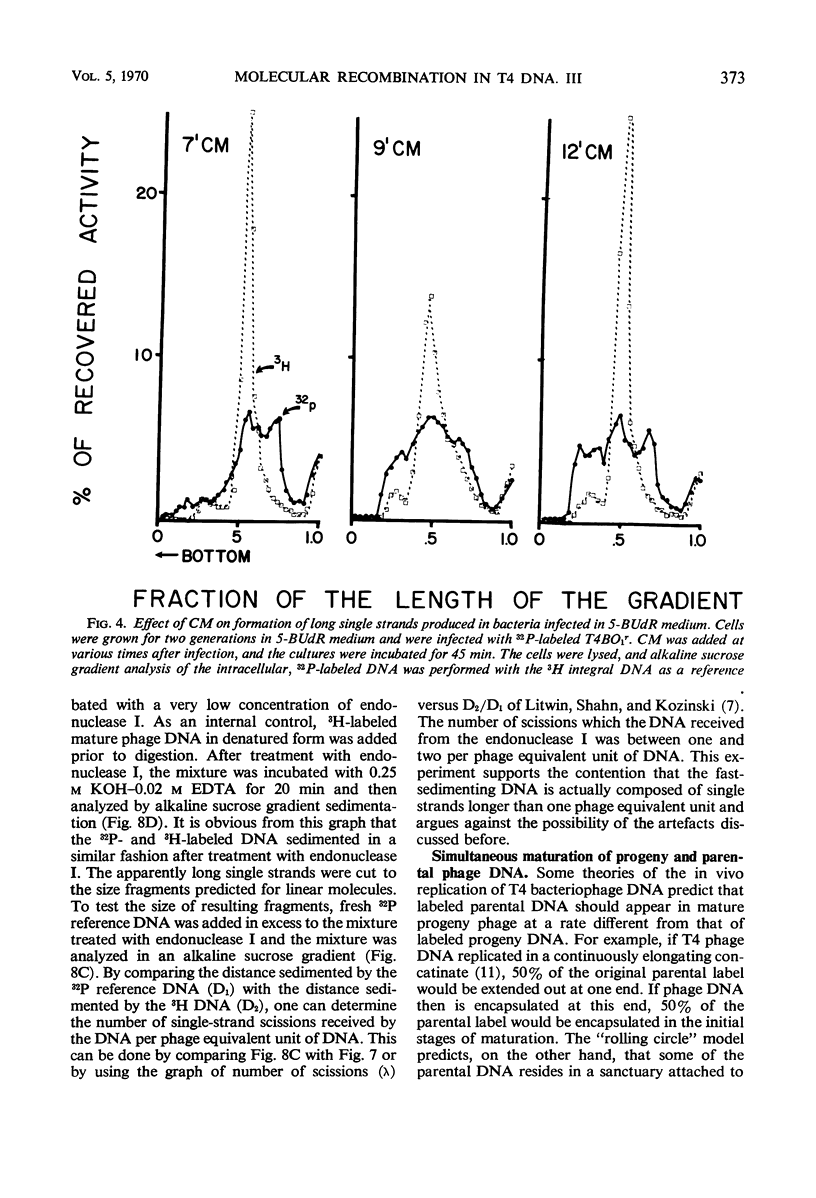
Evidence was presented to support the hypothesis that long single strands appearing at late times (15 min after infection) are produced as a result of recombination and not as a continuous elongation during the replication process. The production of long strands does not depend on the multiplicity of infection, and the first long strands appear at the time when 20 to 50 phage equivalent units of deoxyribonucleic (DNA) are synthesized, and not earlier. The addition of chloramphenicol at 5 min, which prevents molecular recombination but allows replication of DNA, prevents the formation of long, single strands. Chloramphenicol added between 8 and 10 min after infection, a time at which molecular recombination is fully expressed and covalent repair of recombinant molecules is allowed, does not prevent formation of long single strands. Cutting of single-strand DNA with a limited amount of endonuclease I allows confirmation that the fast-sedimenting characteristic of intracellular denatured DNA is caused primarily by the length of the strands, and not by the formation of aggregates. The computer simulation of two recombination models indicates the feasibility of random breakage and rejoining of molecules in generating long concatenates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W. Unbiased participation of T4 phage DNA strands in replication. Biochem Biophys Res Commun. 1969 Apr 29;35(2):294–299. doi: 10.1016/0006-291x(69)90281-2. [DOI] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHattie L. A., Ritchie D. A., Thomas C. A., Jr, Richardson C. C. Terminal repetition in permuted T2 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):355–363. doi: 10.1016/s0022-2836(67)80110-4. [DOI] [PubMed] [Google Scholar]
- Shahn E., Kozinski A. Fragmentary transfer of P32 labeled parental DNA to progeny phage. 3. Incorporation of a single parental fragment to the progeny molecule. Virology. 1966 Nov;30(3):455–470. doi: 10.1016/0042-6822(66)90122-x. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, RUBENSTEIN I. THE ARRANGEMENTS OF NUCLEOTIDE SEQUENCES IN T2 AND T5 BACTERIOPHAGE DNA MOLECULES. Biophys J. 1964 Mar;4:93–106. doi: 10.1016/s0006-3495(64)86771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr Recombination of DNA molecules. Prog Nucleic Acid Res Mol Biol. 1966;5:315–337. doi: 10.1016/s0079-6603(08)60237-8. [DOI] [PubMed] [Google Scholar]


