Abstract
Objective
This exploratory research sought to identify movement related soft tissue changes in the midfacial region in individuals with a cleft lip/palate and unaffected relatives compared to controls. The cleft phenotype (clinically identified cleft or occult Orbicularis oris muscle defect) was hypothesized to affect movement in the midfacial region, leading to furrowing and dimpling during speech.
Design
Changes in the appearance of skin in the midfacial region, including a newly identified feature, nasolabial fold discontinuity (NLF discontinuity), were described and compared across groups.
Participants
Individuals with cleft lip (n= 42) and unaffected relatives of persons with a cleft (n = 57) were compared to healthy controls (n= 41).
Results
NLF discontinuities were observed more frequently in individuals with a cleft phenotype (overt cleft or OOM defect) than in those with no underlying muscular defect (Fisher Exact test, p = .014).
Conclusion
Results suggest that movement of the muscles of facial expression is moderated at least in part by underlying cleft risk factors, indicating certain facial movements as candidate physical marker for extension of the cleft phenotype.
Keywords: facial movement, mid facial region, cleft lip, phenotypes
The cleft lip phenotype is represented by a spectrum of physical variation ranging from complete clefts through the lip and palate, to visible microform clefts, to subepithelial defects in the orbicularis oris muscle that are detectable only with ultrasound inspection or by post mortem histological analysis [Neiswanger et al., 2007; Rogers et al., 2008]. Genetic studies also support the interpretation of mild forms of clefting as part of a spectrum of the cleft lip phenotype [Suzuki et al., 2009]. Investigation of the genetic basis for clefting may be enhanced by the extension of the cleft lip phenotype to include subtle anomalies of muscle movement in the midfacial region. In this exploratory study, we describe movement related facial appearance changes in and around the nasolabial fold during speech that may serve as a marker of these subtle muscle anomalies.
In the mid facial region, the nasolabial fold (NLF) extends from the lateral aspect of the nares to a variable point in the lower cheek, lateral to the angle of the mouth. Typically, the NLF is continuous in formation, although variable in shape and length [Pessa, Zadoo, Adrian et al., 1998]. Medial, central, and lateral portions of the NLF deepen and become more prominent during movement of the elevators of the upper lip (levator labii superioris alaquae nasi, levator labii superioris, zygomaticus minor, levator anguli oris, and zygomaticus major). A strong smile, due primarily to the action of zygomaticus major, produces the greatest deepening of the fold. During mouth closing, i.e., the action of orbicularis oris, the NLF becomes less prominent. The precise fully established [Pessa, Zadoo, Adrian et al., 1998], although the insertion of the upper lip levator muscles in the superior orbicularis oris is well known.
Dimpling on the face is usually due to an underlying muscle attachment [Pessa, Zadoo, Garza et al., 1998]. If the muscles of facial expression are tethered at any point along their length to the dermal layer of the skin, puckering of the skin surface can appear upon contraction of the muscle. Therefore, visible depressions in the skin, i.e., dimples, can reveal underlying discontinuities in the muscular attachments. Although some dimples are apparent in the face at rest, smaller dimples may appear only during facial movement [Bao, Zhou, Li, & Zhao, 2007].
We hypothesized that NLF discontinuities or UL dimpling reflective of possible defects in the upper lip musculature or soft tissue might be apparent during movements of the upper lip (UL) during normal speech. Increased connective tissue, disorganized muscle fibers, and/or disrupted insertions of the levator muscles in the orbicularis oris could affect mid facial skin appearance, particularly during centrally directed movements of the orbicularis oris. Furthermore, individuals with a cleft phenotype, either clinically defined or a sub-clinical phenotype such as subepithelial orbicularis oris muscle defects, might be more likely than controls to show NLF discontinuities or UL dimpling during movement of the upper lip with speech.
METHOD
Participants in the study were drawn from a large family study of cleft lip ± cleft palate (the Pittsburgh Oral-Facial Cleft Study; [Weinberg et al., 2006]). This research study was prospectively reviewed and approved by the Institutional Review Board of the University of Pittsburgh. The sample analyzed in the current study (N = 140 unrelated individuals) consisted of 42 individuals with clefts, 57 unaffected persons related to someone with a cleft, and 41 healthy controls with no personal or known family history of clefting, roughly matched for sex and age. All individuals included in the study had previously undergone ultrasound evaluation for defects in the superior orbicularis oris (OO) muscle [Neiswanger et al., 2007]. Of the 98 participants that did not have an overt cleft, 19 were coded as being positive for the OO muscle defect subphenotype. In the selection of participants from the larger POFC sample, care was taken so that no participant in the current sample was related to any other participant. Average age did not differ across groups in the sample (average age ranging from 29.1 to 31.06 years across groups). Each group consisted of approximately equal numbers of males and females (ranging from 47% to 50% male).
Participants were videotaped reading a passage designed to evaluate speech (the “grandfather passage;” [Darley, Aronson, & Brown, 1975]), and responding to conversational questions from an interviewer, e.g., “What would you do if you won a million dollars?” Videotaped interviews were approximately three minutes in length.
Facial appearance changes in the midfacial region were observed and coded by individuals that had been trained and certified in a standardized facial movement coding system (Facial Action Coding System (FACS) that was designed to capture movements of individual muscles of facial expression [Ekman, Friesen, & Hager, 2002]. Two previously unrecognized midfacial appearance changes occurring during speech were identified and coded from videotaped interviews of the participant sample. NLF discontinuities were defined as transient interruptions in the continuous appearance of the NLF during facial movement (see Figure 1). Upper lip dimpling was defined as the transient appearance of dimples in the upper lip region, lying between the left and right nasolabial folds and bounded superiorly by the nares and inferiorly by the vermilion border. NLF discontinuities and UL dimpling were coded as absent or present, and the side of the defect (left, right, or bilateral) was noted. Permanent discontinuities, depressions, or dimples resulting from scarring—due to cleft repair or other external causes, were not coded as NLF discontinuities or UL dimpling.
Figure 1.
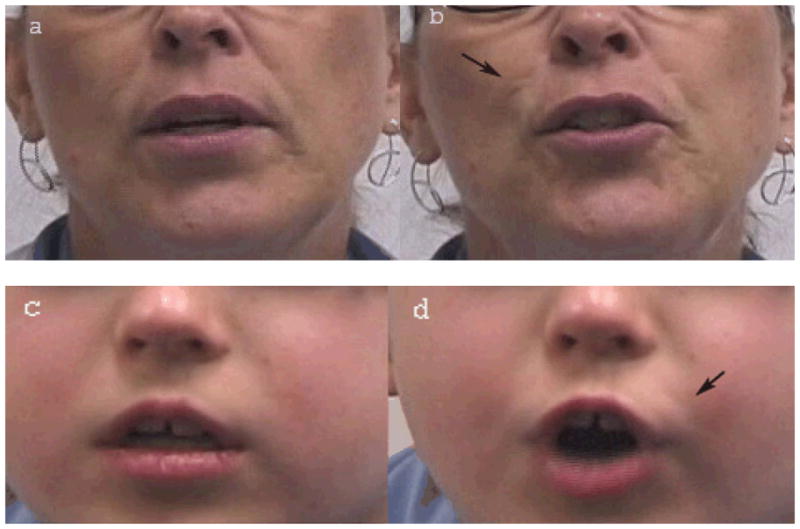
Nasolabial fold (NLF) discontinuities in two subjects. a. Subject 1. NLF discontinuity not obviously apparent. b. Subject 1. NLF discontinuity apparent during speech. Arrow at subject’s right indicates discontinuous NLF. c. Subject 2. NLF discontinuity not obviously apparent. d. Subject 2. NLF discontinuity apparent during speech. Arrow at subject’s left indicates discontinuous NLF.
Intra- and inter-rater reliability tests of the codes for NLF discontinuities and UL dimpling were conducted. For the intra-rater reliability test, coding was performed twice by the first author (KS) on a subset of 20 randomly chosen subjects, with an interval of two to three months between coding sessions. Inter-rater reliability was performed on the same 20 subjects, who were rated by KS and another certified FACS coder [Ekman, Friesen, and Hager, 2002]. Although facial coders were blind to participant status (person with cleft, unaffected relative, or control), they were aware of obvious signs of cleft lip repair and scarring at the site of repair.
Intra-rater agreement was good for NLF discontinuities at .96 (kappa = 0.83) and UL dimpling at .93 (kappa = 0.73). Inter-rater agreement for NLF discontinuities was acceptable at 0.82 (kappa = 0.65), but lower for UL dimpling at 0.61 (kappa = 0.25). Coding for UL dimpling was potentially confounded by the presence of individuals in the study sample who showed multiple depressions and dimples in the upper lip that may have been simply the result of aging processes. Although raters were instructed to disregard UL dimpling that appeared to be due to aging, in practice, coding of UL dimpling was unreliable across coders. Therefore, this appearance change was not analyzed further.
Spontaneous nasal flaring was observed frequently in a preliminary survey of the videotaped speech samples, and was identified as a control movement for comparison with anomalies possibly related to OO movement. Nasal flaring, i.e., the dilation of the nares (FACS action unit 38), has been previously identified as the result of the action of the dilator naris portion of the nasalis muscle [Ekman, Friesen, & Hager, 2002; Hoeyberghs, et al, 1996]. The nasalis muscle is intrinsic to the nose and less likely to be affected by subepithelial or overt forms of clefting than the muscles used to move the upper lip during speech. Nasal flaring was rated by the same coders as above. Inter-rater reliability testing was performed for nasal flaring, with an acceptable agreement of 0.82 (kappa = 0.63).
Three types of participants were included in this analysis—individuals with an overt cleft (n = 42), unaffected relatives of people with clefts (n = 57), and unrelated controls with no family history of clefting (n = 41). To determine if mid facial defects (overt clefting or OOM muscle defects) were related to NLF discontinuities, we grouped all individuals with a clinically observed cleft together with individuals who demonstrated subphenotypic OOM defects, to form a “cleft/OOM defect” group1. This cleft/OOM defect group was then compared with a “no defect” group including all participants with a normal OO muscle on ultrasound, i.e., most unaffected relatives combined with all healthy controls. Our rationale for this grouping was the exploration of appearance changes associated with the wider spectrum of overt and subepithelial forms of clefting.
ANALYSIS AND RESULTS
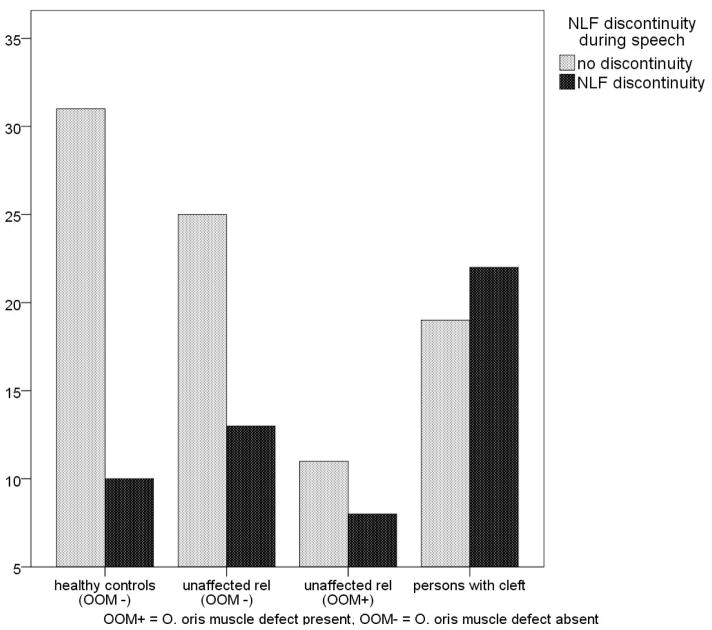
NLF discontinuities were found in both cleft/OOM defect and no defect groups (Figure 2). NLF discontinuities were more frequent in the cleft/OOM defect group than in the no defect group (Fisher’s exact test (one sided), p = 0.014). In comparison, there was no difference in the frequency of nasal flaring between the cleft/OOM defect group and the no defect group (Table 1). The sample of individuals with clefts included seven participants with cleft palate only. Because these individuals can have sub-clinical phenotypes indicating vulnerability to cleft lip (subepithelial OO muscle defects (Weinberg et al, 2008), we reasoned that they might also show NLF discontinuity. While we grouped individuals with cleft palate with the cleft/OOM defect group, we investigated the possible confounding effect of this cleft palate only subgroup by repeating the previous comparison with these seven removed. As we expected, NLF discontinuities remained more frequent in the clef/OOM defect group than in the no defect group (Fisher’s exact test (one sided), p = .016).
Figure 2.
The frequency of nasolabial fold discontinuity during speech in individuals with clefts, relatives without clefts, and unrelated controls
Table 1.
Frequencies (percentages) of nasolabial fold (NLF) discontinuities and nasal flaring in individuals with cleft lip/palate or superior orbicularis oris muscle (OOM) defects
| Facial feature or action
|
||||
|---|---|---|---|---|
| NLF discontinuity, n(%)b | Nasal flaring, n(%)c | |||
| − | + | − | + | |
| No OOM defect | 56 (70) | 24 (30) | 49 (61) | 31 (39) |
| control | 31 (76) | 10 (24) | 27 (66) | 14 (34) |
| relative | 25 (66) | 13 (34) | 21 (55) | 17 (45) |
|
| ||||
| OOM defect or cleft a | 30 (50) | 30 (50) | 37 (61) | 24 (39) |
| relative | 11 (58) | 8 (42) | 11 (58) | 8 (42) |
| cleft affected | 19 (46) | 22 (54) | 26 (63) | 16 (37) |
includes 7 individuals with cleft palate only, as part of the phenotypic spectrum
P = 0.014; Fisher Exact Test
Non-significant
Using standard chi square tests, we tested the difference between groups based on status (person with cleft, person related to someone with cleft, and healthy controls). In this three way comparison, we found that there was a group difference (chi square = 7.51, p = .023). Post hoc pairwise comparisons, however, indicated that unaffected relatives of a person with cleft could not be differentiated from either healthy controls (p =.62) or from persons with a cleft (p =.26).
To address the relation between facial appearance changes (NLF discontinuity) and underlying OOM defects, the laterality of NLF discontinuities was compared with the laterality of OO muscle defects for those unaffected relatives with both features, where data were available (n = 19). Although the small sample precluded testing, there was no clear relation between NLF discontinuity and the side of the OOM defect. Of the 11 relatives with left sided NLF discontinuity, 9 showed left OOM defects, while 2 had a right OOM defect only. Of the 8 relatives with right sided NLF discontinuity, 5 showed right OOM defects, while 3 had a left sided OOM defect only. While numbers are small, it appears that left sided NLF discontinuity may be an indicator of underlying OOM muscle defect, or an additional outcome of disrupted development of soft tissue in this region. The appearance of NLF discontinuity on the opposite side of the OOM defect, as in five of 19 unaffected relatives assessed suggests the possibility that developmental disruption to the muscle layer and disruption to the overlying soft tissues responsible for the NLF discontinuity represent two separate outcomes of similar disruptions processes that may appear individually on different sides of the midface in the same individual. Larger samples will be needed to tease out the relation between the laterality of an OOM defect and an NLF discontinuity.
DISCUSSION
We have identified a previously unreported facial appearance change during facial movement—a transient discontinuity in the nasolabial fold during speech—that differs depending on the presence or absence of underlying defects in the orbicularis oris muscle. This observation suggests that defects in the OO muscle may impact the actions of the elevators of the upper lip, perhaps by influencing their insertion patterns, so as to alter the appearance of the nasolabial fold during speech.
Spontaneous nasal flaring, considered to be unrelated to the action of the orbicularis oris muscle, was not correlated with OO muscle defects. This was expected, as spontaneous dilation of the nares is attributed to the intrinsic muscles of the nose that do not articulate with OO, rather than to the extrinsic muscles such as levator labii superioris alaquae nasi which inserts in the OO muscle and is more closely associated with deliberate nasal flaring [Hoeyberghs et al., 1996].
Our results showing a relation between cleft/OO muscle defect and NLF discontinuity are in contrast to those obtained when participants were grouped by genetic relatedness to the someone with a cleft (person with a cleft, unaffefted relative of a person with cleft, and controls). In that case, unaffected relatives as a group that includes those with and without an OO muscle defect cannot be differentiated from either control or cleft groups on the basis of NLF discontinuity. Demonstrated cleft sub-clinical phenotype in the form of an OO muscle defect status appears to have a greater influence on the frequency of NLF discontinuities than genetic relatedness to a person with a cleft. This interpretation is tempered by the fact that there were no controls with an OOM defect included in the current study. The frequency of OO muscle defects in individuals with no known family history of clefting has been estimated to be 5.8% [Neiswanger et al., 2007]. In general, larger samples are needed to confirm the findings in this study.
A limitation of the current analysis is the relative difficulty in interpreting the causality of discontinuities in appearance. The relation between appearance changes on the surface and underlying muscle and skin defects has not been established. It is possible that NLF discontinuity and OOM defects arise from the same genetic vulnerability but represent different patterns of disruption in the soft tissue of the midface.
Although initial coding revealed a second interesting appearance change, upper lip dimpling, this type of discontinuity in the skin was not reliably coded and therefore not analyzed. Future analyses will extend observation of visible appearance changes in the mid facial region, perhaps enabling UL dimpling to be further developed as a reliably coded phenotype. Focusing on appearance changes in the upper lip in larger samples may help to resolve issues surrounding successful distinction between facial appearance changes due to scarring or aging and appearance changes related to underlying relation between muscle and skin (dimples).
CONCLUSION
The appearance of discontinuities in the nasolabial fold may provide another perspective from which physical anomalies in the unaffected relatives of those with cleft lip or palate can be assessed. As the genetic basis of clefting is explored, the phenotyping of unaffected relatives of persons with a cleft is of increasing importance in understanding the genetic contributions to the process of disrupted development of the midfacial region.
Although the orbicularis oris is the muscle most obviously affected by developmental processes leading to clefting, recognition of the coordinated role of muscles of facial expression in creating facial movement and facial appearance changes may provide an additional method for assessment of the presence of subtle subphenotypic mid facial defects.
Acknowledgments
The authors would like to thank the ultrasound orbicularis oris muscle raters (Dr. Mark Mooney, Dr. Rick Martin, Dr. Seth Weinberg, Dr. Manika Govil, Dr. Carolyn Rogers, Judith Resick, Megan Branning, Pooja Gandhi, Cherise Klotz, Jennifer Moeller, & Anna Kamelin), video editors (Dan Berk, Chris Edborg, Nick DeGrazia, Joel Kraus, & Elise LaGamba), Judith Resick for her efforts in coordinating various aspects of the study, PRDM for helpful comments on the manuscript, and L. Ian Reed for additional coding of facial appearance changes. This research was supported by the following grants from National Institute for Dental and Craniofacial Research: 5 P50 DE 016215 Jeff Murray and 1 R01 DE 016148 Mary Marazita and by a grant from the National Institute of Mental Health to Karen Schmidt (MH067976).
Footnotes
Among the 42 individuals with overt clefts of the lip and/or palate, 10 (24%) did not have an OO muscle defect detectable by ultrasound. However, the midface cannot be considered normal if a visible cleft of the lip or palate is present. Therefore, for the purposes of this analysis, the muscle defect group included all cleft individuals, both with and without a detectable OO defect.
References
- Bao S, Zhou C, Li S, Zhao M. A new simple technique for making facial dimples. Aesthetic and Plastic Surgery. 2007;31:380–383. doi: 10.1007/s00266-006-0191-8. [DOI] [PubMed] [Google Scholar]
- Darley F, Aronson A, Brown J. Motor Speech Disorders. Philadelphia: Saunders; 1975. [Google Scholar]
- Ekman P, Friesen W, Hager J. The Facial Action Coding System. 2. Salt Lake City: Research Nexus eBook; 2002. [Google Scholar]
- Hoeyberghs J, Desta K, Matthews RN. The lost muscles of the nose. Aesthetic and Plastic Surgery. 1996;21:165–169. doi: 10.1007/BF02275537. [DOI] [PubMed] [Google Scholar]
- Neiswanger K, Weinberg SM, Rogers C, Brandon C, Cooper M, Bardi K, Deleyiannis FW, Resick JM, Bowen A, Mooney MP, de Salamanca JE, González B, Maher BS, Martin RA, Marazita ML. Orbicularis oris muscle defects as an expanded phenotypic feature in nonsyndromic cleft lip with or without cleft palate. American Journal of Medical Genetics Part A. 2007;143A:1143–1149. doi: 10.1002/ajmg.a.31760. [DOI] [PubMed] [Google Scholar]
- Pessa J, Zadoo V, Adrian EJ, Yuan C, Aydelotte J, Garza J. Variability of the midfacial muscles: analysis of 50 hemifacial cadaver dissections. Plastic & Reconstructive Surgery. 1998;102:1888–1893. doi: 10.1097/00006534-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Pessa J, Zadoo V, Garza P, Adrian EJ, Dewitt A, Garza J. Double or bifid zygomaticus major muscle: anatomy, incidence, and clinical correlation. Clinical Anatomy. 1998;11:310–313. doi: 10.1002/(SICI)1098-2353(1998)11:5<310::AID-CA3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rogers C, Weinberg S, Smith T, Deleyiannis F, Mooney M, Marazita M. Anatomical Basis for Apparent Subepithelial Cleft Lip: A Histological and Ultrasonographic Survey of the Orbicularis Oris Muscle. Cleft Palate Craniofacial Journal. 2008;45:518–524. doi: 10.1597/07-115.1. [DOI] [PubMed] [Google Scholar]
- Slee JJ, Smart RD, Viljoen DL. Deletion of Chromosome 13 in Moebius Syndrome. Journal of Medical Genetics. 1991;28:413–414. doi: 10.1136/jmg.28.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Marazita M, Cooper M, Miwa N, Hing A, Jugessur A, Natsume N, Shimozato K, Ohbayashi N, Suzuki Y, Niimi T, Minami K, Yamamoto M, Altannamar TJ, Erkhembaatar T, Furukawa H, Daack-Hirsch S, L’heureux J, Brandon CA, Weinberg SM, Neiswanger K, Deleyiannis FW, de Salamanca JE, Vieira AR, Lidral AC, Martin JF, Murray JC. Mutations in BMP4 Are Associated with Subepithelial, Microform, and Overt Cleft Lip. The American Journal of Human Genetics. 2009;84:406–411. doi: 10.1016/j.ajhg.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg SM, Brandon C, McHenry T, Neiswanger KA, Deleyiannis F, de Salamanca J, Castilla EE, Czeizel AE, Vieira AR, Marazita ML. Rethinking isolated cleft palate: evidence of occult lip defects in a subset of cases. American Journal of Medical Genetics Part A. 2008;146A:1670–1675. doi: 10.1002/ajmg.a.32291. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Neiswanger K, Martin RA, Mooney MP, Kane AA, Wenger SL, Losee J, Deleyiannis F, Ma L, De Salamanca JE, Czeizel AE, Marazita ML. The Pittsburgh Oral-Facial Cleft study: Expanding the cleft phenotype. Background and justification. Cleft Palate Craniofacial Journal. 2006;43:7–20. doi: 10.1597/04-122r1.1. [DOI] [PubMed] [Google Scholar]