Abstract
Molluscum contagiosum poxvirus (MCV) type 1 and type 2 encode two chemokine-like proteins MC148R1 and MC148R2. It is believed that MC148R proteins function by blocking the inflammatory response. However, the mechanism of the proposed biological activities of MC148R proteins and the role of the additional C-terminal cysteines that do not exist in other chemokines are not understood. Here, we demonstrated in two different assay systems that His-tagged MC148R1 displaces the interaction between CXCL12α and CXCR4. The N-terminal cysteines but not the additional C-terminal cysteines modulate this displacement. His-tagged MC148R1 blocked both CXCL12α-mediated and MIP-1α-mediated chemotaxis. In contrast, MC148R2 blocked MIP-1α-mediated but not CXCL12α-mediated chemotaxis. Immunoprecipitation by antibodies to MC148R1 or CXCL12α followed by immunoblotting and detection by antibodies to the other protein demonstrated physical interaction of His-tagged CXCL12α and His-tagged MC148R1. Interaction with chemokines might mask the receptor interaction site resulting in decreased binding and impairment of the biological activities.
Keywords: Chemokines, Chemotaxis, CXCL12, MCV, HIV
INTRODUCTION
Chemotactic cytokines (Chemokines) are small proteins (70–100 amino acid residues) with well-characterized three-dimensional structures usually stabilized by two disulfide bridges. Chemokines are involved in attracting and activating distinct leukocyte subsets (reviewed in (Fernandez and Lolis, 2002)). They exert their function through interacting with the seven-transmembrane (7TM) G-protein coupled receptors (GPCR). Chemokines are classified into four different families on the basis of the pattern of the conserved two N-terminal cysteine residues that form disulfide bridges with the two C-terminal cysteines of the molecules. In CC chemokines, the two N-terminal cysteines are adjacent; in CXC chemokines the two cysteines are separated by a single amino acid; and in CX3C chemokines the cysteines are separated by three amino acids. The XC family has only one cysteine near the N-terminus. Chemokines have similar three-dimensional structures comprising an N-terminal unstructured region, three antiparallel β-strands connected by loops, and a carboxyl terminal α-helix (Clore and Gronenborn, 1995). Previous studies on structure-function of chemokines determined that the first five amino acids constitute the activation site and the next five constitute the receptor-binding site (reviewed in (Fernandez and Lolis, 2002)).
Some viruses have evolved novel anti-chemokine strategies to evade the host immune system (Murphy, 2000). Anti-chemokines comprise a major group of virus-encoded chemokine modulators and consist of three groups based on structure and mechanism of action: 1) chemokine receptor homologues, 2) chemokine-binding protein, and 3) chemokine homologues. Herpesviruses and poxviruses have been found to encode proteins that target the human chemokine system. The biological activity of these virus-encoded proteins could represent a viral strategy to subvert immunity impairing the generation of an effective anti-viral immune response (reviewed in (Murphy, 2000)). Molluscum contagiosum virus (MCV) is a member of the poxvirus family that produces small, papular tumors in the skin of immunocompetent children and young adults and opportunistic infections in immunodeficient AIDS patients (Gur, 2008). MCV infection occurs as a late manifestation of HIV-1 infection and is a cutaneous correlate of cellular immune deficiency. It has been proposed that MCV inhibits the activity of chemokines that bind to specific G protein-coupled membrane receptors and recruit leukocytes to areas of infection and injury (Alcami et al., 1998; Lalani et al., 1998; Smith et al., 1997). The discovery of an ORF sequence (MC148R) encoding a CC chemokine homologue, within the 190,000-bp genome, provided a clue to the mechanism used to prevent an inflammatory response (Senkevich et al., 1996).
MCV type 1 and the closely related MCV type 2 encode open reading frames (ORF), MC148R1 and MC148R2 respectively, which code for two 104 amino acid chemokine-like proteins (Damon et al., 1998; Krathwohl et al., 1997). The literature reported contradictory findings in terms of MC148R1 activity. Two groups independently demonstrated that MC148R2 and/or MC148R1 could inhibit the chemotaxis induced by various CC and CXC chemokines (Damon et al., 1998; Krathwohl et al., 1997). These studies implicated a broad-spectrum effect for MC148R1 on CC and CXC chemokine receptors. MC148R1 purified from vaccinia-infected cells has been shown to bind CCR2 but not CCR8. Interestingly, MC148R1 inhibited calcium mobilization through CCR2 and CCR8 (Damon et al., 1998). However, MC148R1 purified from transfected monkey COS-7 cells showed specificity for CCR8 but found no agonist activity against CCR2 (Luttichau et al., 2000). The chemotaxis studies provided little data on individually cloned MC148R proteins (Damon et al., 1998; Krathwohl et al., 1997). However, Luttichau et al found MC148R1 to act as a selective antagonist of CCR8 in binding, calcium mobilization, and chemotaxis assays (Luttichau et al., 2000).
In addition to the two conserved N-terminal cysteines, MC148R1 contains two additional cysteines at its carboxyl terminus (residues 95 and 102). The primary amino acid sequences of all human CC chemokines except for CCL21/SLC lack these additional C-terminal cysteines (Arenberg et al., 2001). The deduced amino acid sequence of mature MC148R1 predicted a shorter N-terminus (Senkevich et al., 1996). Since the N-terminus of the chemokine is the region involved in receptor activation, it was hypothesized that MC148R1 might function as a chemokine antagonist (Senkevich et al., 1996). In support of this hypothesis it was demonstrated that MC148R1 blocked the chemokine response of monocytes to MIP-1α (Krathwohl et al., 1997) and inhibited chemotaxis of human lymphocytes, and neutrophils in response to a large number of CC and CXC chemokines (Damon et al., 1998). However, the mechanism of MC148R1 antagonistic activity has not been investigated. Additionally, the role of the N-terminal and C-terminal cysteine residues in the observed biological activity of MC148R1 has not been investigated.
In this study we analyzed the mechanism of blocking the chemokine response by MC148R1 and investigated the role of the conserved N-terminal and the non-conserved C-terminal cysteines. We demonstrate in two different assay systems that His-tagged MC148R1 displaces the interaction between CXCL12α and CXCR4, and that the N-terminal cysteines modulate this displacement. The results suggest physical interaction with the chemokines as a mechanism for the observed anti-chemokine activities of MC148R1.
RESULTS
Construction, expression and purification of the recombinant proteins
In order to investigate the mechanism of MC148R1 activity we constructed a number of point mutations in its primary amino acid structure. The first mutant knocks out the two conserved N-terminal cysteines and the next three knock out the additional C-terminal cysteines in MC148R1 either individually or in combination (Figure 1A). We hypothesized that the additional C-terminal cysteines could cause folding changes that would provide the MC148R1 protein its antagonistic properties. Another point mutant was constructed that changed the Leucine at residue 94 into arginine crating a consensus BBXB domain found in CC chemokine. We hypothesized that creating a BBXB domain might confer a chemotactic activity for the chemokine-like protein MC148R1. Compared to other chemokines, MC148R1 contains a significantly shorter N-terminus preceding the conserved cysteines. It has been hypothesized that the receptor activation domain of the N-terminus found in other chemokines is deleted from MC148R1, leaving only a receptor-binding domain that is hypothesized to be responsible for the observed antagonistic activities. We have generated a mutation (Δ26–30) with the first five amino acids deleted in order to create a nonfunctional mutant that lacks the antagonistic activities.
Figure 1.
A) The predicted amino acid sequences of mature human MIP-1β/CCL4, wild-type MC148R1, and mutated MC148R1. Amino acid sequences are indicated by capital letters with added methionine residues in lower case. All purified proteins contain a carboxyl terminal 6-histidine tag that is not depicted. Mutations are indicated as the amino acid before mutation, followed by the numerical position based on the precursor protein, and the resulting amino acid after mutation. Bold letters indicate the mutated amino acid residues. Asterisks indicate deleted amino acids. BBXB domains are underlined. Dashes have been added in order to align the cysteine residues.
B) Silver stained SDS-PAGE of bacterially expressed and His-affinity purified chemokines. Approximately 0.5 µg of purified protein was loaded in each lane. The concentration of the total cell lysates was not determined. One µl of the total 10ml lysate (Total lys) was loaded.
(C) Western blot analysis of MCV and CXCL12α/SDF-1α proteins expressed by recombinant vaccinia viruses. HeLa cells were infected with WR (control vector), vCXCL12α, or vMC148R1 and cell lysates were prepared and fractionated in SDS-PAGE and blotted. The blots were probed with polyclonal anti-histidine tag antibodies or anti-CXCL12-specific monoclonal antibodies. The blots were washed, reacted with HRP-conjugated secondary antibodies and blots exposed to X-ray after reacting with the substrate.
To provide a reliable source for the MC148R1 and its mutants we cloned the His-tagged construct into the bacterial expression vector pET-32a (+). The CXCL12α coding sequence is separately cloned within the same vector and therefore, serves as a positive control for expression and purification from E. coli (Altenburg et al., 2007). The pET-32a vector contains the sequence coding for CXCL12α, MC148R1, or mutant proteins along with a C-terminal His tag.
Silver-stained SDS-PAGE gels demonstrated that the high purity of our bacterialy expressed and His-affinity purified proteins (Figure 1B). The silver-stained gel only shows wild type MC148R1 and CXCL12α and two other cysteine mutants. The purity of other proteins used in this study was basically similar and examined in other gels. Western blot analysis using the His-tag antibodies as a probe demonstrated expression of wild type and mutant proteins in bacteria (data not shown). We have also constructed two recombinant vaccinia viruses that encode either His-tagged MC148R1 or His-tagged CXCL12α. Western blot analysis confirmed the expression of either protein in infected cells (Figure 1C).
Anti-Chemotactic activities of MC148R proteins
In order to examine the chemotactic activities of the MC148R proteins we performed a chemotaxis assay where the chemokines are incubated in the lower chamber and cells in the upper chamber. The readout of this assay is the number of cells migrated into the lower chamber. As expected, CXCL12α induced CEM cell migration (Fig. 2A), and MIP-1α induced migration of peripheral blood mononuclear cells (PBMCs) (Fig. 2B). MC148R1 blocked CXCL12α-induced (A) and MIP-1α-induced (B) cell migration in a dose dependent manner. In contrast, MC148R2 had no significant effect on cell migration induced by CXCL12α but significantly inhibited cell migration induced by MIP-1α (B). Mixing IL-8 with either MIP-1α or CXCL12α at similar concentrations had no effect on cell migration by either chemokine (Figure 2A&B).
Figure 2. Anti-chemotactic activity of His-tagged MC148R1 and MC148R2.
The chemotactic activities of the His-tagged MC148R1 and MC148R2 were examined in a two-chamber chemotaxis assay. All chemokines used were from R&D except for the His-tagged MC148R1 and its mutants. Chemotaxis assays were performed as outlined in Materials and Methods. The effect on CXCL12α-induced chemotaxis was analyzed in CEM cell (A) while effects on MIP-1α -induced chemotaxis was analyzed using peripheral blood mononuclear cells (PBMCs) purchased from StemCell Technologies (B). Approximately 2×105 cells in a volume of 0.1 ml were added to each transwell membrane. The chemokines (100ng/ml) with or without the MCV proteins (at the indicated concentration) were added to the lower chamber in a 0.6 ml volume. The results are represented as the number of cells migrated into the lower chamber. This experiment is representative of at least four other experiments performed in duplicates.
Similar chemotaxis experiments were performed to examine the role of the conserved and the additional C-terminal cysteines in the antagonistic activities of the MC148R1 protein. The experiments were performed with CEM cells (Figure 3A) and commercially available PBMCs (Figure 3B). The MC148R1 inhibitory effect on cell migration was only abolished when the two conserved N-terminal cysteins were mutated (Figure 3A&B). The loss of the anti-chemotactic activity by C30/31A mutant was consistent and was never observed with either the wild type His-tagged MC148R1, wild type MC148R2, C95A, C102A, C95/102A, L94R, or the Δ26–30 mutant proteins (Fig 3A&B). The L94R mutant that acquired the BBXB domain did not induce chemotaxis (data not shown). The IL-8 chemokine was used as a negative control for the antagonistic activities of MC148R proteins. As shown in Figure 3A&B, IL-8 had no inhibitory effects on the chemotactic activities of CXCL12α (Fig. 3A) or MIP-1α (Fig. 3B). The results demonstrate a significant role for the conserved N-terminal cysteines in the anti-chemotactic activity observed with the His-tagged MC148R1.
Figure 3. Anti-chemotactic activity of MC148R1 and the role of the cysteine residues.
The chemotactic activities of the indicated chemokine proteins were examined in a two-chamber chemotaxis assay. All chemokines used were from R&D except for the MC148R1 and its mutants. Chemotaxis assays were performed as outlined in Materials and Methods with CEM T cells. Approximately 2×105 cells in a volume of 0.1 ml were added to each transwell membrane. The chemokines (100ng/ml) with or without the MCV proteins (100ng/ml) were added to the lower chamber in a 0.6 ml volume. A) Commercial CXCL12α used with CEM cells. B) Commercial MIP-1α with peripheral blood mononuclear cells. Error bars indicate standard deviations of mean values obtained from triplicate assays. This experiment is representative of three experiments done at different times with different MC148R1 purification sets. The chemokine inhibition activities of MC148R1 and the respective mutations are reported as the percent migration of cells that were stimulated with CXCL12α alone. A baseline value for spontaneous cell migration in the absence of CXCL12α or MIP-1α was calculated to be less than 1% of the cells migrated in the presence of the chemokine. The results are represented as the number of cells migrated into the lower chamber. This experiment is representative of at least three other experiments performed in duplicates.
Effect of MC148R1 and mutant proteins on CXCL12α-inhibition of Env-mediated fusion
It is well established that CXCL12 inhibits HIV Env-mediated cell fusion and viral entry of X4 strains (reviewed in (Alkhatib, 2009)). We have previously demonstrated that the His-tagged CXCL12α was as functional in blocking X4 fusion as the commercially available CXCL12α that lacks the C-terminal His-Tag (Altenburg et al., 2007; Altenburg et al., 2010). We next investigated the effects of His-tagged MC148R1 on CXCL12-mediated inhibition of X4 fusion in a cell-based fusion assay. To determine whether the His-tagged MC148R1 affects CXCL12α-mediated blocking of X4 fusion we performed experiments involving incubating mixtures of MC148R1 or mutant proteins and CXCL12α with the target cells before challenging with Env-expressing cells. As expected, pre-treating the target cells with affinity-purified CXCL12α alone resulted in efficient inhibition of X4 Env-mediated fusion (Figure 4A). This inhibitory activity was not observed with either MC148R1 or the affinity-purified vector-transformed bacterial lysates. Pre-treatment of the target cells with a mixture of CXCL12α and MC148R1, C95/102A, L94, or Δ26–30 abolished the inhibitory activity of CXCL12α. In contrast, pre-treatment of the target cells with a mixture of CXCL12α and C30/31A had no effect on the inhibitory activity of CXCL12α (Figure 4A). The results demonstrate that the conserved N-terminal cysteines are critical for MC148R1 to inactivate the observed inhibitory activity of CXCL12α. The two additional cysteines at the C-terminus do not seem to be involved in the critical structure needed for the observed MC148R1 activity.
Figure 4. MC148R1 inactivates the antiviral activity of CXCL12α.
A) Purified MC148R1 or its mutants were mixed with purified CXCL12α and incubated with the target TZM-bl cells for 1 hr at 37°C before mixing with X4 Env-expressing cells and incubating for 2.5 hr. The effect of the chemokine mixture on HIV-1 Env-mediated fusion was measured by β-gal production. B) The CXCR4+CCR5+ TZM-bl cells expressing vaccinia virus-encoded T7 RNA polymerase were used as target cells. The TZM-bl cells expressing T7 RNA polymerase were either infected with recombinant vaccinia viruses encoding MC148R1 or CXCL12α or co-infected with both recombinant viruses. Effector HeLa cells were coinfected with recombinant vaccinia virus encoding the PT7-lacZ reporter gene and vaccinia virus encoding the X4 LAV Env. The infected target cells were mixed with Env-expressing cells and the extent of Env-mediated cell fusion was measured by β-gal production. The Western Reserve (WR) vector strain was used as a control for virus infection. The results are representative of at least three different experiments performed in duplicate. Error bars indicate standard deviations of mean values obtained from duplicate wells.
To demonstrate the biological activity of the His-tagged MC148R1 in mammalian cells we used recombinant vaccinia viruses to express the His-tagged CXCL12α and His-tagged MC148R1 either individually or co-express them in the target cells. Expression of CXCL12α in the target cells caused significant blocking of X4 Env-mediated fusion. Interestingly, the blocking effect of CXCL12α was abolished upon co-expressing the MC148R1 protein in the same target cell population (Figure 4B). Cells co-expressing CXCL12α and WR were used as a control for multiple virus infection and did not result in abolishing the blocking effect of CXCL12α. These results provided strong evidence for the inactivation effect of MC148R1 on CXCL12α antiviral activity. This evidence has been provided through co-expressing the native proteins in mammalian cells. Taken together, these observations further confirm the inhibitory effect of MC148R1 on CXCL12α binding with CXCR4.
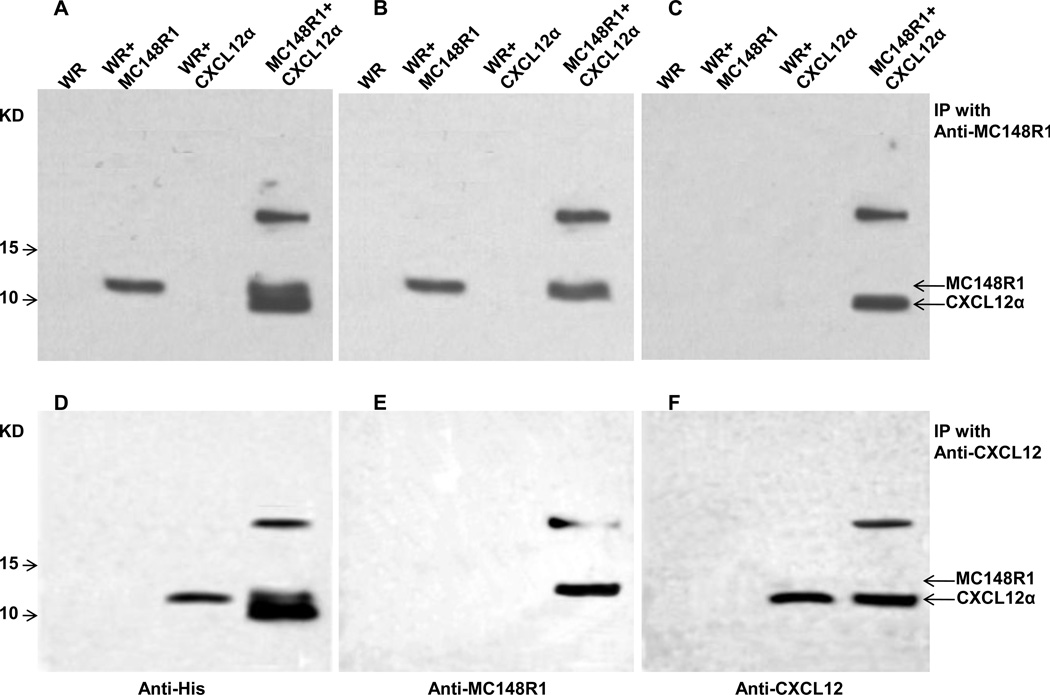
Physical interaction of MC148R1 and CXCL12α
To determine whether MC148R1 and CXCL12α physically interact we co-infected NIH 3T3 cells with recombinant vaccinia viruses expressing either protein. Immunoprecipitation (IP) with anti-MC148R1 antibodies and immunoblotting and probing with antibodies to either protein showed the expected expression of MC148R1 and CXCL12α protein bands in cells infected with either virus (Figure 5). High molecular weight band representing heterodimers were only detectable in lysates prepared from cells co-infected with both viruses (Figure 5A, B, C, D, E&F). IP with MC148R1 antibodies or anti-CXCL12α antibodies then immunoblotting with Anti-His antibodies revealed the expected chemokine proteins and a high molecular weight band (Fig. 5A&D). Stripping and re-probing the blot with anti-MC148R1 identified the bands specific to the MC148R1 protein (Fig. 5B&E). Re-stripping and re-probing the blot with anti-CXCL12α antibodies identified the CXCL12α-specific protein bands (Fig. 5C&F). The results demonstrate physical interaction between MC148R1 and CXCL12α.
Figure 5. Physical interaction of His-tagged MC148R1 and CXCL12α.
HeLa cells were infected or co-infected with the indicated viruses. Following 16 hr incubation of infected cells at 31°C, cell lysates were prepared and immunoprecipitated with anti-MC148R1 antibodies (Panels A, B, and C) or immunoprecipitated with anti-CXCL12α (Panels D, E, and F), fractionated on SDS-PAGE and immunoblotted. The blot was probed with anti-His antibodies (Panels A &D), stripped, reprobed with anti-MC148R1 antibodies (Panels B &E), stripped and finally probed with anti-CXCL12α antibodies (Panels C & F). Following primary antibody reactions the blots were washed and probed with anti-rabbit (MC148R1 or His tag antibodies) or anti-mouse (CXCL12α) antibodies conjugated to HRP. The blots were exposed to X-ray film after reacting with the HRP substrate.
MC148R1 but not MC148R2 displaces CXCL12α binding
To determine whether physical interaction with chemokine altered binding of CXCL12α to CXCR4 we performed experiments where control IL-8, MC148R1, MC148R2, C30/31A, or C95/101A were mixed with cold CXCL12α. The mixtures were incubated at room temperature for 30 min then 10ng/ml of I−125-labeled CXCL12α was added to each protein mixture. The mixtures containing the I−125-labeled CXCL12α were then added to CEM cells. The controls in this experiment were either cold CXCL12α or cold CXCL12α+IL-8 at increasing concentrations of cold proteins. We have previously determined that binding of CXCL12α to CEM cells is very sensitive to AMD3100 indicating it is mediated through CXCR4 (Altenburg et al., 2007; Altenburg et al., 2010). The results show that cold CXCL12α alone or mixed with IL8, C30/31A and MC148R2 had no significant effect on the binding affinity of CXCL12α. However, the binding was significantly reduced in the presence of either MC148R1 or C95/101A. For example, in the MC148R1+cold CXCL12α or C95/101A+cold CXCL12α mixtures higher concentrations of cold CXCL12α were required to result in 50% displacement of bound 125I-CXCL12α (Fig. 6). We consistently observed at least a 4–5 times higher binding affinity for CXCL12α alone or treated with IL-8, C30/31A, MC148R2 than cold CXCL12α treated with MC148R1 or C95/101A (Fig. 6). Reduced binding affinity was observed as a result of mixing cold CXCL12α with C95/101A but not with C30/31A (Fig. 6). These results demonstrate that interaction of CXCL12α with MC148R1 but not MC148R2 results in the displacement of CXCL12α by reducing the chemokine’s binding affinity.
Figure 6. Binding analysis of CXCL12α in the absence and presence of MC148R proteins.
Cold CXCL12α alone or mixed with equivalent amounts of the indicated proteins at increasing concentrations were incubated for 30 min at room temperature. A fixed amount of I−125-labeled CXCL12α (10ng/ml) was added to each chemokine mixture then added to CEM cells. The cell-chemokine mixtures were incubated at 4°C for 1 h and then washed three times with PBS-0.1% BSA. The cell pellet-associated counts were measured in a gamma counter. The 0 ng/ml point represent the binding of I−125-labeled CXCL12α alone with no cold chemokine added. The results are representative of three different experiments performed in triplicates. CPM, counts per minute.
DISCUSSION
Chemokines are defined by four invariant cysteine residues in their N-termini that form disulfide bonds (reviewed in (Fernandez and Lolis, 2002)). The first cysteine in the sequence forms a covalent bond with the third, and the second cysteine forms a disulfide bond with the fourth cysteine. The chemokine-like proteins MC148R1 and MC148R2 are the only viral chemokine homolog that has been shown to inhibit chemotaxis of multiple leukocyte subsets induced by CC and CXC chemokines (Damon et al., 1998; Krathwohl et al., 1997). In contrast, another study found no inhibition of chemotaxis of monocytes, lymphocytes and neutrophils using several CC and CXC chemokines (Luttichau et al., 2000). MC148R1 and MC148R2 are among the few virally encoded chemokine homologues that contain two additional cysteines in their C-termini. vMIP-III encoded by KSHV has 6 cysteines in its primary sequence, but the additional two cysteines are placed at different positions. We hypothesized that the two additional cysteines might provide the optimal structure for the MC148R1 to act as a chemokine antagonist. Our results demonstrated that mutating the two extra C-terminal cysteines had no significant effect on the antagonistic activity of MC148R1. In contrast, mutating the conserved N-terminal cysteines in MC148R1 resulted in the loss of its functions in terms of inhibiting chemotaxis and abolishing CXCL12α-mediated X4 fusion. We show for the first time that vaccinia-expressed MC148R1 abolished the CXCL12α-mediated inhibition of HIV Env-mediated cell fusion. The results suggested that the determinants of MC148R1 involved in blocking chemotaxis might overlap with those involved in inhibiting CXCL12α-mediated blocking of X4 fusion.
The results reported here are not due to the fact that our proteins contain an N-terminal methionine and a C-terminal His-tag or due to any protein impurities that might represent a minor population of molecules that get eluted form the His-affinity columns. Several lines of evidence are provided to support our conclusions. First, we have previously demonstrated that met-CXCL12α-His-Tag was functionally indistinguishable from the commercially available CXCL12α that lacks the first methionine and the His-tag (Altenburg et al., 2007; Altenburg et al., 2010). Among the seven mutants of MC148R1 protein treated and purified the same way the only mutant that lost the MC148R1 antagonistic function was the C30/31A mutant. The other six mutants of MC148R1 were always functional in terms of blocking chemotaxis. Second, we have demonstrated that our purification procedure results in >90% pure chemokines as visualized by silver-stained gels. Finally, several of the commercially available chemokines also contain C-terminal His-tags. For example, the met-CXCL16-His-tag sold by R&D systems is functionally indistinguishable from the met-CXCL16-Flag (Flag sequence, DYKDDDDK) purified on Flag-affinity columns (Matloubian et al., 2000; Wilbanks et al., 2001).
The core of the chemokine structure is stabilized largely by the two disulfides and by hydrophobic interactions from one side of the C-terminal helix and a portion of the β-sheet (reviewed in (Fernandez and Lolis, 2002)). Previous studies have demonstrated that alanine mutagenesis of any of the cysteines of CXCL8 (IL-8) leads to loss of structure and function. The results of our current study demonstrated that the loss of the antagonistic function of MC148R1 resulted from mutating the conserved N-terminal cysteines. This suggested a critical role for the conserved cysteines and the N-terminus of MC148R1 in the observed antagonistic functions. Since the N-terminal cysteines form disulfide bonds with the third and fourth cysteines it is likely that mutating either of the conserved N-terminal cysteines will have a similar effect to our double cysteine mutant.
The first evidence for the importance of the N-terminus for the chemokine activity came from studies with IL-8, which binds with high affinity to CXCR1 and CXCR2 (reviewed in (Loetscher and Clark-Lewis, 2001)). It has been demonstrated by mutagenesis and by selective substitution or deletion that the chemotactic activity of IL-8 critically depends on the Glu-Leu-Arg residues, the so-called ELR motif, immediately preceding the first cysteine (reviewed in (Loetscher and Clark-Lewis, 2001)). The ELR motif is a characteristic feature of all CXC chemokines that act through CXCR1 and/or CXCR2. It contributes to the high-affinity binding and is the receptor-triggering moiety of the molecule. The CXCL12α, however, lacks the ELR motif and its chemotactic activity against CXCR4-expressing cells had been determined to be within the first eight N-terminal residues (reviewed in (Loetscher and Clark-Lewis, 2001)). As a mechanism for the antagonistic activity of MC148R1 we propose physical interaction and masking the chemokine’s functional N-terminus that is critical for binding the chemokine receptor. In support of this hypothesis we demonstrated decreased binding affinity of CXCL12α when mixed with MC148R1 but not with MC148R2.
The chemotaxis results reported in this study are in agreement with the previously published data by Damon et al and Krathwohl et al (Damon et al., 1998; Krathwohl et al., 1997) but not by Lattichau et al who reported a selective blocking of CCR8-mediated chemotaxis by MC148R1 (Luttichau et al., 2000). It is hard to reconcile all the previous literature since every study was different in terms of the source of the MC148R1 protein and the target cells used in the different assays.
However, our study is the first to report that MC148R2 does not block CXCL12α-mediated chemotaxis. The primary amino acid sequence of MC148R2 indicates 10 non-homologous residues when compared to MC148R1 (Krathwohl et al., 1997). It is possible that these residues contributed to the loss of CXCL12α-mediated chemotaxis by MC148R2. The ten non-homologous amino acid residues in MC148R1 and MC148R2 proteins might account for the observed difference in their biological activities. Detailed mutational analysis of both proteins might provide insight into the different functional domains involved.
Since N-terminal deletions in several homologous CC chemokines abolished their function we examined the effect of deleting the first 5 residues on MC148R function (reviewed in (Loetscher and Clark-Lewis, 2001)). The Δ26–30 MC148R1 mutant is a deletion of the first 5 amino acids after the signaling peptide and before the conserved cysteines. The idea behind doing this was that MC148R1 has a much shorter amino terminus than the other functional chemokines. We reasoned the shorter amino terminus resulted in efficient binding to the receptor but a lack of signaling through the receptor, making it a receptor antagonist. Our results showed that this N-terminal deletion (Δ26–30) was as functional as the wild type MC148R1 in terms of its antagonistic activity. The results suggested no critical role for the first five N-terminal residues of MC148R1 in its antagonistic activity.
It has been previously demonstrated that the BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity (Proudfoot et al., 2001). Since the MC148R1 primary amino acid sequence does not contain any BBXB motifs we hypothesized that introducing a BBXB motif might change the functional properties of this chemokine-like protein. L94R mutation introduced a BBXB domain in a homologous location in RANTES. Although the L94R bound better to mammalian cells (data not shown) it did not induce chemotaxis but preserved its wild type antagonistic function. Among all the MC148R1 constructs described in this study only the C30/31A mutant lost the antagonistic function. Even though MC148R1 is a chemokine-like protein it does not act as a chemokine, however, its antagonistic function is lost by the loss of the conserved cysteines whose loss in the CC chemokine results in the loss of the chemotactic function. The structural determinants involved in the antagonistic activities of MC148R1 remain to be investigated.
MATERIALS AND METHODS
Cells and antibodies
The HeLa, NIH 3T3, and LM (tk-) cell lines were maintained in Dulbecco Modified Eagle Medium (DMEM; Quality Biologicals, Gaithersburg, MD) containing 10% fetal bovine serum (FBS) and antibiotics. The CEM T Lymphoblast cell line was maintained in RPMI-1640 containing 10% FBS and antibiotics. Peripheral blood mononuclear cells were purchased from StemCell Technologies. Polyclonal antibodies against the MC148R1 protein were generated in rabbits against a KLH-conjugated peptide corresponding to the C-terminus of the protein (HKDLCPQIWSGCESL). The immunizations and serum collections were performed by Sigma Genosys (Sigma, TX). Monoclonal antibodies to CXCL12α were purchased from R&D Systems. Anti-His antibodies were purchased from Santa Cruz Biotechnology.
Construction of recombinant vaccinia viruses encoding MC148R1 or CXCL12α
The cDNA encoding MC148R1 was obtained from Dr. Bernard Moss, LVD, NIAID, NIH. Construction of recombinant vaccinia viruses encoding either His-tagged MC148R1 or CXCL12α protein was performed as previously described (Agrawal et al., 2004; Broder and Earl, 1999). Briefly, the cDNA fragment encoding the MC148R1 protein was inserted into pSC59 under the strong vaccinia virus early/late promoter and transfected into TK- cells that have been infected with the Western Reserve (WR) wild-type strain of vaccinia virus. WR was used as a negative vector control that does not encode any foreign protein. Positive plaques were identified by dot blot hybridization using P32-labeled MC148 cDNA. After 3 cycles of plaque purification on TK- cells virus stocks were prepared by infecting HeLa cells and frozen at −70°C until used.
Purification of E. Coli expressed recombinant His-tagged chemokines
We transfected the recombinant pET-32a/CXCL12α or pET-32a/MC148R1 vectors into AD494 (DE3) pLysS E. coli host for optimal expression. We have previously described the construction, expression, and purification of CXCL12 variants (Altenburg et al., 2007; Altenburg et al., 2010). Briefly, transformed bacteria harboring each recombinant plasmid were isolated into single colonies. A single colony was picked from each sample and used to inoculate 5 ml of LB containing the antibiotics 100µg/ml ampicillin, and 30µg/ml chloramphenicol and incubated at 37°C with shaking for 12–16 hours. The culture was then centrifuged for 5 min at 5000g and the supernatant discarded. The bacterial pellets were resuspended in 500ml LB containing 50µg/ml ampicillin and grown until the optical density (at wavelength 600nm) of the cultures reached 0.6. At this point, isopropyl β-D-thiogalacto-pyranoside (IPTG) was added to a final concentration of 0.6 mM and incubated at 30°C for 4 hrs to induce expression of recombinant proteins. The cultures were placed on ice for 5 min and the bacterial cells harvested by centrifugation. The cells were then frozen at −70°C until ready to use.
Bacterial pellets were suspended in 5 ml lysis buffer per pellet and rotated at room temperature for 1 hour. The lysates were then centrifuged, and the pellets containing inclusion bodies were denatured in buffer containing 6 M guanidine-HCl. The clarified supernatants were poured onto a Ni-NTA histidine-binding column (Sigma, St. Louis, MO) equilibrated with 10 column volumes of denaturing binding buffer. Both ends of the column were immediately capped and the columns were rotated at 4° C overnight. The denatured proteins were refolded on the column by a stepwise removal of the denaturant as previously described (Matsumoto et al., 2003). The columns were washed with decreasing levels of urea, followed by increasing levels of imidazole to remove nonspecific bacterial contaminants. The proteins were eluted with 2 ml elution buffer, and the elution buffer was exchanged for phosphate buffered saline (PBS) on a PD-10 desalting column. Protein concentrations were quantified by the Bradford assay (Bio-Rad, Hercules, CA), and analyzed for size and purity by SDS-PAGE. The proteins were also analyzed by western blotting using a polyclonal antibody to the histidine tag that is present in all constructs (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The relative concentrations of the purified proteins were converted to nanomolarity by using the predicted molecular weight of each recombinant.
Chemotaxis Assays
Chemotaxis assays were performed as previously described by our group (Altenburg et al., 2007; Altenburg et al., 2010). Briefly, for CXCL12α-mediated chemotaxis CEM T lymphoblast cells were suspended in chemotaxis medium (Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 0.5% bovine serum albumin (BSA) at a density of 2×106 cells/ml. The purified CXCL12α (100nM) with or without wild type or mutant MC148R1 (100nM) were suspended in chemotaxis medium. The suspended mixtures (in 0.6 ml) were first added to the wells of a 24-well plate followed by a 5-micron pore transwell membrane to each well (Costar, Corning Incorporated, Acton, MA). The cells were added to the inside of the transwell membrane at a volume of 0.1 ml (2×105 cells). The plates were then capped and incubated for four hours at 37°/6% CO2. After the incubation period, the transwell membranes were removed from the wells and the migrated cells counted by flow cytometry. For MIP-1α-mediated chemotaxis we used commercially available PBMCs (StemCell Technologies). PBMCs were resuspended in Dulbecco’s minimal essential medium (GIBCO/BRL) with antibiotics at a density of 2×106/ml. Chemotaxis was assayed as described above.
HIV-1 Env-mediated cell fusion assay
HIV-1 Env-mediated fusion was performed by a vaccinia-based reporter assay as previously described (Agrawal et al., 2004). Briefly, the target CD4+CXCR4+CCR5+ TZM-bl cells (AIDS Reagent Program, Bethesda, Maryland) (Chackerian et al., 1997) were infected by vaccinia virus encoding the bacteriophage T7 RNA polymerase. Effector HeLa cells were co-infected by PT7-lacZ vaccinia virus (lacZ reporter gene under the T7 promoter) and vaccinia virus encoding either the X4 LAV Env, or the control Unc, an uncleaved HIV-1 Env that has its cleavage site mutated and therefore cannot engage in membrane fusion. The Unc Env is used to measure the nonspecific background in the fusion assay. The target cells were plated in a 96 well plate at 1×105 cells per well and treated for one hour with either CXCL12α alone or a mixture of CXCL12α and MC148R1 protein or its mutants at 10µg/ml each. After one hour incubation at 37°/ 6% CO2, the Env-expressing HeLa cells were mixed with the target cells at 1:1 ratio. The cell mixtures were incubated for 2.5 hours at 37°C then lysed and the substrate chlorophenolred-β-D-galactopyranoside (CPRG) was added. The extent of cell fusion was assayed by measuring β-galactosidase produced. In other Env-mediated fusion experiments the vaccinia viruses encoding either CXCL12α or MC148R1 were used to express the chemokines.
Immunoprecipitation and immunoblotting
NIH 3T3 cells were either infected with recombinant vaccinia viruses vMC148R1 or vCXCL12α or co-infected with both viruses. The optimum time of expression of each protein was determined by time course experiments and found to be 16 hr post-infection. Cytoplasmic extracts were cleared and prepared for immunoprecipitation experiments. Cell lysates were immunoprecipitated with polyclonal antibodies (purified IgG fraction) specific for the MC148R1 or monoclonal antibodies to CXCL12α and proteins fractionated on a 15% SDS-PAGE. The gel was blotted and the blot sequentially probed with anti-His, stripped and re-probed with anti-MC148R1 antibodies, stripped then probed with anti-CXCL12α antibodies. The blots probed with anti-MC148R1 rabbit antibodies were washed and reacted with anti-rabbit-HRP conjugated antibodies. Following washing and reacting with the HRP substrate the blot was exposed and to X-ray film and developed.
Binding assays
Direct binding experiments involved the use of 125I-labeled CXCL12α (home-made using standard labeling procedures) and cold recombinant CXCL12α and MCV proteins. Cold CXCL12α alone or mixed with IL-8, MC148R1, MC148R2, C30/31A, or C95/101A were incubated for 30 minutes at room temperature then 125I-labeled CXCL12α added to each mixture. The new chemokine mixtures containing 10ng/ml 125I-labeled CXCL12α (specific activity of 2,200 Ci/mmol) were then added to CEM cells (1 million cells/tube, in triplicate). The cell-chemokine mixtures were incubated at 4°C for 1 h and then washed three times with PBS-0.1% BSA. The cell pellet-associated counts were measured in a gamma counter and 125I-labeled CXCL12α binding measure in counts per minute (CPM).
ACKNOWLEDGMENTS
We acknowledge the help of the core facility of the Biomedical Sciences at Texas Tech University Health Sciences Center, El Paso for providing DNA sequencing data. We thank Jon Mohl for an outstanding technical assistance. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc. This study was supported by NIH grant # 2R01AI052019 for GA and by a grant from National Natural Science Foundation of China (Grant # 30870125) for J.Q.
The Abbreviations used are
- PBMC
peripheral blood mononuclear cells
- Env
envelope glycoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal L, Lu X, Qingwen J, VanHorn-Ali Z, Nicolescue V, McDermott D, Murphy PM, Alkhatib G. Role for CCR5Δ32 protein in resistance to R5, R5×4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. J Virol. 2004;78:2277–2287. doi: 10.1128/JVI.78.5.2277-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcami A, Symons JA, Collins PD, Williams TJ, Smith GL. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- Alkhatib G. The biology of CCR5 and CXCR4 +Curr Opin HIV AIDS. 2009;4:96–103. doi: 10.1097/COH.0b013e328324bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburg JD, Broxmeyer HE, Jin Q, Cooper S, Basu S, Alkhatib G. A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities. J Virol. 2007;81:8140–8148. doi: 10.1128/JVI.00268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburg JD, Jin Q, Alkhatib B, Alkhatib G. The Potent Anti-HIV Activity of CXCL12{gamma} Correlates with Efficient CXCR4 Binding and Internalization. J Virol. 2010 doi: 10.1128/JVI.00342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenberg DA, Zlotnick A, Strom SR, Burdick MD, Strieter RM. The murine CC chemokine, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol Immunother. 2001;49:587–592. doi: 10.1007/s002620000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Earl PL. Recombinant vaccinia viruses. Design, generation, and isolation [In Process Citation] Mol Biotechnol. 1999;13:223–245. doi: 10.1385/MB:13:3:223. [DOI] [PubMed] [Google Scholar]
- Chackerian B, Long EM, Luciw PA, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore GM, Gronenborn AM. Three-dimensional structures of alpha and beta chemokines. Faseb J. 1995;9:57–62. doi: 10.1096/fasebj.9.1.7821760. [DOI] [PubMed] [Google Scholar]
- Damon I, Murphy PM, Moss B. Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc Natl Acad Sci U S A. 1998;95:6403–6407. doi: 10.1073/pnas.95.11.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annual review of pharmacology and toxicology. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS. 2008;19:503–506. doi: 10.1258/ijsa.2008.008186. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Hromas R, Brown DR, Broxmeyer HE, Fife KH. Functional characterization of the C---C chemokine-like molecules encoded by molluscum contagiosum virus types 1 and 2. Proc Natl Acad Sci U S A. 1997;94:9875–9880. doi: 10.1073/pnas.94.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani AS, Ness TL, Singh R, Harrison JK, Seet BT, Kelvin DJ, McFadden G, Moyer RW. Functional comparisons among members of the poxvirus T1/35kDa family of soluble CC-chemokine inhibitor glycoproteins. Virology. 1998;250:173–184. doi: 10.1006/viro.1998.9340. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Clark-Lewis I. Agonistic and antagonistic activities of chemokines. Journal of leukocyte biology. 2001;69:881–884. [PubMed] [Google Scholar]
- Luttichau HR, Stine J, Boesen TP, Johnsen AH, Chantry D, Gerstoft J, Schwartz TW. A highly selective CC chemokine receptor (CCR)8 antagonist encoded by the poxvirus molluscum contagiosum. J Exp Med. 2000;191:171–180. doi: 10.1084/jem.191.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Misawa S, Tsumoto K, Kumagai I, Hayashi H, Kobayashi Y. On-column refolding and characterization of soluble human interleukin-15 receptor alpha-chain produced in Escherichia coli. Protein Expr Purif. 2003;31:64–71. doi: 10.1016/s1046-5928(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Murphy PM. Viral antichemokines: from pathogenesis to drug discovery. The Journal of clinical investigation. 2000;105:1515–1517. doi: 10.1172/JCI10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. Journal of Virology. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot AE, Fritchley S, Borlat F, Shaw JP, Vilbois F, Zwahlen C, Trkola A, Marchant D, Clapham PR, Wells TN. The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity. J Biol Chem. 2001;276:10620–10626. doi: 10.1074/jbc.M010867200. [DOI] [PubMed] [Google Scholar]
- Senkevich TG, Bugert JJ, Sisler JR, Koonin EV, Darai G, Moss B. Science. Vol. 273. New York, N.Y: 1996. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes; pp. 813–816. [DOI] [PubMed] [Google Scholar]
- Smith CA, Smith TD, Smolak PJ, Friend D, Hagen H, Gerhart M, Park L, Pickup DJ, Torrance D, Mohler K, Schooley K, Goodwin RG. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236:316–327. doi: 10.1006/viro.1997.8730. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrobial agents and chemotherapy. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]