Summary
The mammalian target of rapamycin (mTOR) is an intracellular kinase that regulates cell growth and metabolism. Its specific inhibitor rapamycin is currently used in transplant recipients as an immunosuppressive drug to prevent allograft rejection. Studies have shown complex and diverse mechanisms for the immunosuppressive effects of rapamycin. The drug has been reported to inhibit T-cell proliferation, induce anergy, modulate T-cell trafficking, promote regulatory T cells, and also prevent maturation of dendritic cells as well as production of type I IFN production. However, several other studies have paradoxically demonstrated immunostimulatory effects of rapamycin by improving antigen presentation and regulating cytokine production from macrophages and myeloid dendritic cells. Recently, it has been shown that rapamycin also exhibits immunostimulatory effects on memory CD8+ T-cell differentiation. The drug improved both quantity and quality of memory CD8+ T cells induced by viral infection and vaccination, showing that mTOR is a major regulator of memory CD8+ T-cell differentiation. These discoveries have implications for the development of novel vaccine regimens. Here we review the role of mTOR in memory CD8+ T-cell differentiation and compare the effect of rapamycin between CD8+ T cells, CD4+ T cells, and dendritic cells. Also we discuss potential application of these findings in a clinical setting.
Keywords: mTOR, rapamycin, vaccination, infection, T cells, immune memory
Introduction
Memory CD8+ T cells are distinct in both quantity and quality from their naive predecessors (1-5). It is important to understand how the differentiation of memory CD8+ T cells results in the acquisition of such distinct properties and to use this information to develop new vaccines as well as improve upon existing vaccine regimens. There has been substantial progress made over the past few years in better defining the phenotypic and functional changes that occur in the course of memory CD8+ T-cell differentiation that results in long-lasting protective T-cell immunity.
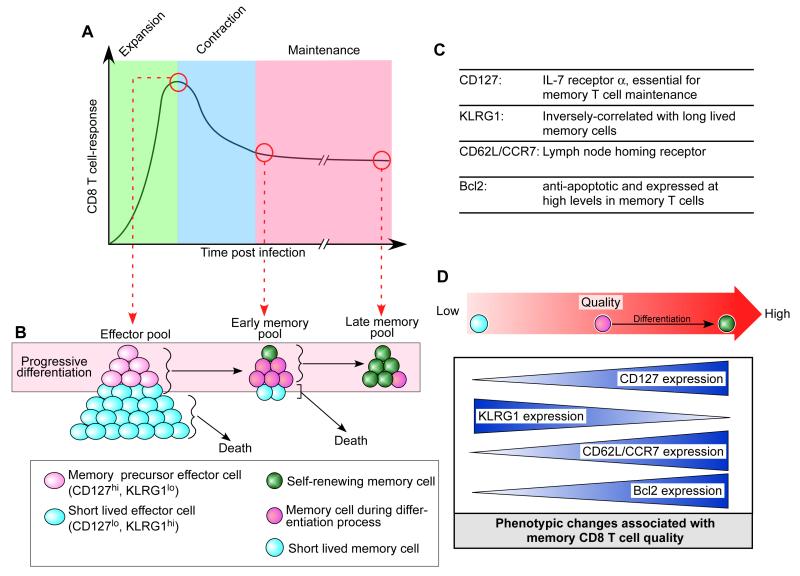
During an acute infection, where pathogens are cleared, antigenic stimulation and inflammatory signals such as type I interferon (IFN) and interleukin-12 (IL-12) initiate a rapid and substantial clonal expansion of naive antigen-specific T cells that become effector T cells 1-2 weeks after infection (6-10) (Fig. 1A). In this expansion phase, many phenotypic and functional changes occur in antigen specific T cells. One important phenotypic change for memory T-cell differentiation is CD127 (IL-7 receptor α) re-expression. It is known that CD127 re-expression distinguishes memory T-cell precursors in an effector T-cell pool at the peak of CD8+ T-cell response (11, 12). CD127 is highly expressed on naive T cells but it is uniformly downregulated on all antigen-specific CD8+ T cells after activation. This CD127lo T-cell population includes KLRG1hi and KLRG1lo/int cells, and the latter form memory T-cell precursors by re-expressing CD127 subsequently (7, 13). Thus, at the peak of a CD8+ T-cell response, naive T-cell expansion results in two distinct subsets (short-lived effector cells and memory precursor effector cells) that can be distinguished by expression of the cell surface markers CD127 and KLRG1 (7, 11-13) (Fig. 1B, C). The short-lived effector cells account for 90~95% of the effector population and have a CD127lo KLRG1hi phenotype with the majority of them dying during the effector to memory transition phase (Fig. 1B, C), albeit with some remaining in the memory phase. However, these persisting short-lived CD127lo KLRG1hi cells in the memory pool have poor longevity as observed by a waning in their quantity over time compared to the memory cells that arise from memory precursor effector cells (7, 13)(Fig. 1B). However, the memory precursor effector cells that express high levels of CD127 and lower levels of KLRG1 efficiently survive the contraction phase, acquire memory T-cell properties, and constitute the majority population of cells in the memory pool (7, 11-13) (Fig. 1B, C). Finally, a memory CD8+ T-cell pool consists of a high quantity of antigen specific T cells, 10-1000-fold greater than the original naive population (10, 14, 15) (Fig. 1A).
Fig. 1. Memory CD8+ T-cell differentiation.
(A) Antigen-specific CD8+ T-cell responses after an acute infection. During the expansion phase, naive CD8+ T cells proliferate and then become effector cells. After clearance of the pathogen, 90 to 95% of the effector T cells die during a contraction phase. The surviving 5-10% of the antigen-specific T cells become the memory population. (B) Progressive memory CD8+ T-cell differentiation and the subsets of effector and memory CD8+ T cells. (C) Several key cell surface and intracellular markers for memory CD8+ T-cell differentiation. (D) Some of the key phenotypic changes associated with memory CD8+ T-cell quality.
These memory CD8+ T cells generated from the precursors further differentiate into self-renewing memory T cells that can persist in the absence of antigen (16-19)(Fig. 1B). The extended life span is in part dictated by IL-7-/IL-15-dependent homeostatic proliferation characterized by slow cell division with minimal changes of the total number (20-23). In addition, these self-renewing memory T cells have an enhanced recall response to a cognate antigen and heightened protective capacity against re-infection compared to early-stage memory T cells (19)(Fig. 1D). This progressive improvement of memory T-cell qualities occurs along with many phenotypic changes including upregulation of Bcl2 (anti-apoptotic) (24), re-expression of CD62L/CCR7 (lymph node homing receptors), and higher levels of CD127 (11, 19, 25) (Fig. 1B-D). These phenotypic changes seem to provide memory T cells superior functional qualities for effective immunological memory.
It is known that several transcription factors such as T-bet, eomesodermin, and Blimp-1 play an important role in the differentiation and phenotypic changes of memory T cells (7, 26-30). Recently, mTOR has also been identified as a major regulator of memory CD8+ T-cell differentiation (31). Specifically, it has been shown that the mTOR inhibitor rapamycin, a common immunosuppressive drug currently used in transplant recipients, can modulate memory T-cell differentiation (31, 32). In this review, we discuss our current understanding of the role of mTOR in memory CD8+ T-cell differentiation and also discuss potential application of rapamycin and its concerns in a clinical setting.
The mTOR signaling cascade
Rapamycin was initially discovered as a potent anti-fungal macrolide that is produced by the bacterial strain Streptomyces hygroscopicus, isolated from Easter Island (33)(Fig. 2). Thereafter, it was found that rapamycin was able to inhibit proliferation of mammalian cells. This discovery spawned new areas of research focused on the effects of rapamycin in hopes of generating anti-cancer compounds and immunosuppressive drugs (33-35). Recent clinical data have shown that rapamycin and its analogs have some anti-cancer activity against certain types of cancers such as endometrial cancers and renal cell carcinoma (35, 36). Furthermore, rapamycin has now became one of the more well known immunosuppressive drugs currently used in transplant recipients (37). The interesting and attractive manipulative ability that rapamycin has on cell growth also promoted research efforts towards understanding the primary mechanism of action by rapamycin (33).
Fig. 2.
Structure of rapamycin.
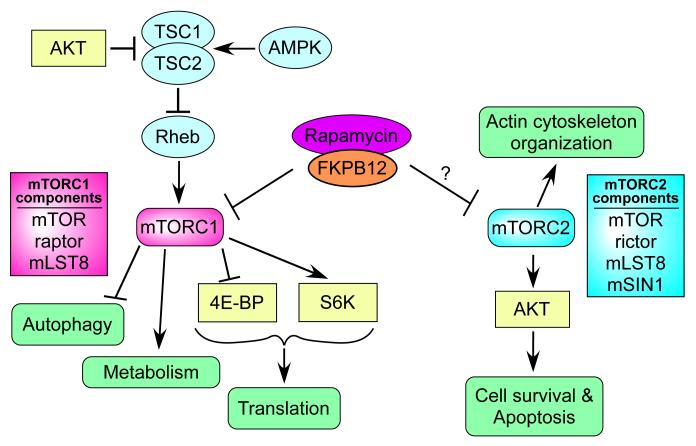
The target of rapamycin (TOR) was originally identified in yeast (38) followed by the discovery of the mammalian homolog (mTOR) (39-41). mTOR is a protein kinase ubiquitously expressed by many cells with the downstream signals regulating cell growth and metabolism in response to environmental cues (33). mTOR forms two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 consists of mTOR, raptor, and mLST8, and controls several important aspects for cell growth and homeostasis (34, 42)(Fig. 3). One of the best-studied areas of mTORC1 research is its translational control induced by phosphorylating 4E-BP1 and S6K1 (43) (Fig. 3). In addition, it is now known that mTORC1 regulates other key cellular activities such as macroautophagy, transcription, and metabolism (33). The activity of mTORC1 can be controlled by TSC-Rhe pathway (44). TSC (TSC1-TSC2 complex) is a negative regulator for mTORC1 and inhibits Rhe, which directly activates mTORC1 (Fig. 2). Further, the activation of TSC is regulated by upstream signaling molecules such as Akt and AMPK, TSC inhibiting and activating molecules respectively (33, 43, 44) (Fig. 3). Thus, an Akt signal activates mTORC1 while AMPK suppresses activation of mTORC1 indirectly. Rapamycin potently inhibits mTORC1 activity. Specifically, rapamycin binds to its intracellular partner FKBP12 and this FKBP12-rapamycin complex effectively prevents activation of mTORC1 through a direct interaction with mTORC1 (39-41) (Fig. 3).
Fig. 3. The mTOR signaling cascade.
The kinase mTOR forms two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Rapamycin needs to bind to the intracellular partner FKBP12 to exert its effect in the target cell. Rapamycin-FKBP12 complex inhibits activation of mTORC1 by directly binding to mTORC1. mTORC2 is not inhibited directly by rapamycin, but prolonged rapamycin treatment reduces the mTORC2 activity in some cells. Arrows and bars represent activation and inhibition, respectively.
Less is known about the biological role of mTORC2 and the signals necessary for its activation. In contrast to mTORC1, mTORC2 is generally insensitive to rapamycin, although the reduction of mTORC2 activity was reported in some cell types after prolonged rapamycin treatment (45). mTORC2 consists of mTOR, mLST8, rictor, and mSIN1 and regulates the organization of actin cytoskeleton most likely mediated through protein kinase C-α (PKCα) and the small guanosine triphosphatases (GTPases) Rho and Rac (33) (Fig. 3). mTORC2 can also regulate activation of Akt by phosphorylating the specific site at serine 473 (46, 47). This phosphorylation causes strong activation of Akt, and promotes cell survival (Fig. 3).
mTOR and memory CD8+ T-cell differentiation
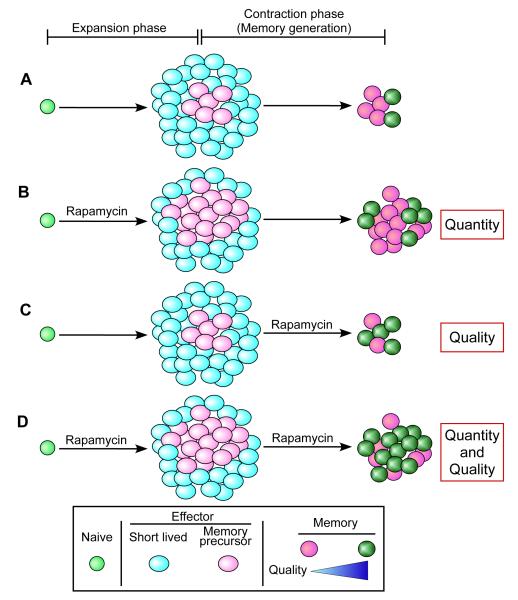
Memory CD8+ T-cell differentiation after infection or vaccination is controlled by complex mechanisms in which several molecules such as T-bet, eomesodermin, and Blimp-1 are known to play an important role (7, 26-30). Recently, we have identified mTOR as a major regulator of memory CD8+ T-cell differentiation (31). In vivo administration of rapamycin into mice infected with the acute strain of lymphocytic choriomeningitis virus (LCMV) significantly changes the fate of antigen-specific CD8+ T cells (31). Rapamycin treatment during the T-cell expansion phase (days 0-8 post infection) increases the quantity of memory CD8+ T cells compared to untreated animals (Fig. 4A, B). This result is due to decreased contraction of the antigen-specific T cells in rapamycin-treated mice. Thus, rapamycin treatment results in a similar number of antigen-specific effector CD8+ T cells at the peak of the T-cell response on day 8 post infection compared to untreated mice but reduces the number of apoptotic cells in drug-treated animals (31). The underlying mechanism for this decreased contraction is that rapamycin promotes the generation of memory precursor effector cells that survive and differentiate into long-lived memory (Fig. 4B). Thus, inhibiting mTOR alters the development process of short-lived and memory precursor effector cells.
Fig. 4. Rapamycin improves the quantity and quality of memory CD8+ T cells.
(A) Without rapamycin treatment. (B) Rapamycin treatment during T-cell expansion phase. Rapamycin increases memory precursor effector cells that survive during the contraction phase. Higher quantity of memory T cells is formed compared to control. (C) Rapamycin treatment during T-cell contraction phase. Rapamycin accelerates effector to memory T-cell formation, and improves quality of memory T cells. (D) Rapamycin treatment during both the expansion and contraction phase. Rapamycin improves both quality and quantity of memory CD8+ T cells.
In addition to modulating the T-cell expansion phase, we have found that mTOR signaling influences the T-cell contraction phase when memory properties are acquired (31). Inhibition of mTOR with rapamycin only during the T-cell contraction phase (days 8-30) accelerates effector to memory T-cell transition and improves the quality of memory CD8+ T cells (31) (Fig. 4C). Interestingly, rapamycin treatment during the contraction phase does not enhance the number of memory T cells formed (31) (Fig. 4C). Thus, the rapamycin effects are qualitative and not quantitative, and the number of highly functional memory CD8+ T cells (CD127hi, CD62Lhi, KLRG1lo, CD27hi, Bcl2hi) generated in rapamycin-treated mice is significantly increased (31) (Fig. 4C). Indeed, memory CD8+ T cells derived from rapamycin-treated mice exhibit a better recall response and protective capacity as well as longevity (31). Consequently, rapamycin treatment during both the expansion and contraction of a T-cell response enhances not only the magnitude but also the quality of antigen-specific memory CD8+ T cells (31) (Fig. 4D).
In an independent study, Pearce et al. (32)found similar effects of rapamycin in their system using mice with a conditional deletion of tumor necrosis factor receptor-associated factor 6 (TRAF6) in T cells. These mice with TRAF6-deficient CD8+ T cells formed normal effector responses after immunization, but failed to generate long-lived memory T cells. TRAF6-deficient CD8+ T cells were defective in adenosine monophosphate (AMP)-activated kinase activation and mitochondrial fatty acid oxidation (FAO) in response to growth factor withdrawal (32). When they administered rapamycin, it restored FAO and promoted memory CD8+ T-cell development not only in the TRAF6-defective T cells but also wildtype T cells (32). Thus, this study performed by Pearce et al. (32) also shows that in vivo administration of rapamycin accelerates memory CD8+ T-cell differentiation.
Does rapamycin directly target antigen-specific CD8+ T cells to modulate memory T-cell differentiation, or does another cell affected by rapamycin mediate the acceleration of memory T-cell formation? The answer to this question is essential in understanding and defining the fundamental role of mTOR in memory CD8+ T-cell differentiation. Experiments using RNA interference performed by us resolved this critical issue (31). Knocking down mTOR or raptor (another critical component of mTORC1) (Fig. 3) expression in antigen-specific CD8+ T cells resulted in enhancement of memory CD8+ T-cell quality similar to rapamycin treatment (31), suggesting that rapamycin exerts its effect on memory T-cell differentiation by directly acting on mTORC1 in antigen-specific CD8+ T cells. Furthermore, experiments where FKBP12 was knocked down also supported intrinsic effect of rapamycin (31). FKBP12 is an intracellular binding partner of rapamycin, and this FKBP12-rapamycin complex inhibits the mTORC1 pathway. Knocking down FKBP12 in antigen-specific CD8+ T cells makes these cells insensitive to any intrinsic effects of rapamycin, while the drug could still act effectively on all the other cells in the mouse. Using this system, we showed that in vivo administration of rapamycin failed to improve memory T-cell differentiation in rapamycin-insensitive antigen-specific CD8+ T cells with FKBP12 knockdown (31). In the same mouse, however, the treatment still promoted memory formation of wildtype antigen-specific CD8+ T cells (31). These data obtained by RNA interference (RNAi) experiments show that rapamycin acts intrinsically in antigen-specific CD8+ T cells to accelerate memory CD8+ T-cell differentiation (31).
Immunosuppressive effects of rapamycin
It has been known for a long time that rapamycin has an ability to suppress immune responses in animal models, and the primary effect was thought to be due to inhibition of T-cell proliferation. However, it appears that rapamycin’s strong inhibition on proliferation is only observed when T cells receive T-cell receptor (TCR) stimulation without costimulatory molecules or IL-2 receptor signaling (48, 49). These findings suggest that the anti-proliferative effect is only one aspect of rapamycin ability to induce immunosuppression. Indeed, it is now known that rapamycin causes anergy of proliferated T cells even in the presence of costimulation (50).
Recent findings have demonstrated that the immunosuppressive effect of rapamycin may also be mediated through T-regulatory (Treg) cells (51). Treg cells play an essential role in preventing autoimmune diseases and express the transcription factor forkhead box protein 3 (Foxp3), which confers Treg cell function (52, 53). Treatment of Treg cells with rapamycin in vitro does not impair the ability to suppress effector T-cell proliferation. Rather, it promotes expansion of preexisting Treg cells (54) or induces regulatory function by expressing Foxp3 in conventional T cells (55, 56). Interestingly, the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 similarly upregulates Foxp3 expression after in vitro culture (57), and naive CD4+ T cells transduced with a recombinant retrovirus expressing a constitutively active Akt fail to upregulate Foxp3 in the presence of transforming growth factor-β (TGF-β), which is a strong inducer of Foxp3 under normal circumstance (58). Furthermore, rapamycin treatment counteracts the effect of a constitutively active Akt and promotes Foxp3 expression (58). A recent study (59) has suggested that the Akt/mTOR signaling that negatively regulates both thymic development and function of Treg cells is mediated by sphingosine 1-phosphate (S1P) through S1PR1, a cell surface receptor for S1P. Taken together, these results indicate that the PI3K/Akt/mTOR signal negatively regulates Foxp3 expression, thereby controlling the number of Treg cells.
A recent report using mice with a conditional deletion of mTOR in T cells has shown that mTOR regulates not only the generation of Treg cells but also effector T-cell differentiation (60). The data show that mTOR-deficient CD4+ T cells efficiently differentiate into Treg cells upon stimulation compared to wildtype. In contrast, differentiation into T-helper 1 (Th1), Th2, or Th17 cells was severely inhibited in mTOR-deficient CD4+ T cells even in the presence of appropriate polarizing cytokines (60). Consistent with this observation, mTOR deficiency results in decreased phosphorylation of signal transducer and activator of transcription 3 (STAT3)/STAT4/STAT6 in response to the polarizing cytokines and a failure to upregulate lineage specific transcription factors (60). Thus, a functioning mTOR signal in CD4+ T cells promotes activation and differentiation of effector T cells and inhibits induction of Treg cells.
In addition to the role mTOR plays in CD4+ T-cell differentiation, mTOR can modulate T-cell trafficking by regulating expression of the key lymph node-homing receptors CD62L and CCR7 (61). Activated T cells downregulate CD62L and CCR7 and migrate into peripheral tissues to the site of infection. Treatment of activated T cells with rapamycin increases expression of both CD62L and CCR7. The upregulation of these molecules is associated with elevated levels of the transcription factor KLF2, a key modulator of these homing receptors (61). These data suggest that rapamycin might exert immunosuppressive effects by changing T-cell trafficking properties and preventing cells form going to effector sites.
Rapamycin also has a variety of effects on dendritic cells (DCs) (51). DCs play a critical role in producing inflammatory cytokines providing a first line of defense against infectious pathogens (62). DCs also promote T-cell activation by presenting antigens to T cells (63, 64). mTOR inhibition seems to suppress differentiation and maturation of DCs, such that DCs exposed to rapamycin have an impaired ability to stimulate T cells (65). Furthermore, rapamycin treatment of DCs enhances their capacity to induce activation and proliferation of Treg cells (66). Therefore these effects of rapamycin on DCs may also contribute to the immunosuppression in patients being treated with rapamycin. In addition to controlling proper maturation of DCs, mTOR signaling regulates cytokine production from DCs. Plasmacytoid DCs (pDCs) are an essential immune component that produce type I IFN for host defense to viral infection (67). Rapamycin treatment of pDCs in vitro inhibits type I IFN production through suppression of IRF7 activity (68). Consistently, in vivo administration of encapsulated rapamycin, which is taken up by only antigen-presenting cells, reduces type I IFN production in serum after viral infection (68). Thus, the mTOR pathway in DCs plays a critical role in both innate and adaptive host defense.
Immunostimulatory effects of rapamycin
The examples mentioned above describe immunosuppressive effects of rapamycin on both innate and adaptive immunity through several different mechanisms. However, in addition to the stimulatory effects of rapamycin on CD8+ T-cell responses (31, 32), recent reports have shown that rapamycin can also enhance immune responses by modulating cytokine production. Macrophages and myeloid DCs (mDCs) treated with rapamycin produce larger amounts of IL-12 and less IL-10 upon stimulation with Toll-like receptor (TLR) ligands or bacteria compared to cells without rapamycin treatment (69, 70). Since IL-12 induces CD4+ T-cell differentiation into Th1 or Th17 cells and IL-10 suppresses immune responses, T cells stimulated with rapamycin treated mDCs or macrophages induce higher levels of IFN-γ and IL-17 (69, 70). Consistent with these in vitro immunostimulatory studies, brief rapamycin treatment of mice in vivo before infection with Listeria monocytogenes results in better protection and faster control of the bacteria compared to untreated mice (69). In addition, mTOR appears to regulate antigen presentation in macrophages and DCs by modulating autophagy (71), a lysosomal degradation pathway. Rapamycin-induced autophagy of DCs enhances the ability of the DCs to prime T cells in vitro (71). Similarly, in vivo, mice vaccinated with live attenuated mycobacteria-infected DCs that are pretreated with rapamycin induce higher Th1 responses and show better protection after challenge with virulent Mycobacterium tuberculosis (71).
Are CD4+ and CD8+ T-cell responses differentially influenced by mTOR signaling?
As previously discussed, experiments using mTOR-deficient T cells demonstrate that mTOR signaling is essential for the differentiation of effector CD4+ T cells including Th1, Th2, and Th17 cells (60). However, it appears that inhibiting mTOR signal by rapamycin enhances CD8+ T-cell responses (31). This observation suggests that mTOR may differentially regulate CD4+ and CD8+ T-cell responses. However, in contrast to the results seen with mTOR-deficient CD4+ T cells (60), we observed that rapamycin-treated LCMV-infected mice had higher number of antigen-specific memory CD4+ T cells (unpublished data), suggesting the drug also enhanced memory CD4+ T-cell differentiation. This discrepancy between genetic deletion of mTOR and rapamycin administration might be explained by the degree of inhibition of the mTOR signal. Experiments describing inhibition of effector CD4+ T-cell differentiation were performed with mTOR-deficient CD4+ T cells in which the gene encoding mTOR was genetically deleted. Genetic deletion of mTOR results in a complete block on mTOR signaling, quite different from rapamycin-mediated inhibition of mTOR.
Rapamycin can effectively and specifically inhibit mTOR, but in vivo administration of the drug cannot completely block the signal and provides dose-dependent mTOR inhibition. Similarly, incomplete inhibition of mTOR is seen in CD8+ T cells when mTOR expression was knocked down by RNA interference. This finding might suggest that rapamycin stimulates memory CD8+ T-cell formation by incompletely inhibiting the mTOR signal. Consistent with this notion, efficient inhibition of mTOR using a higher dose of rapamycin, relative to the optimal dose which accelerates memory T-cell differentiation, resulted in suppression of CD8+ T-cell expansion (31). Thus, it seems that mTOR signaling has evolved as a mechanism to both restrain memory T-cell development while providing an essential signal for CD8+ T-cell expansion. This model may also help explain why complete loss of mTOR inhibits CD4+ T-cell activation but in vivo rapamycin administration improves memory CD4+ T-cell formation.
Another major difference between mTOR-deficient T cells and rapamycin treatment is mTORC2 signaling. mTOR-deficient T cells completely lack both mTORC1 and mTORC2 signals. Delgoffe et al. (60) have suggested that along with mTORC1, mTORC2 may also have an effect on CD4+ T-cell differentiation. As stated previously, rapamycin can inhibit both mTORC1 and mTORC2 but the inhibitory effect on mTORC2 is limited to certain cell types (33, 42, 45). It remains to be determined if in vivo administration of the drug suppresses mTOCR2 in CD8+ T cells. Therefore, more detailed studies are needed to elucidate the role of mTORC2 in memory CD8+ T-cell differentiation.
Viral infection vs. tissue transplant: the observed differential effects of rapamycin in two types of antigenic environments
How can rapamycin, a drug administered to transplant recipients to suppress their immune system (37), improve the quality and quantity of memory CD8+ T cells during viral infection (31)? It seems that something other than the dose of rapamycin contributes to the different effects of rapamycin between viral infection and a transplant setting, because similar blood concentration levels of rapamycin were used in both situations. One possible explanation is that viral infection has a potent ability to activate the innate immune system. DCs play an integral role in the initiation of an immune response. There are several reports suggesting that rapamycin inhibits the maturation and activation of DCs (65, 66), but virus infection efficiently activates DCs through TLR signaling compared to organ transplantation. Thus, rapamycin may still allow for a basal level of DC activation after viral infection in vivo. In contrast to inhibitory effects of rapamycin on DCs, other studies have shown that myeloid DCs treated with rapamycin have an improved ability to activate T cells during infections (69, 70). Such DCs activated by infection might diminish immunosuppressive effects of rapamycin during the course of infection.
Another possible explanation is that transplant recipients take several immunosuppressive drugs together to avoid organ rejection (37). This is indeed the case in rapamycin-based immunosuppressive regimens. Rapamycin is usually given to transplant patients along with other drugs such as corticosteroid and mycophenolate mofetil (37). Therefore, rapamycin might inhibit the immune response only when combined with other therapies, or rapamycin’s immunostimulatory effects may be masked by other drugs.
A recent large clinical trial in renal transplant recipients in which rapamycin was administered right after transplantation showed that the rate of biopsy-proven acute rejection was significantly higher in patients treated with rapamycin than in those treated with the calcineurin inhibitors tacrolimus or cyclosporine (72). Conversely, there are several reports suggesting beneficial protective effects of rapamycin against viral diseases. Rapamycin-based immunosuppressive regimens reduced the risk of cytomegalovirus infection in transplant recipients compared with other drug regimens (73, 74). Also, in another study, conversion from cyclosporine to a rapamycin-based treatment regimen was associated with a restoration of herpesvirus-8-specific T-cell responses (75). Further basic and clinical studies will be needed to investigate rapamycin effects on antiviral T-cell responses and on allograft survival.
Targeting mTOR to enhance vaccine immunogenicity
To develop a successful vaccine against pathogens such as human immunodeficiency virus (HIV), tuberculosis (TB), hepatitis C virus (HCV), etc., it will be essential for the vaccine to induce not only neutralizing antibody responses but also to generate highly effective T-cell immunity (2, 76). In particular, inducing a higher number of long-lasting memory CD8+ T cells that have enhanced functional qualities is a critical factor in controlling such pathogens. Considerable effort has gone into developing various vaccine vectors and vaccine regimens that will induce high frequencies of memory CD8+ T cells. However, there has been minimal emphasis on developing strategies to improve the functional qualities of the vaccine-induced memory T cells.
Targeting mTOR by rapamycin is a very attractive strategy to enhance immunogenicity of vaccines. As discussed above, in vivo rapamycin administration into mice infected with LCMV enhances the magnitude of memory CD8+ T cells and more importantly improves their functional quality, characterized by high proliferative and protective capacity and increased longevity (31). The immunostimulatory effect of rapamycin is also observed during secondary T-cell responses (31). This has implications for synergistic effects when coupled with prime-boost vaccine regimes. In addition to virus infection-induced immunity, rapamycin treatment also augments CD8+ T-cell responses generated by a non-replicating virus like particle vaccine derived from hepatitis B virus core antigen (31, 77). Improved CD8+ T-cell responses by rapamycin is also seen in rhesus macaques vaccinated with modified vaccinia virus Ankara (31), a highly attenuated strain of vaccinia virus currently in clinical trials as an HIV vaccine vector (78). In light of the beneficial effects that rapamycin treatment has in many animal vaccine models, it appears to have potential for being used as a vaccine adjuvant in humans.
The mechanism for improved memory CD8+ T-cell responses by rapamycin is unlike any of the currently used adjuvants. The mode of action of rapamycin as an adjuvant is to target the intracellular kinase mTOR in antigen-specific CD8+ T cells (31). In contrast, most commonly used adjuvants stimulate DCs and act through membrane bound receptors such as TLRs (79). The different molecules and cells that the two adjuvant strategies employ, if combined, might serve to broaden the effective vaccine regimens. It will be of interest to examine whether combinational treatment of rapamycin with other adjuvants has synergistic effects and further enhances vaccine-induced T-cell immunity.
In vivo rapamycin administration provides a number of advantages for improving CD8+ T-cell immunity as discussed above. One additional advantage of using rapamycin as an adjuvant is that it can be taken orally and does not need to be combined with the vaccine; hence there will be no issues with vaccine formulation. However, because rapamycin is a common immunosuppressive drug for organ transplantation, the treatment will raise safety concerns (37). This is a very important issue that needs to be addressed before this approach can be used in humans. Thus, it will be essential to investigate and optimize the dose and regimens of rapamycin treatment that have maximal immunostimulatory effects without any adverse events.
Acknowledgements
Work from our laboratory was supported by NIH grants (AI030048 and N01-AI-50025) and Bill and Melinda Gates Foundation Grant (CAVD 38645). B.Y. was supported by a postdoctoral fellowship from the American Cancer Society (PF-09-134-01-MPC).
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8(+) T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 6.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, et al. Human effector and memory CD8(+) T cell responses to smallpox and yellow Fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 12.Huster KM, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 17.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 18.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 19.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 20.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne LC, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R{alpha} mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108:1949–1956. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]
- 23.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–3088. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 24.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar S, et al. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 26.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 30.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Dowling RJ, Pollak M, Sonenberg N. Current status and challenges associated with targeting mTOR for cancer therapy. BioDrugs. 2009;23:77–91. doi: 10.2165/00063030-200923020-00002. [DOI] [PubMed] [Google Scholar]
- 36.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 37.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 38.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 39.Brown EJ, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 40.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 42.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 43.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 44.Avruch J, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 47.Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Slavik JM, Lim DG, Burakoff SJ, Hafler DA. Rapamycin-resistant proliferation of CD8+ T cells correlates with p27kip1 down-regulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:910–919. doi: 10.1074/jbc.M209733200. [DOI] [PubMed] [Google Scholar]
- 49.Slavik JM, Lim DG, Burakoff SJ, Hafler DA. Uncoupling p70(s6) kinase activation and proliferation: rapamycin-resistant proliferation of human CD8(+) T lymphocytes. J Immunol. 2001;166:3201–3209. doi: 10.4049/jimmunol.166.5.3201. [DOI] [PubMed] [Google Scholar]
- 50.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 54.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 55.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valmori D, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 57.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 63.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 64.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 65.Hackstein H, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 66.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 67.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008:91157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Ohtani M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter Rl, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 72.Ekberg H, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 73.Haririan A, et al. Sirolimus exposure during the early post-transplant period reduces the risk of CMV infection relative to tacrolimus in renal allograft recipients. Clin Transplant. 2007;21:466–471. doi: 10.1111/j.1399-0012.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 74.Demopoulos L, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40:1407–1410. doi: 10.1016/j.transproceed.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 75.Barozzi P, et al. Changes in the immune responses against human herpesvirus-8 in the disease course of posttransplant Kaposi sarcoma. Transplantation. 2008;86:738–744. doi: 10.1097/TP.0b013e318184112c. [DOI] [PubMed] [Google Scholar]
- 76.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 77.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172:1777–1785. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 78.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 79.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]