Abstract
Oscillations in gene transcription that occur in response to biological daily clocks coordinate the physiological workings of living organism. But turnover in cellular energy may be sufficient to make a clock tick.
Last spring, a visitor at the biennial meeting of the Society for Research on Biological Rhythms in Florida approached the geneticist Sydney Brenner inquiring as to what it was that scientists studying circadian rhythms actually do. With a glimmer in his eye, Brenner responded that the meeting concerned “those things that only happen once each day”. Indeed, all forms of life undergo circadian (roughly 24-hour) fluctuations in energy availability that are tied to alternating cycles of light and darkness. Biological clocks organize such internal energetic cycles through transcription–translation feedback loops. But two papers1,2 in this issue show that, in both humans and green algae, rhythmic cycles in the activity of peroxiredoxin enzymes can occur independently of transcription.
Biological circadian oscillators have long been recognized as a self-sustained phenomenon, their 24-hour length being both invariant over a wide range of temperatures and responsive to light. Early indications that genes underlie the clocks came3 from the isolation of mutant fruitflies carrying altered, and yet heritable, circadian rhythms. This and subsequent work4,5 established that endogenous molecular clocks consist of a transcription–translation feedback loop that oscillates every 24 hours in cyanobacteria, plants, fungi and animals.
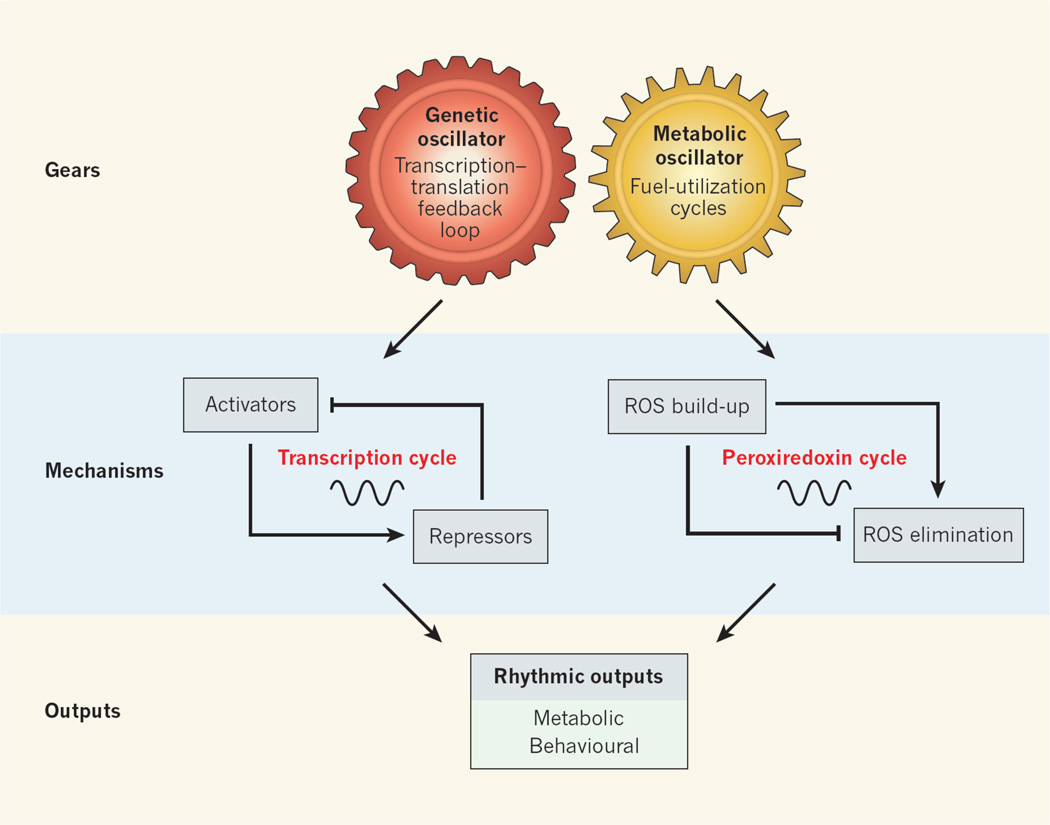
Although the specific clock genes are not evolutionarily conserved across distinct phyla, their architecture is similar. The forward limb of the clock involves a set of transcriptional activators that induce the transcription of a set of repressors. The latter comprise the negative limb, which feeds back to inhibit the forward limb. This cycle repeats itself every 24 hours (Fig. 1).
Figure 1. Coupling of genetic and metabolic clocks.
Two types of circadian oscillator maintain synchrony between the light–dark environment and internal biochemical processes. These are genetic oscillators, which consist of a transcription–translation feedback loop, and — as two new studies1,2 show — metabolic oscillators, which are involved in fuel-utilization cycles and consist of the cycle of oxidation and reduction of peroxiredoxin enzymes. The two oscillator types are coupled, both driving rhythmic outputs (such as photosynthesis reaction cycles in plants and the feeding–fasting cycle in animals) in synchrony with Earth’s rotation.
Energetic cycles are one type of physiological process that shows transcription-dependent circadian periodicity6,7; such cycles include the alternating oxygenic and nitrogen-fixing phases of photosynthesis, and the glycolytic and oxidative cycles in eukaryotes (organisms with nucleated cells). The idea that biochemical flux per se may couple circadian and energetic cycles was first suggested by a report of McKnight and colleagues8 showing that varying the redox state of the metabolic cofactor NAD(P) affects the activity of two clock proteins, and it gained further support from subsequent studies9–14. But exactly how transcriptional and non-transcriptional cycles may be interrelated was still not fully understood.
To address this relationship, O’Neill and Reddy1 (page 498) examined the rhythmic properties of human red blood cells (RBCs). In their mature form, these cells lack both a nucleus and most other organelles, including energy-producing mitochondria. They function mainly as oxygen shuttles, utilizing the protein haemoglobin as the delivery vehicle.
Some of the most abundant proteins in mature RBCs are the evolutionarily conserved enzymes of the peroxiredoxin family, which can inactivate reactive oxygen species (ROS). Class-2 peroxiredoxins contain a cysteine amino-acid residue in their active site that undergoes oxidation when ROS accumulate. This results in the enzyme’s transition from a monomeric to a dimeric state. Excess ROS accumulation induces the formation of even higher-order oligomers. Peroxiredoxin function is essential for RBC survival, as defects in the expression or activity of these enzymes lead to the breakdown of the cells.
A previous survey15 searching for proteins that show circadian rhythms of expression in liver identified peroxiredoxins. In their study, O’Neill and Reddy monitored the monomer– dimer transition of these proteins in RBCs from three humans. They observed two main circadian features in these enucleated cells. First, the oligomerization pattern was self-sustained over several cycles within an approximate 24-hour period and was not affected by temperature. Second, peroxiredoxin oxidation cycles were synchronized in response to temperature cycles, a property called entrainment that is a hallmark of circadian oscillators.
These results, which should be replicated in larger numbers of individuals, clearly show that circadian patterns of peroxiredoxin oxidation persist even in the absence of gene transcription. To rule out the contribution of other, nucleated, blood cells, the authors show that inhibitors of translation (cycloheximide) and transcription (α-amanitin) do not interfere with the peroxiredoxin oxidation rhythm.
In seeking to connect the observed peroxiredoxin oxidation rhythm with the broader physiological functions of RBCs, O’Neill and Reddy also examined the oligomeric transitions of haemoglobin. They detected a rhythmic pattern in the dimer–tetramer transition of haemoglobin, which suggests that the oxygen-carrying capacity of an RBC also exhibits circadian variation. Whether cycles of haemoglobin oxidation similarly show temperature compensation and responsiveness — and the robustness of such oscillations — remains unknown.
What drives the rhythmic cycles of oligomerization for peroxiredoxin and haemoglobin? One possibility is flux in metabolic cycles such as glycolysis — the only source of energy in RBCs. Indeed, O’Neill and Reddy report weak oscillations in the levels of ATP, the cellular energy molecule that can be generated by glycolysis. Nonetheless, further analyses are necessary to determine the exact relationship between oxidation-state transitions and energy production.
Rhythmic variation in levels of NAD(P)H, albeit of low amplitude, also corresponded with the variation in oligomerization state of both peroxiredoxin and haemoglobin1 — a finding that points to this reduced form of NAD(P) as being a cofactor coupling energy flux with changes in the oxidation of these proteins. This observation echoes those of previous studies8.
RBCs do not represent a special case for gene–energy coupling. O’Neill and colleagues2 (page 554) frame their inquiry in a broader context by examining the rhythmic activity of the same family of peroxiredoxins in one of the most primitive eukaryotes known — the green alga Ostreococcus tauri.
The authors took advantage of a curious observation — that simply maintaining O. tauri out of the light suspends all gene transcription. Shifting this microorganism back into the light reinitiates transcription and restarts the clock. However, the clock does not simply reset following transfer into light at any time of day. Instead, it begins ticking again according to the time when the lights were shut off initially. In other words, the alga seems to keep track of time even in the dark, when transcription has ceased. This implies that additional mechanisms in the cell provide a ‘sense of time’ independent of gene transcription.
To investigate how O. tauri senses time, O’Neill et al. tested the idea that the persistence of oscillations in peroxiredoxin oxidation may offer a clue to the ‘invisible’ factor responsible for keeping time in the absence of transcription. In contrast to the previously established ‘transcriptional’ oscillator of O. tauri, the peroxiredoxin oscillation was still detected in the dark, further proving pharmacologically that the enzyme’s rhythm is independent of new gene or protein synthesis. Exploring the interrelationship between transcriptional feedback oscillators and post-transcriptional mechanisms, the authors show that drugs that inhibit transcription affect circadian oscillation within restricted phases of the daily cycle.
So, are RBCs and O. tauri exceptions to the generally accepted idea that the origins of biological clocks can be traced to genetic mechanisms? Intriguingly, O’Neill and Reddy find that peroxiredoxin rhythms were altered in mouse embryonic connective-tissue cells harvested from mutant animals possessing a genetically disrupted clock. This indicates that, in nucleated cells, transcriptional and non-transcriptional oscillators are normally coupled. Similarly, cyclic phosphorylation of the protein KaiA, which can occur in the absence of transcription16, is coupled with transcriptional rhythms in intact cyanobacteria17.
The provocative models provided by these studies1,2 return us to the question of the interdependence of circadian and energetic systems: just how do these processes communicate reciprocally? The cofactor signalling molecules that link the two systems remain of great interest, especially given the potential role of circadian disruption in metabolic disorders. Besides, the fact that oscillators exist in the absence of transcription does not negate the selective advantage that circadian genes confer. At the very least, these genes enhance organismal adaptation to the energetic environment.
Footnotes
J.B. declares competing financial interests. See online article for details.
Contributor Information
Joseph Bass, Email: j-bass@northwestern.edu, Division of Endocrinology and Molecular Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611, USA. He is also at the Center for Sleep and Circadian Biology, Northwestern University, Evanston..
Joseph S. Takahashi, Department of Neuroscience, Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, Texas 75390-9111, USA.
References
- 1.O’Neill JS, Reddy AB. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill JS, et al. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konopka RJ, Benzer S. Proc. Natl Acad. Sci. USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell-Pedersen D, et al. Nature Rev. Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi JS, Hong H-K, Ko CH, McDearmon EL. Nature Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmer SL. Annu. Rev. Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 7.Bass J, Takahashi JS. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter J, Reick M, McKnight SL. Annu. Rev. Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 9.Nakahata Y, et al. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asher G, et al. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey KM, et al. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asher G, et al. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Lamia KA, et al. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy AB, et al. Curr. Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima M, et al. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 17.Kitayama Y, Nishiwaki T, Terauchi K, Kondo T. Genes Dev. 2008;22:1513–1521. doi: 10.1101/gad.1661808. [DOI] [PMC free article] [PubMed] [Google Scholar]