Abstract
BACKGROUND
Pediatric patients with severe pulmonary arterial hypertension (PAH) are treated with intravenous epoprostenol or intravenous or subcutaneous treprostinil. Little is known about longitudinal hemodynamics and outcomes of epoprostenol, treprostinil, and transitions from epoprostenol to treprostinil.
METHODS
This was retrospective study of 77 pediatric patients (47 idiopathic PAH, 24 congenital heart disease-PAH) receiving epoprostenol or treprostinil from 1992 to 2010 at 2 centers. Outcomes were defined as living vs dead/transplant.
RESULTS
Mean age at baseline was 7.7 ± 5.2 years, with follow-up of 4.3 ± 3.4 years. Thirty-seven patients were treated with epoprostenol, 20 with treprostinil, and 20 were transitioned from epoprostenol to treprostinil. Mean pulmonary-to-systemic vascular resistance ratio (Rp/Rs) for epoprostenol was 1.0 ± 0.4, 0.8 ± 0.4, 0.8 ± 0.4, 1.0 ± 0.4, and 1.2 ± 0.4, respectively, at baseline, 1, 2, 3, and 4 years. For treprostinil, Rp/Rs was 0.9 ± 0.3, 0.7 ± 0.3, 0.5 ± 0.2, (p < 0.01 vs baseline), and 1.1 ± 0.2, respectively, at baseline, 1, 2, and 3 to 4 years, respectively. There were similar changes in mean pulmonary artery pressure and pulmonary vascular resistance index. The Rp/Rs 1 year after epoprostenol to treprostinil transition increased from 0.6 to 0.8 (n = 7). Changes not statistically significant unless noted. Eight patients died or received a transplant within 2 years of baseline; compared with the rest of the cohort, mean baseline Rp/Rs, right atrial pressure, and pulmonary vascular resistance index were significantly worse in this group. Thirty-nine patients remain on prostanoids, 17 are off, 16 died, and 5 received heart-lung transplant. Kaplan-Meier 5-year transplant-free survival was 70% (95% confidence interval, 56%-80%).
CONCLUSION
There was improvement in Rp/Rs on both therapies at 1 to 2 years that was not sustained. The 5-year transplant-free survival was better than in similar adult studies.
Keywords: pediatric patients, pulmonary arterial hypertension, epoprostenol, treprostinil, hemodynamics
Pulmonary arterial hypertension (PAH) is characterized by elevation of pulmonary vascular resistance (PVR) and pulmonary arterial pressures leading to progressive right heart failure and, ultimately, death. Before currently available treatment, mean adult 5-year survival was less than 40% and pediatric outcomes were worse.1 Current therapeutic options include 3 major classes of medications: prostacyclins, endothelin receptor antagonists, and type-5 phosphodiesterase inhibitors.2,3 Multiple studies have demonstrated hemodynamic improvements with sildenafil4,5 or bosentan.6,7 Iloprost, an inhaled prostacyclin, has been shown to produce early hemodynamic improvement without significant long-term changes, even in those who had initial improvement.8 Limited pediatric studies have shown improved survival since therapies specifically indicated for PAH have become available,9,10 although it remains unclear whether this is due to earlier diagnosis, improved follow-up, or the actual therapies.
Epoprostenol is an arachidonic acid metabolite produced by vascular endothelium. It is a short-acting, potent pulmonary and systemic vasodilator with anti-platelet activity. Epoprostenol was first approved for patients with advanced PAH as a continuous intravenous infusion in 1995.11 Since that time, multiple adult trials of epoprostenol have shown clinical and hemodynamic improvement with increased survival.1,12-15 Treprostinil, via subcutaneous delivery, was initially approved in 2002 for use in patients with PAH to reduce symptoms or to transition from epoprostenol to improve quality of life with its longer half-life. Treprostinil is now available by continuous intravenous administration (approved in 2004), subcutaneous infusion, or inhalation (approved in 2009).
Previous reports in small populations have shown that pediatric patients with idiopathic PAH (IPAH) or PAH associated with congenital heart disease (CHD) have experienced clinical improvement16 and hemodynamic improvement17-19 with epoprostenol therapy. Adult studies have shown that the transition to treprostinil is safe and generally well tolerated; however, there are limited data on hemodynamic changes over time.20-22 The only study on treprostinil use in pediatric patients recently reported hemodynamic improvements in a small cohort.23
There are no widely accepted standards for initiation of prostanoid therapy in adult or pediatric patients. Indications vary between clinical centers, and therapy is titrated according to symptoms because longitudinal hemodynamic data are limited. Intravenous therapy is typically reserved for those patients in whom oral therapy is not successful or those with severe disease, defined as systemic pulmonary arterial pressure with symptoms (New York Heart Association class III or IV), supra-systemic pulmonary arterial pressure, or concerning symptoms.
This study examined the hemodynamic changes over time in pediatric patients who received intravenous epoprostenol or intravenous or subcutaneous treprostinil for treatment of PAH. In addition, we sought to determine whether treprostinil produces similar hemodynamic changes as epoprostenol de novo or in transition from epoprostenol.
Methods
This was a 2-center, retrospective, descriptive study. The study was approved by the Stanford University Administrative Panel on Human Subjects in Medical Research and by the University of Colorado, Colorado Multiple Institutional Review Board.
All patients who were treated for PAH or repaired CHD-PAH at Lucile Packard Children’s Hospital in conjunction with the Vera Moulton Wall Center for Pulmonary Vascular Diseases at Stanford University and Children’s Hospital Colorado were identified through clinical databases. The study included all patients who were currently receiving or had received intravenous epoprostenol or intravenous or subcutaneous treprostinil for treatment of PAH. Cardiac catheterization data were extracted from existing institutional databases.
Rp/Rs, the ratio of pulmonary-to-systemic vascular resistances, was used as the primary hemodynamic parameter instead of PVR to standardize the data by eliminating the high variability in systemic blood pressure between catheterizations and the need to use assumed oxygen consumption in calculations, because thermodilution was not consistently performed.24 When used, PVR was recalculated from raw hemodynamic data using oxygen consumption from the Bergstra equation to ensure standardization across patients and between institutions.25 Each patient’s underlying diagnosis, age, weight, height, body surface area, and hemoglobin at catheterization were obtained. Outcomes tracked included transition off therapy, heartlung transplantation, and death.
At both institutions, epoprostenol therapy is initiated in the cardiac catheterization laboratory or intensive care unit, and the dose is increased to 4 ng/kg/min. The dose is incrementally increased as an outpatient to the target dose of 30 to 50 ng/kg/min. Selected patients, based on clinician and family preference, are offered treprostinil as an alternative to epoprostenol as initial therapy or to transition from current epoprostenol therapy. Owing to the longer half-life of treprostinil, patients are first started on epoprostenol in the catheterization laboratory and transitioned to treprostinil at a dose 130% to 150% higher than the epoprostenol dose in the intensive care unit within the first 24 hours, if epoprostenol is tolerated. The outpatient target dose of treprostinil is 50 to 70 ng/kg/min, but may be higher. A single-lumen Broviac catheter is used for infusion with a CADD legacy infusion pump (Pharmacia Deltec, St. Paul, MN) for epoprostenol or a CADD Legacy, CADD MS3, or Crono Five pump (Cane, Torino, Italy) for treprostinil.
The indications for initiation of epoprostenol or treprostinil include clinical deterioration, failure to improve on conventional therapy, syncope, hemodynamic instability, or markedly abnormal hemodynamic parameters. Patients receiving prostanoid therapy undergo repeat catheterization yearly or sooner if there are significant clinical concerns. Baseline catheterization in this study was defined as catheterization before initiation of prostanoid therapy.
Results
Between 1992 and 2010, 79 pediatric patients were treated with epoprostenol or treprostinil for PAH between the 2 institutions. The study excluded 2 patients with left heart obstructive lesions. Of the 77 patients eligible for inclusion in this study, 46 (61%) were girls. There were 47 patients with IPAH, 24 with repaired CHD-PAH, and 6 with other forms of World Health Organization Group 1 IPAH. Thirty-seven patients were treated with epoprostenol alone, 20 with treprostinil alone, and 20 transitioned from epoprostenol to treprostinil. Median dose (range) of epoprostenol was 31 (2–98), 34 (6–86), 34 (10–91), and 34 (23–77) ng/kg/min at 1, 2, 3, and 4 years into therapy respectively. Median dose (range) of treprostinil was 36.5 (16–75), 61.5 (11–95), 48 (36–60), and 104 (100–108) ng/kg/min at 1, 2, 3, and 4 years into therapy respectively (n = 2 at 3 and 4 years).
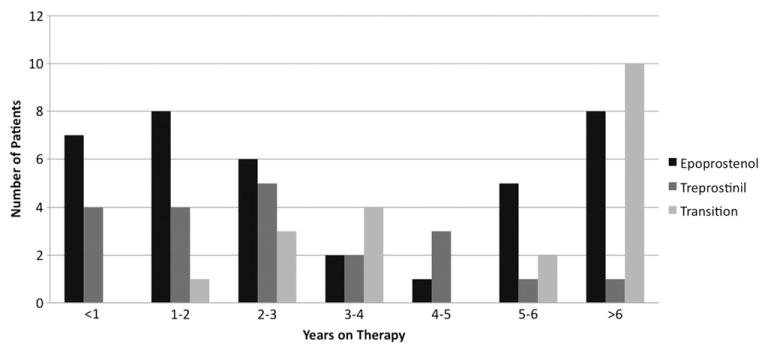
Baseline cardiac catheterization data were available for 74 patients before prostanoid therapy was initiated. Average age at baseline catheterization was 7.7 ± 5.2 years. Sixty-four patients had at least 1 follow-up cardiac catheterization after prostanoid therapy was initiated. Average length of follow up was 4.3 ± 3.4 years (range, 0.04–13.3 years), and average length of prostanoid therapy was 4.0 ± 3.3 years (de novo treprostinil, 2.9 ± 2.3 years; Figure 1). Of the patients without follow-up hemodynamic data, 6 died, 1 received a heart-lung transplant, 1 is off therapy, and 5 did not receive a follow-up catheterization or complete hemodynamic data were not available for review.
Figure 1.
Length of prostanoid therapy for the entire cohort by drug treatment group.
Demographic and baseline hemodynamic data were compared among the groups (epoprostenol vs treprostinil vs transition). There were no statistically significant differences among the groups at baseline except age between the treprostinil and transition groups (Table 1). Similarly, demographic and baseline hemodynamic data were compared between patients with IPAH and repaired CHD-PAH, with no statistical differences.
Table 1.
Comparison of Baseline Characteristics Among Drug Treatment Groups
| Variablesa | Epoprostenol (n = 37) |
Treprostinil (n = 20) |
Transition (n = 20) |
|---|---|---|---|
| Age, years | 7.8 ± 4.7 | 9.7 ± 5.8 | 5.5 ± 4.4b |
| Sex | |||
| Female | 21 (57) | 13 (65) | 12 (60) |
| Male | 16 (43) | 7 (35) | 8 (40) |
| Diagnosis | |||
| IPAH | 26 (70) | 11 (55) | 10 (50) |
| CHD-PAH | 7 (19) | 9 (45) | 8 (40) |
| Other WHO Group I | 4(11) | 0 | 2 (10) |
| RAP, mm Hg | 7.6 ± 4.6 | 7.4 ± 3.6 | 6.5 ± 2.3 |
| mPAP, mm Hg | 67.0 ± 22.8 | 57.7 ± 20.0 | 68.1 ± 18.7 |
| PVRI, Wood units | 20.1 ± 13.5 | 15.6 ± 10.8 | 20.1 ± 16.5 |
| CI, liters/min/m2 | 3.7 ± 1.7 | 4.2 ± 2.0 | 3.7 ± 1.4 |
| Rp/Rs | 1.0 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.3 |
CHD-PAH, congenital heart disease-pulmonary artery hypertension; CI, cardiac index;IPAH, idiopathic pulmonary artery hypertension; mPAP, mean pulmonary artery pressure;PVRI, peripheral resistance index;RAP, right atrial pressure;Rp/Rs, pulmonary-to-systemic vascular resistance ratio;WHO, World Health Organization.
Continuous data are shown as mean ± standard deviation and categoric data as number (%).
p < 0.05 (transition vs treprostinil only).
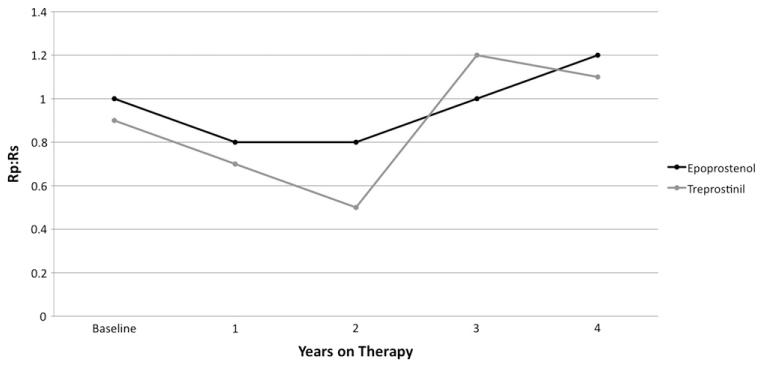
The baseline mean Rp/Rs was 0.9 ± 0.4 for the 61 patients with follow-up and was 0.8 ± 0.4 at the most recent catheterization (p = NS). Longitudinal hemodynamic data are shown for epoprostenol and treprostinil (Figure 2). Mean Rp/Rs was compared at baseline with Rp/Rs at 1, 2, 3, and 4 years into therapy for epoprostenol and treprostinil. Patients were grouped according to initial therapy; therefore, the epoprostenol group includes patients who later transitioned. Over time, there was improvement in Rp/Rs for patients treated with epoprostenol that was not statistically significant and was not sustained (Table 2). For treprostinil, there was a statistically significant improvement in Rp/Rs at 2 years on therapy that was not sustained. The improvement in both groups was lost by 3 to 4 years on therapy.
Figure 2.
Change in pulmonary-to-systemic vascular resistance ratio (Rp/Rs) over time on epoprostenol or treprostinil. There was an initial improvement in Rp/Rs at 1 to 2 years on therapy that was not sustained long-term.
Table 2.
Change in Pulmonary-to-Systemic Vascular Resis tance Ratio Over Time in Patients Receiving Epoprostenol or Treprostinila
| Rp/Rs | Entire cohort |
(−) Censored patients |
||
|---|---|---|---|---|
| Epoprostenol (n = 54) Mean ± SD |
Treprostinil (n = 20) Mean ± SD |
Epoprostenol (n = 47) Mean ± SD |
Treprostinil (n = 19) Mean ± SD |
|
| Baseline | 1.0 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.3 |
| 1 year | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.8 ± 0.4 | 0.7 ± 0.3 |
| 2 years | 0.8 ± 0.4 | 0.5 ± 0.2b | 0.8 ± 0.4 | 0.5 ± 0.2b |
| 3 years | 1.0 ± 0.4 | 1.0 ± 0.4 | ||
| 4 years | 1.2 ± 0.4 | 1.1 ± 0.2 | 1.2 ± 0.4 | 1.1 ± 0.2b |
Rp/Rs, Pulmonary-to-systemic vascular resistance ratio SD, standard deviation.
Rp/Rs was assessed at yearly intervals was compared with baseline Rp/Rs (treprostinil data at 3 and 4 years was combined for analysis due to small number of patients). An initial improvement in Rp/Rs at 1 to 2 years on therapy was not sustained long-term. Similar trends wereseen when censored patients were removed from analysis.
p < 0.05.
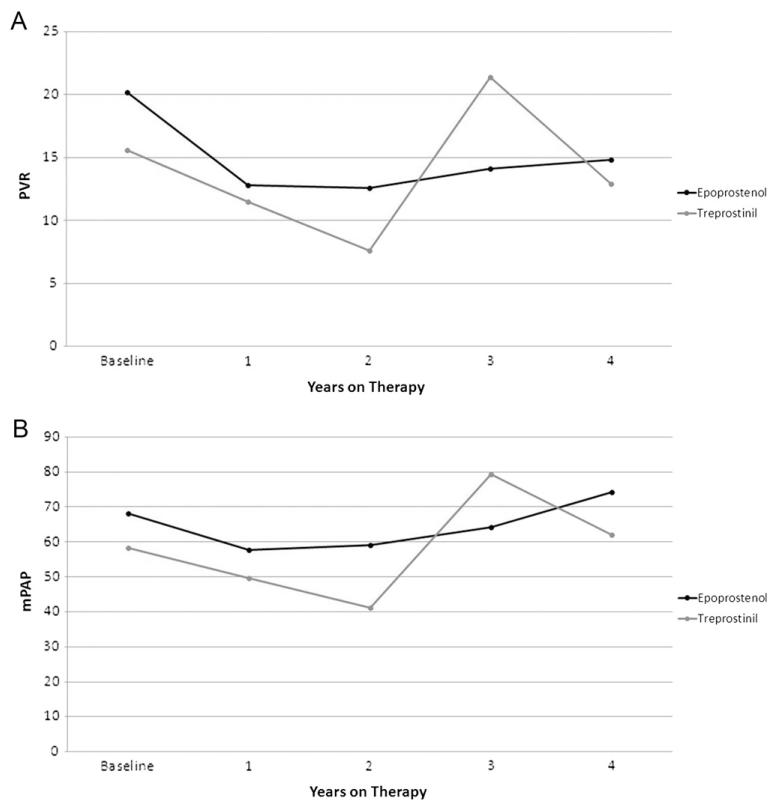
Similar changes occurred in PVR index (PVRI) and mean pulmonary artery pressure (mPAP; Figure 3.) For epoprostenol, mean PVRI was 20.2 ± 13.4, 12.8 ± 10.1 (p = 0.01), 12.6 ± 5.9, 14.1 ± 7.8 and 14.8 ± 3.8, respectively, at baseline, 1, 2, 3, and 4 years, and mean mPAP was 68.1 ± 21.4, 57.7 ± 23.0 (p = 0.04), 59.1 ± 22.8, 64.2 ± 22.6, and 74.2 ± 15.8. For treprostinil, mean PVRI was 15.6 ± 10.8, 11.5 ± 7.7, 7.6 ± 3.7, 21.4 ± 7.5, and 12.9 ± 0.8, respectively, at baseline, 1, 2, 3 and 4 years, and mean mPAP was 58.3 ± 19.7, 49.7 ± 17.1, 41.1 ± 17.7 (p = 0.04), 79.3 ± 5.7, and 62.0 ± 14.1. None of these changes were statistically significant, unless noted.
Figure 3.
(A) Change in pulmonary vascular resistance (PVR) index over time on epoprostenol or treprostinil. (B) Change in mean pulmonary artery pressure (mPAP) over time on epoprostenol and treprostinil. There was an initial improvement in both parameters at 1 to 2 years on therapy that was not sustained long-term.
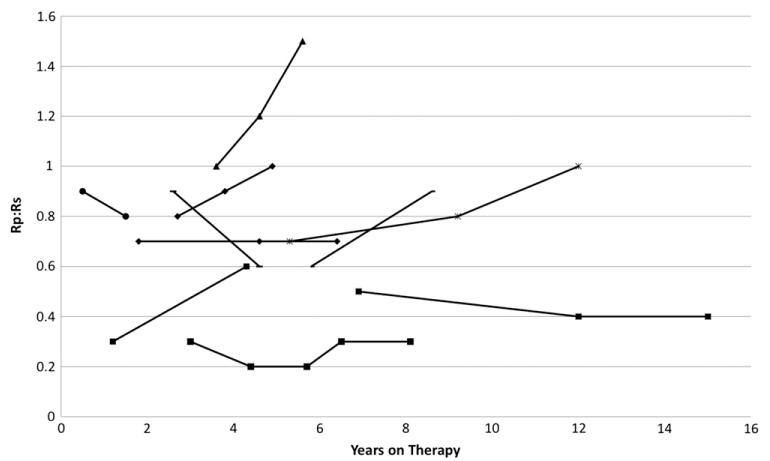
Of 20 patients who transitioned from epoprostenol to treprostinil, 5 ultimately transitioned off prostanoid therapy and 3 died. In 5 additional children, the transition was considered a failure due to clinical deterioration shortly after transition: 2 patients died, 1 patient transitioned back to epoprostenol and subsequently died, 1 patient underwent heart-lung transplant, and 1 patient tolerated initial transition and then failed weaning to iloprost, was restarted on treprostinil without improvement, and ultimately transitioned back to epoprostenol. Longitudinal hemodynamic data were available for 10 patients who underwent transition from epoprostenol to treprostinil. At 9 to 15 months after transition from epoprostenol to treprostinil, there was a change in mean Rp/Rs from 0.6 to 0.8 (n = 7; Figure 4).
Figure 4.
Change in pulmonary-to-systemic vascular resistance ratio (Rp/Rs) from baseline catheterization on epoprostenol to follow-up catheterizations over time on treprostinil for 10 patients who transitioned from epoprostenol to treprostinil. Mean Rp/Rs at 9 to 15 months after transition increased from 0.6 to 0.8 (n = 7), which was similar to the increase in Rp/Rs in the entire cohort several years into therapy.
Eight patients died or received heart-lung transplant within 2 years of baseline catheterization. Mean Rp/Rs, right atrial pressure (RAP), and PVRI were significantly different for this group compared with the rest of the cohort. The cardiac index (CI) did not differ significantly in this group compared with the rest of the cohort (Table 3). These patients were excluded from longitudinal mean Rp/Rs data for epoprostenol and treprostinil in a separate analysis to ensure that the improvements seen were not ascribed to these patients with early death or transplant falling out of the cohort. The hemodynamic changes over time were unchanged, although baseline Rp/Rs was lower in both groups. The Rp/Rs at 4 years for epoprostenol (1.2 ± 0.4, p = 0.05) and at 3 to 4 years for treprostinil (1.1 ± 0.2, p = 0.04) was worse than the baseline Rp/Rs.
Table 3.
Baseline Characteristics of Censored Patients Compared With the Remainder of the Cohort
| Variable | Early death/Tx (n = 8) Mean ± SD |
Rest of cohort (n = 66) Mean ± SD |
|---|---|---|
| RAP, mm Hg | 11.9 ± 6.3a | 7.0 ± 3.5 |
| PVRI, Wood units | 28.3 ± 13.9a | 17.9 ± 12.3 |
| CI, liters/min/m2 | 3.2 ± 1.5 | 3.8 ± 1.7 |
| Rp/Rs | 1.3 ± 0.5a | 0.9 ± 0.4 |
CI, cardiac index;PVRI, peripheral vascular index;RAP, right atrial pressure;Rp/Rs, pulmonary-to-systemic vascular resistance ratio;SD, standard deviation; Tx, transplant.
p < 0.05.
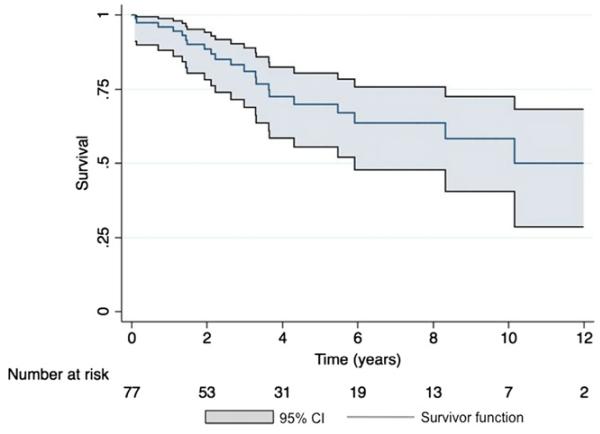
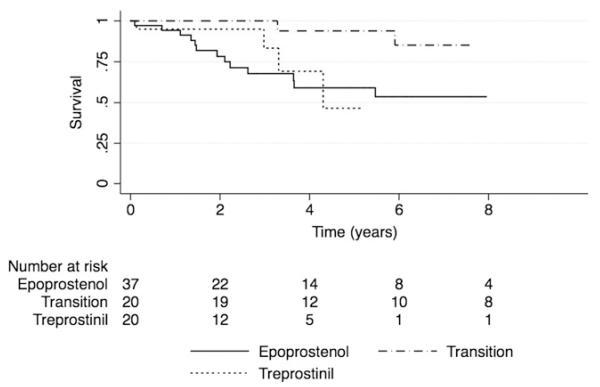
Thirty-nine patients are still receiving prostanoid therapy, 17 have transitioned to other therapy (phosphodiesterase inhibitors, endothelin receptor antagonists or inhaled prostacyclins), 16 died, and 5 received a heart-lung transplant. Only 1 patient who died had transitioned off prostanoid therapy. For 17 patients who transitioned off intravenous or subcutaneous prostanoids, baseline (n = 15) mean Rp/Rs, RAP, PVRI, and CI were significantly different compared with the rest of the cohort (Table 4). The average Rp/Rs before transition off prostanoid therapy was 0.5 ± 0.2 and did not change on follow-up catheterization. The group demographics of age (6.2 ± 5.6 vs 8.1 ± 4.9 years, p = 0.2), sex, and diagnosis for those who transitioned off were similar to the rest of the cohort. Kaplan-Meier 5-year transplant-free survival for the entire cohort was 70% (95% confidence interval 56%–80%;Figure 5). The 5-year survival in the epoprostenol, treprostinil, and transition groups was also compared (Figure 6).
Table 4.
Baseline Characteristics of Patients who Later Transitioned off Epoprostenol or Treprostinil Compared With the Rest of the Cohort
| Variable | Transitioned off (n = 15) Mean ± SD |
Rest of cohort (n = 58) Mean ± SD |
|---|---|---|
| RAP, mm Hg | 5.5 ± 2.9a | 8.1 ± 4.3 |
| PVRI, Wood units | 12.6 ± 9.8a | 20.8 ± 13.0 |
| CI, liters/min/m2 | 4.9 ± 2.4a | 3.4 ± 1.3 |
| Rp/Rs | 0.7 ± 0.3a | 1.0 ± 0.4 |
CI, cardiac index;PVRI, peripheral vascular index;RAP, right atrial pressure;Rp/Rs, pulmonary-to-systemic vascular resistance ratio.
p < 0.05.
Figure 5.
Kaplan-Meier survival curve for the entire cohort, comprising patients on epoprostenol, treprostinil, and those who transitioned, with 95% confidence intervals (CI) depicted. Transplant-free 5-year survival was 70% (95% CI, 56%–80%).
Figure 6.
Survival curve by drug treatment group. Sub-groups were too small to permit meaningful statistical comparison of the curves. Transition occurred on average at 3.3 years into therapy.
Discussion
Before the availability of specific drugs for PAH, median survival in adults with IPAH was 2.8 years, with a 5-year survival of 34%.1 Since the introduction of prostacyclin therapy, long-term outcomes and survival have improved in adults with PAH.12-15 Sitbon et al15 reported hemodynamic improvement at 1 year in 156 patients with IPAH on epoprostenol with a 5-year survival of 55%. Similarly, several large adult studies consistently demonstrated a reduction in PVR on follow-up catheterizations.12-14
Initial reports of pediatric survival were far worse than that for adults.1 With the advent of multiple available therapies (although none are approved by the U.S. Food and Drug Administration for pediatric patients), pediatric survival has improved.17 Specifically for prostanoid therapy, the largest study of 37 pediatric patients demonstrated functional improvement on epoprostenol. This study did not, however, report hemodynamic changes.16 Several other studies demonstrated short-term significant improvements in hemodynamics for pediatric patients on epoprostenol. Rosenzweig et al18 reported 16 patients with CHD on epoprostenol for 1 year, and Yung et al17 reported 35 patients with IPAH on epoprostenol for 53 ± 28 months, both with significant improvement in mPAP, CI, and PVRI.19 Finally, a recent report in 8 patients demonstrated short-term (6-18 month) improvement in CI and PVRI on subcutaneous treprostinil.
This study reports hemodynamic data in pediatric patients on epoprostenol and treprostinil, including those who underwent transition. Hemodynamic changes over time on treprostinil were similar to epoprostenol. There was a significant improvement in Rp/Rs on treprostinil after 2 years of therapy and a trend toward improvement on epoprostenol after 1 to 2 years of therapy. Neither of these changes was sustained over a period of 4 years. We postulate that the lack of long-term improvement may be due to progression of underlying disease. Alternatively, there could be tolerance to prostanoid therapy with inadequate dose titration over time, although this has not been substantiated. This supports a recent report that initiation of new PAH therapies early in the disease course resulted in early clinical improvement that diminished over time.9
There was no significant statistical difference in hemodynamic data after transition from epoprostenol to treprostinil, although there was a trend toward a higher Rp/Rs after transition. This may be explained by the higher dosage per kilogram required to obtain the same physiologic effect with treprostinil and by our limited understanding of pharmacodynamics in pediatric patients. In addition, baseline catheterization on epoprostenol occurred at an average of 3.3 years after therapy was initiated, and increased Rp/Rs was seen in patients receiving epoprostenol alone after several years of therapy. Overall, patients who transitioned from epoprostenol to treprostinil demonstrated similar changes in Rp/Rs trends as patients who only received one prostanoid. Similar to the adult data, there were transition failures,21 which may have resulted from inadequate dose titration or to disease progression. However, successful transition of carefully selected pediatric patients with favorable hemodynamics to oral or inhaled therapies has been reported.26
Overall 5-year pediatric survival was reported at 81% by Yung et al,17 although survival without transplantation or atrial septostomy was 57% . Recently, Van Loon et al10 reported 5-year survival of 66% in pediatric patients with IPAH or CHD-PAH when current era drugs became available during the disease course without improvement in survival when the drugs were available at diagnosis. A United Kingdom registry documented 216 pediatric PAH patients with 5-year survival of 64.2%, although this included patients without treatment.9 The survival reported in this study is similar to or slightly better than previous pediatric and adult studies, suggesting that treprostinil treatment results in similar long-term outcomes to epoprostenol and that both are associated with a survival benefit compared with no therapy. Survival could not be compared among groups (epoprostenol vs treprostinil vs transition) due to limited numbers of patients.
This study examined predictors of survival in pediatric patients with PAH on prostanoid therapy. Adult studies demonstrated decreased survival, with elevated RAP, mPAP, PVRI and decreased CI.1,3-15 Similarly, elevated Rp/Rs, RAP, and PVRI (but not CI) were significantly associated with early death or transplant in this cohort. Patients with significant baseline elevations in Rp/Rs, RAP, and PVRI should be considered early for prostanoid therapy and possibly even for heart-lung transplant evaluation, if desired.
This study has several limitations, all somewhat inherent in the general, experienced-based approach to pediatric PAH: there was no well-defined evidence-based treatment algorithm for pediatric PAH nor dosing strategy for epoprostenol or treprostinil, no standard protocol for repeat catheterizations or adjustments in therapy, and no standardization of protocols between the institutions.
These limitations notwithstanding, this study demonstrates efficacy of treprostinil equivalent to epoprostenol, with similar long-term outcomes. The largest changes in PVRI and Rp/Rs are seen, in general, within the first 1 to 2 years of therapy. This supports the emerging interest in initial, aggressive treatment of all but the most mild cases of disease, with subsequent transition to other therapies in an attempt to reverse as much of the disease as possible, as early as possible, in an attempt to delay long-term sequelae and death. In most patients receiving epoprostenol therapy, transition to treprostinil appears to be safe and well tolerated and should be considered, although close observation and follow-up for transition failure is warranted. Further studies are needed to refine longer-term hemodynamic changes and survival for both prostanoids.
Acknowledgments
This study was supported by the Jayden DeLuca Foundation, the Leah Bult Foundation, and UL1 RR025780 Colorado Clinical Translational Science Institute, National Center for Research Resources, and National Institutes of Health.
Footnotes
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Rashid A, Ivy D. Severe paediatric pulmonary hypertension: new management strategies. Arch Dis Child. 2005;90:92–8. doi: 10.1136/adc.2003.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary arterial hypertension in children. Pediatr Pulmonol. 2004;38:2–22. doi: 10.1002/ppul.20051. [DOI] [PubMed] [Google Scholar]
- 4.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009154:379–84. 384, e371–2. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 6.Barst R. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Therapeut. 2003;73:372–82. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig EB, Ivy DD, Widlitz A, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Ivy DD, Doran AK, Smith KJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–9. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart. 2009;95:312–7. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 10.van Loon RL, Roofthooft MT, Delhaas T, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol. 2010;106:117–24. doi: 10.1016/j.amjcard.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Rubin LJ, Groves BM, Reeves JT, Frosolono M, Handel F, Cato AE. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation. 1982;66:334–8. doi: 10.1161/01.cir.66.2.334. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338:273–7. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–82. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 15.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 16.Lammers AE, Hislop AA, Flynn Y, Haworth SG. Epoprostenol treatment in children with severe pulmonary hypertension. Heart. 2007;93:739–43. doi: 10.1136/hrt.2006.096412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 18.Rosenzweig EB, Kerstein D, Barst RJ. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation. 1999;99:1858–65. doi: 10.1161/01.cir.99.14.1858. [DOI] [PubMed] [Google Scholar]
- 19.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J. 2006;28:1195–203. doi: 10.1183/09031936.06.00044406. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin VV, Gaine SP, Barst RJ, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol. 2003;41:293–9. doi: 10.1097/00005344-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Gomberg-Maitland M, Tapson VF, Benza RL, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172:1586–9. doi: 10.1164/rccm.200505-766OC. [DOI] [PubMed] [Google Scholar]
- 23.Levy M, Celermajer DS, Bourges-Petit E, Del Cerro MJ, Bajolle F, Bonnet D. Add-on therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr. 2011;158:584–8. doi: 10.1016/j.jpeds.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Balzer DT, Kort HW, Day RW, et al. Inhaled nitric oxide as a preoperative test (INOP test I: The INOP Test Study Group. Circulation. 2002;106:I76–81. [PubMed] [Google Scholar]
- 25.Bergstra A, van Dijk RB, Hillege HL, Lie KI, Mook GA. Assumed oxygen consumption based on calculation from dye dilution cardiac output: an improved formula. Eur Heart J. 1995;16:698–703. doi: 10.1093/oxfordjournals.eurheartj.a060976. [DOI] [PubMed] [Google Scholar]
- 26.Melnick L, Barst RJ, Rowan CA, Kerstein D, Rosenzweig EB. Effectiveness of transition from intravenous epoprostenol to oral/inhaled targeted pulmonary arterial hypertension therapy in pediatric idiopathic and familial pulmonary arterial hypertension. Am J Cardiol. 2010;105:1485–9. doi: 10.1016/j.amjcard.2009.12.075. [DOI] [PubMed] [Google Scholar]