Abstract
Background
Complex behavioral traits such as coping strategies in response to stress are usually formed by genetic and environmental influences.
Methods
By exploiting the phenotypic and genotypic differences between the Wistar Kyoto (WKY) and Fischer 344 (F344) inbred rat strains, we recently identified three X chromosome-linked quantitative trait loci contributing to differences in coping strategies in the defensive burying (DB) paradigm. In this article we study the influence of postnatal maternal environment in these behaviors by characterizing the maternal behavior of these strains and the effect of cross-fostering on DB behavior of male offspring from reciprocal crossing (F1).
Results
Maternal behavior of WKY rats can be quantitatively characterized by less contact and more periods of neglect of their F1 pups. In contrast, F344 mothers engaged in more active behaviors such as licking/grooming and arched-back nursing. Cross-fostering male F1 pups at birth did not influence the latency to bury measure in DB; however, duration of burying and prod approaches were influenced by both genotype and maternal environment in an additive manner.
Conclusions
These results demonstrate that different measures of behavioral coping in the DB paradigm are influenced by maternal environment to differing degrees and in addition by genetic factors.
Keywords: Wistar-Kyoto, genetics, stress, cross-fostering, reciprocal breeding, Fischer 344
Inbred strains have frequently and traditionally been used to genetically map disease or susceptibility loci. This technique has been quite successful in mapping complex traits, where genotypic variation witnessed in the genetically diverse second generation (F2) offspring is correlated with the phenotypic variation displayed by the offspring (Flint 2003; Rapp and Deng 1995; Nadeau et al 2000; Shimomura et al 2001). Chromosomal regions with a high probability of association with the phenotype of interest are most probably regions where important genes influencing the expression of the behavior lie. Despite the evidence for the genetic bases of complex traits, it is generally accepted that environment accounts for a significant proportion (Meaney 2001a)—up to 65% (Gomez-Serrano et al 2001)—of the variance in any given complex trait in animals. One critical environmental factor is that of the postnatal maternal environment.
In general, parental care in humans has been shown to have a profound influence on the health of the offspring into adulthood (Chrousos and Gold 1992; Sapolsky 1994; McEwen 1998). A recent review by Meaney (2001b) shows that those whose scores on parental bonding scales reflected cold and distant parent-child relationships were found to be at a significantly greater risk of depression and anxiety in later life (Canetti et al 1997; Parker 1981). Animal studies show the effect of pre-and postnatal environment on various measures (Rhees et al 1999) including behavior (Francis et al 2003). Specifically, maternal care in early life has been shown to have significant modulating effects through adulthood on stress reactivity and stress-response systems (Liu et al 1997), fear behaviors (Caldji et al 1998), heart rate (Myers et al 1989) and blood pressure (McMurty et al 1981; Cierpial and McCarty 1987). Furthermore, specific patterns of maternal behavior have been shown to transmit non-genomically across several generations (Meaney 2001a; Francis et al 1999b; Champagne and Meaney 2001). Thus, maternal environment can contribute to the phenotype of the offspring additively or interactively with their genotype.
We have recently reported (Ahmadiyeh et al 2003) an interaction between grand-maternal lineage and genetic loci on the X chromosome influencing the behavior in the defensive burying (DB) test. This test is modeled after naturalistic observations of rodents’ responses to predators in the wild and is thought to measure—with ethological validity (Treit 1991)—an animal’s endogenous, instinctual coping responses necessary for its survival in the wild. In the laboratory environment, the DB test assays an animal’s behavioral responses to the proximal threat afforded by a shock prod (Treit et al 1993), which delivers a shock to the animal whenever it touches the prod. In our earlier study (Ahmadiyeh et al 2003), the Wistar Kyoto (WKY) and Fischer 344 (F344) rats served as parental inbred strains showing significant phenotypic differences in the defensive burying paradigm and more passive response (longer latency to bury, shorter duration of burying, and greater number of prod approaches) in the WKY than in the F344. The phenotypic differences between the inbred strains were present in both males and females. Reciprocal breeding crosses generated first-generation (F1) offspring identical (heterozygous) at all loci except for the maternal X and paternal Y chromosomes in males.
Male F1 offspring from a WKY or F344 mother exhibited coping behaviors in the defensive burying test that were similar to their mothers’. This pattern of transmission suggested a sex-linked (X or Y chromosomal) pattern of inheritance. Our F2 phenotypic data, however, favored an X-linked mode of transmission, and genetic analysis of the second-generation (F2) offspring confirmed a significant X-chromosomal genetic component impacting the development of behavioral responses in the DB test (Ahmadiyeh et al 2003); however, each of these X-chromosomal loci accounted for no more than 3%–11% (dependent on specific phenotype) of the variance of the traits. We hypothesized, therefore, that other maternal factors—whether mitochondrial, cytoplasmic, prenatal, or postnatal—were acting in either an additive or interactive fashion upon the X-chromosomal genetic factors in ultimately shaping behavior in the DB test.
In the present study we seek to determine the influence of early postnatal maternal environment on the development of DB behaviors in male F1 rats in adulthood while controlling for X-chromosomal genotype. We are interested in two primary questions: 1) Do the WKY and F344 mothers respond differently toward their own F1 pups that are (except for the sex chromosomes in males) genetically comparable in litters of both strains? 2) Is the pre-weaning postnatal maternal environment sufficient to significantly alter the development of DB behaviors in adulthood? To answer the first question, we studied the maternal behavior of WKY and F344 mothers toward their F1 pups during the first 10 days of life. To answer the second question, we cross-fostered at birth F1 male pups from a F344 mother with those from a WKY mother and studied their DB behavior as adults. No more than 2 pups were crossed into or out of any litter, since ample evidence shows that drastically altering the conditions of the nest, as in wholesale fostering of pups, alters the maternal behavior of the mother toward her pups (Maccari et al 1995; Myers et al 1989). Native pups within each strain were also studied as a control comparison group. As a separate internal control, we also studied the maternal behavior of WKY and F344 foster mothers to determine whether the conservative cross-fostering technique employed in our study had any effect on the mothering behavior of the foster mothers of either strain.
Methods and Materials
Animals
Animals were housed on a 14:10 light/dark cycle (14 hours light, 10 hours dark) and were fed regular rat chow and water ad libitum. Only litters with 8–12 pups were used in this study; furthermore, there were no differences in mean litter size between WKY and F344 mothers. Behaviors observed during dark periods were done under red lights. Animals used in the cross-fostering study were weaned and weighed at postnatal day 21, housed in groups of 4, tested in the DB test at 3 months of age, and weighed again at 4 months of age just before sacrifice.
Mating and Cross
Maternal Behavior
Eight-week-old WKY and F344 males (n = 4 of each strain) and females (n = 20 of each strain) (Harlan Industries, Indianapolis, Indiana) were left undisturbed for 2 weeks upon arrival. Reciprocal crosses were then set up (WKY females X F344 males and F344 females X WKY males; Figure 1A) until 15 WKY and 13 F344 females became pregnant within a 4-day span. Pregnancy and expected delivery date were determined by observing vaginal smear for sperm on the morning after mating. Of these pregnant dams, only 13 WKY and 12 F344 fit the litter size criteria and were retained for the study. Of these, 5 WKY females were timed to give birth within 8 hours of 5 F344 females; these litters were cross-fostered according to the cross-fostering protocol, and the behavior of the mothers was observed to determine whether our cross-fostering technique had any effect on maternal behavior. The remaining litters of each strain were left undisturbed, and the behavior of the mothers was observed to determine whether there were any differences between the maternal behavior of the two inbred strains.
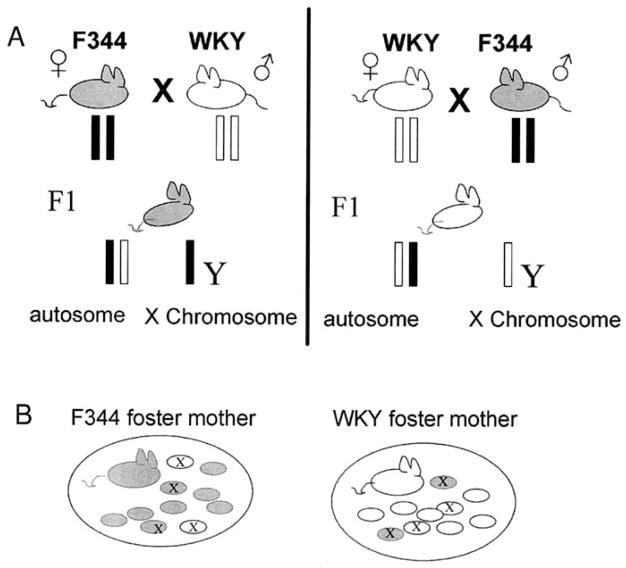
Figure 1.
Schematic showing the reciprocal cross/matings and the cross-fostering design employed in this study. (A) Reciprocal crosses between F344 and WKY inbred strains produce male F1 rats that are heterozygous on all autosomal loci and contain the X chromosome from the maternal strain. (B) The cross-fostering design consisted of transferring 2 male F1 pups from a WKY mother (white) to be raised by a F344 foster mother, while simultaneously fostering 2 F1 males from a F344 (gray) to a WKY litter. In addition, 2 native control pups from each litter were identified at the time of cross-fostering. All 4 experimental animals (2 native, and 2 cross-fostered) are marked with an X in the schematic. In all, 100 animals were analyzed: 25 pups from each group (F344 native, F344 foster, WKY native, WKY foster), 25 native pups from a F344 biological mother raised by its own F344 mother [F 1(F X W)/F344], 25 cross-fostered pups from a WKY biological mother raised by a F344 foster mother [F1(W X F)/F344], 25 native pups from a WKY biological mother raised by the WKY mother [F1(W X F)/WKY], and 25 cross-fostered pups from an F344 biological mother raised by a WKY foster mother [F1(F X W)/WKY]. F344, Fischer 344; WKY, Wistar Kyoto; F1, first-generation.
Cross-Fostering
For the cross-fostering experiment, the procedure for mating and the setup of the crosses were identical to that for maternal behavior, with the exception that 13 WKY females were timed to give birth with 13 F344 females (within 8 hours of each other).
Maternal Behavior
Pup-directed maternal behavior during the first 10 days of the pups’ life was measured using the standard protocol (Liu et al 2000; Caldji et al 1998) with slight modification including the addition of neglect (NEG) of pups. The first 10 days of life were chosen because these correspond to the time of most rapid growth in central nervous system development and in the development of the stress-response systems (Liu et al 2000; Liu et al 1997). In addition, we wanted to retain consistency for ease of comparison with other maternal behavior studies where the 10-day protocol is considered standard. Daily observation consisted of six 2-hour long observation segments (four segments in light: 07:00, 10:00, 13:00, 16:00; two segments in darkness: 21:00, 24:00) and was performed by an observer blinded to the strain and cross-fostering history of the litters. Within each 2-hour period, 40 observations were made for each animal, one every 3 min. Pup-directed behaviors recorded include arched-back nursing; blanket nursing (mother lies over pup); nursing in a passive posture (mother lies on her side or back); licking/grooming; mother carrying pup; contact with pup but mother is not engaged in any specific behaviors listed above; no contact; and neglect of pup. Whereas a mother got a score for no contact if she simply did not have physical contact with any of her pups (e.g., drinking water, sleeping away from nest), neglect was more a measure of the capacity of the mother to care for her pups in the social construct of the nest. Neglect behavior was typically seen when the mother failed to retrieve all her pups and place them back into the nest after a nest bout; it was defined as the pup being left alone more than half the length of the cage away from the nest without other pups huddled close and without the mother present.
Cross-Fostering
The cross-fostering procedure was similar to that described previously (Francis et al 1999b). WKY (n = 13) and F344 (n = 13) females gave birth, and the cross-fostering procedure only took place if the births were timed such that pups from both strains could be crossed within 2–12 hours after birth. Dams were removed from the home cage, and two male pups from each litter were cross-fostered to their new litter to be raised by a mother of the opposite strain. Animals were identified by toe clips. At the same time, 2 native male pups were removed, toe clipped, and returned to their home cage to remain with their native mother and to serve as controls (Figure 1B). The cross-fostering procedure was completed in less than 15 min per litter, and the dams returned to their respective litters. At weaning, the 2 native controls and the 2 fostered pups from each litter were housed together, for a total of 4 per cage. Overall, 100 animals were analyzed in this experiment: 25 native pups from a F344 biological mother raised by its own F344 mother [F1(F x W)/F344], 25 cross-fostered pups from a WKY biological mother raised by an F344 foster mother [F1(W x F)/F344], 25 native pups from a WKY biological mother raised by the WKY mother [F1(W x F)/WKY], and 25 cross-fostered pups from a F344 biological mother raised by a WKY foster mother [F1(F x W)/WKY].
Defensive Burying Test
At 3 months of age, the animals were tested in the DB test, as described previously (Ahmadiyeh et al 2003). Briefly, animals are habituated (4 cage-mates together) to a Plexiglas chamber (40 cm square, 60 cm high) with bedding (wood shavings; 7 cm deep, 1 cm below the hole for the prod) for 15 minutes each day for 3 consecutive days. On the fourth day, a continuously electrified prod, which delivers a shock when the rat touches it, is introduced into the chamber. The shock is generated from a shock generator (Lafayette Instruments, San Diego, California) set at 4.5 milliamperes. Animals were singly and randomly introduced into the chamber on the fourth day. The rats typically explored the novel prod and received a shock, thereby starting the 15-minute test, which was videotaped. Once shocked, animals typically did not approach the prod, retreated to the back of the cage, and began spraying bedding toward the prod in an effort to cover it. Behaviors recorded and subsequently scored by an observer blind to the identity of the animal included the latency to begin burying, the total time spent burying (duration of burying), and the number of times a rat approached the prod (snout within 1.0 cm from prod).
Statistical Analyses
Differences in maternal behavior between the two inbred strains toward their undisturbed native litters, and differences in maternal behavior of inbred strains serving as foster mothers (where 2 foster pups were crossed into their litters) were determined by two-way analysis of variance (ANOVA). These maternal behavioral measures were analyzed independently, as sometimes a mother exhibits two behaviors at the same time, and not all behaviors fit into any one of these categories. The effects of the postnatal maternal environment provided by the foster mother and the prenatal and genetic effects of the biological mother were analyzed against adult DB behavior using two-way ANOVA with genetic and fostering mothering as factors. Posthoc (Tukey) test was used when appropriate to assess differences in behavior between groups. NCSS software (Kaysville, UT) was used for all analyses.
Results
Mothering Behavior Differs Between F344 and WKY Inbred Strains
Figure 2 shows the maternal behaviors that were significantly different between F344 and WKY dams regardless of whether the pups were their own or adopted into the litter. Two-way ANOVA revealed a significant main effect of strain for all behaviors shown, with no effect from fostering and no interaction between strain and foster motherhood. F344 dams exhibited far more arched-back nursing [F(1,97) = 173.3, p < .001] and licking/grooming [F(1,97) = 27.5, p < .001] toward their F1 pups than did WKY dams. In addition, WKY dams exhibited far greater periods of no contact with their pups [F(1,97) = 124.6, p < .001] as well as far greater periods of neglect of pups [F(1,97) = 177.7, p < .001] than did F344 dams. The frequencies of blanket nursing, passive nursing posture, mother carrying pup, and times when mother contacted pup but exhibited no other behavior listed were the same for the two strains.
Figure 2.
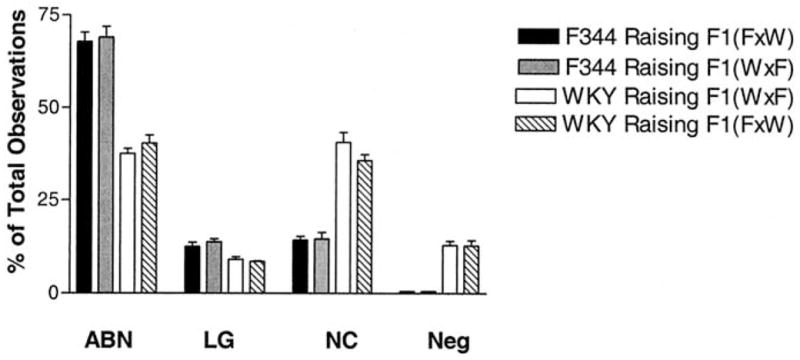
Strain differences and effect of cross-fostering on maternal behavior. Means ± SEM for maternal behaviors of arched-back nursing (ABN), licking/grooming (LG), no contact (NC), and neglect (Neg) for regular F344 mothers (F344 raising [F1(F X W)], n = 7) as compared with F344 foster mothers (F344 raising [F1(W X F)], n = 5) and for regular WKY mothers (WKY raising [F1(W X F)], n = 8) as compared with WKY foster mothers (WKY raising [F1(F X W)], n = 5) during postnatal days 1–10. There were no differences between foster mothers and regular mothers of either strain. Each behavior is represented as the mean percentage of the total number of observations. F344, Fischer 344; WKY, Wistar Kyoto.
Cross-Fostering Transiently Affects Body Weight of Pups, Leading to No Weight Differences Between Foster and Native Pups
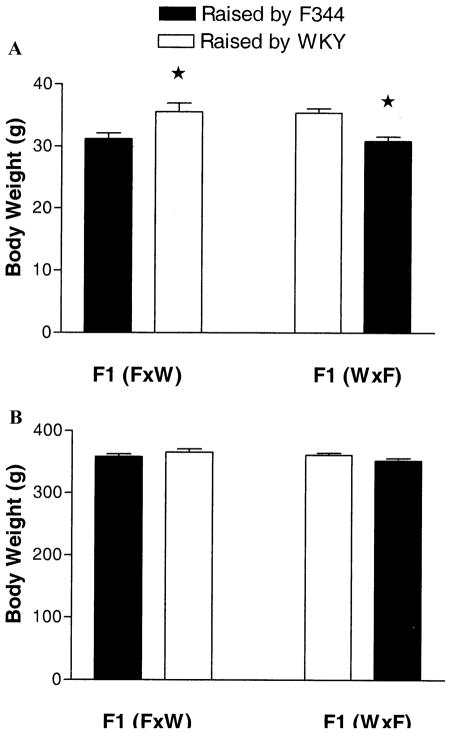
We weighed the native and foster pups at weaning and again in adulthood to determine overall health of offspring. Figure 3 shows the body weight of pups at 21 days (A) and at 4 months of age (B). Interestingly, postnatal maternal environment determined body weight at weaning. Specifically, pups (foster and native) raised by WKY dams weighed significantly [F(1,97) = 16.9; p > .001] more than pups (foster and native) raised by F344 dams. This effect of postnatal maternal environment had disappeared by 4 months of age.
Figure 3.
Effect of cross-fostering on body weight of pups at weaning (21 days of age, n = 25 pups/group) and adulthood (4 months of age, n = 25 adults/group). (A) Means ± SEM for body weight at weaning, separated by cross and the maternal environment in which the pups were raised. There was a significant main effect of environment, with offspring raised by WKY weighing more at weaning than offspring raised by F344, p < .001. Importantly, fostering status did not affect body weight of pups. That is, foster pups and native pups raised by WKY mothers (and foster pups and native pups raised by F344 mothers) weighed the same. (B) Body weight at 4 months of age is not significantly different in any of the conditions, irrespective of genotype or environment. F344, Fischer 344; WKY, Wistar Kyoto.
Postnatal Maternal Environment Significantly Influenced Some Defensive Burying Behaviors in Adulthood
In this experiment, three specific behavioral components of the DB test (latency, duration, and approach) were assessed in adult male F1 offspring of reciprocal crosses, reared by their own mother or foster mother from the other strain.
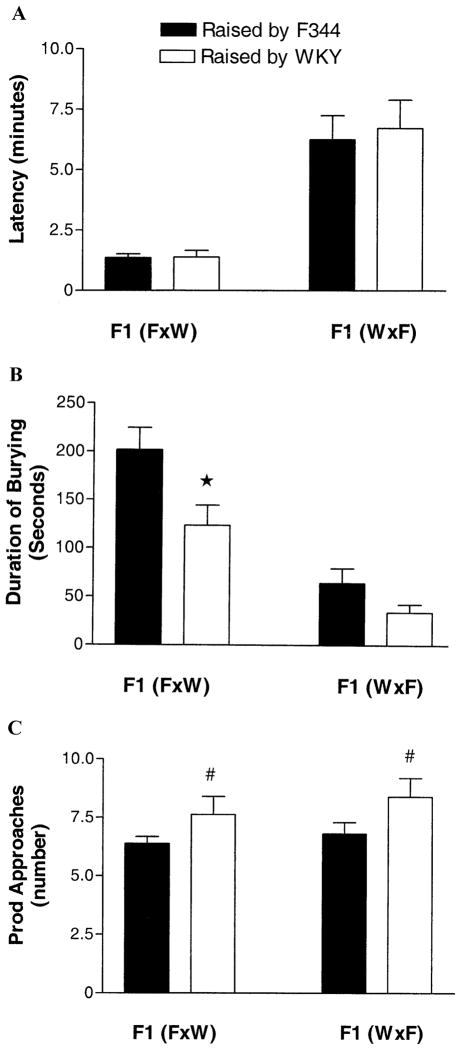
Male F1 offspring of F344 mothers [F1(F X W)] begin spraying bedding toward the shock prod after receiving a shock with a significantly shorter latency (Figure 4A); [F(1,97) = 38.2, p < .001] than male offspring of WKY mothers [F1(W F)], irrespective of the pre-weaning, postnatal maternal environment of the pups.
Figure 4.
Means + SEM of coping behaviors of adult rats by X chromosomal genotype and pre-weaning postnatal maternal environment. (A) Latency to bury: significant main effect of genotype, p < .001. B) Duration of burying: significant effect of genotype, p < .001, as well as a significant effect of maternal environment, p < .01; asterisk indicates significant difference from F1(F X W) raised by F344. (C) Prod Approaches: significant main effect of maternal environment, p < .05; number sign indicates significant difference (main effect) from offspring raised by F344. F1, first-generation; F344, Fischer 344.
In general, adult male offspring of F344 mothers [F1(F X W)], buried significantly (Figure 4B); [F(1,97) = 31.4, p < .001] more than the offspring of WKY mothers [F1(W X F)] (independently of maternal environment); however, postnatal environment significantly [F(1,97) = 7.12, p = .008] affected the duration of burying. Specifically, male offspring of F344 mothers [F1(F X W)] raised by WKY foster mothers bury for a significantly (p < .05) shorter time than their genetic littermates raised by their genetic F344 mother [F1(F X W)]. Similarly, male F1 pups of WKY mothers [F1(W X F)] raised by F344 foster mothers tended (p = .09) to bury longer than their genetic littermates raised by their genetic WKY mother, suggesting an additive genetic and environmental contribution to the development of this behavior.
The postnatal maternal environment also had a significant effect on the number of prod approaches the male F1 offspring made in the DB test as adults. Male F1 offspring raised in a WKY environment had more prod approaches [F(1,97) = 4.8, p < .05] than those reared by F344 mothers regardless of their genetic mother (Figure 4C). Male F1 offspring of WKY mothers [F1(W X F)] reared by their genetic mother showed only a tendency toward approaching the prod more frequently than F1(W X F) offspring raised F344 foster mothers; a finding probably due to a relatively small number of animals in each group as compared with our previous study (Ahmadiyeh et al 2003).
Discussion
In this study, we investigated the role of maternal environment in the development of behavioral coping responses to stress using two genetically and phenotypically distinct inbred strains of rats, WKY and F344. In our previous study, an F2 intercross of these rats found three quantitative trait loci on the X chromosome that significantly contributed to DB behaviors (Ahmadiyeh et al 2003). By cross-fostering F1 males (genetically identical except for gender chromosomes), we were able to simultaneously assess the contribution of X genotype and postnatal maternal environment toward the development of adult coping behaviors. Specifically, we have shown that F344 mothers who engaged in more arched-back nursing and licking/grooming maternal behaviors (active, stimulatory behaviors) raised offspring who—independent of X chromosomal genotype or biological parent—employed a more active coping strategy in the DB test as adults. Likewise, WKY mothers who exhibited less of these stimulatory behaviors with their litters, while simultaneously exhibiting greater periods of no contact and neglect of pups, raised offspring who grew up to employ a more passive coping strategy in the DB test. This effect of the postnatal maternal environment was significant for both the duration of burying and prod approach measures; latency to bury, however, was a genetically robust trait and was not influenced by the maternal environment. While the magnitude of these maternal effects may seem fairly modest, they clearly imply the moderating effect of maternal environment on these genetically determined behavioral traits.
We were able to observe these subtle maternal effects because our cross-fostering technique did not alter the maternal behavior of foster mothers, as evidenced by the lack of difference in weaning weight between foster and native pups within each litter. Interestingly, the genotype of foster mother altered the influence of maternal genotype on weaning weight. The most likely explanation for this finding is that the nutrient/fat composition of milk differs between these strains. This is particularly likely in light of the fact that litter size and blanket and passive posture nursing were not different between the strains, and arched-back nursing was actually greater in F344 mothers. Although we did not directly study milk composition in our experiments, differences in milk composition between inbred strains has been shown to influence the diabetic susceptibility of F1 offspring in intercross populations (Reifsnyder et al 2000).
It is expected that behaviors in the DB test would be affected by the postnatal maternal environment, as maternal behavior has been shown to influence stress responses, fear, and anxiety in rats (Weaver et al 2002; Francis et al 1999a; Caldji et al 1998; Romeo et al 2003). Furthermore, maternal behavior moderates the effect of specific genotype on novelty seeking in children (Keltikangas-Jarvinen et al 2003). However, when no differences in maternal care are observed, no effect of postnatal maternal environment can be ascertained, for example, on levels of anxiety in a genetic rat model of anxiety (Wigger et al 2001). This latter finding strongly supports the role of specific maternal behaviors in the effect of cross-fostering on the adult DB behavior.
The DB test was originally developed as an ethologically valid behavioral test of anxiety (Treit et al 1981). In this test, after receiving the first shock, the animal has primarily two options to avoid further shock. It may move itself to the corner of the cage, or it may actively bury the prod with the bedding material. These two behavioral strategies could be interpreted as passive or active coping responses to stress. The concept of coping styles has been used and debated in a variety of animal species and behavioral paradigms (Koolhaas et al 1999). Individual coping strategies are thought to be affected by genetic, developmental, environmental, and learned components. As coping is primarily an adaptive response to changes in the environment (Francis et al 1999a), and the relative benefit of active or passive coping strategies can change depending on alterations in the environment; both active and passive coping in the DB paradigm can be considered adaptive. On the other hand, WKYs, who show passive coping behaviors in the DB test, express stable behavioral characteristics that suggest the continuous presence of behavioral inhibition or despair (Pare 1989; Pare and Redei 1993; Lahmame and Armario 1996; Lopez-Rubalcava and Lucki 2000; Redei et al 2001). Thus, this passive coping behavior of WKYs in the DB test is not likely the result of a successful coping strategy but rather a genetically determined predictable stress response strategy across different behavioral paradigms, which our findings suggest are amenable to some degree of environmental influence.
The current finding that the contribution of maternal environment and genotype is additive in affecting DB behavior is of particular interest. Although not specifically addressed in this study, the fact that maternal behavior can influence some of the genetically inherited DB phenotypes might imply that maternal behavior can influence coping behavior across generations. Thus, just like in humans (Keltikangas-Jarvinen et al 2003), rearing environment can alter the impact of genotype in this trait of behavioral coping with stress.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH 60789 (EER).
References
- 1.Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE. X-linked and lineage-dependent inheritance of coping responses to stress. Mamm Genome. 2003;14:748–757. doi: 10.1007/s00335-003-2292-x. [DOI] [PubMed] [Google Scholar]
- 2.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canetti L, Bachar E, Galili-Weisstub E, De-Nour AK, Shalev AY. Parental bonding and mental health in adolescence. Adolescence. 1997;32:381–394. [PubMed] [Google Scholar]
- 4.Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos G, Gold P. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. 1074. [PubMed] [Google Scholar]; BIOL PSYCHIATRY. 2004;55:1069–1074. doi: 10.1016/j.biopsych.2004.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cierpial MA, McCarty R. Hypertension in SHR rats: Contribution of maternal environment. Am J Physiol. 1987;253:H980–H984. doi: 10.1152/ajpheart.1987.253.4.H980. [DOI] [PubMed] [Google Scholar]
- 7.Flint J. Analysis of quantitative trait loci that influence animal behavior. J Neurobiol. 2003;54:46–77. doi: 10.1002/neu.10161. [DOI] [PubMed] [Google Scholar]
- 8.Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor—norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999a;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 9.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999b;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 10.Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Serrano M, Tonelli L, Listwak S, Sternberg E, Riley AL. Effects of cross fostering on open-field behavior, acoustic startle, lipopolysaccharide-induced corticosterone release, and body weight in Lewis and Fischer rats. Behav Genet. 2001;31:427–436. doi: 10.1023/a:1012742405141. [DOI] [PubMed] [Google Scholar]
- 12.Keltikangas-Jarvinen L, Raikkonen K, Ekelund J, Peltonen L. Nature and nurture in novelty seeking. Mol Psychiatry. 2004;9:308–311. doi: 10.1038/sj.mp.4001433. [DOI] [PubMed] [Google Scholar]
- 13.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 14.Lahmame A, Armario A. Differential responsiveness of inbred strains of rats to antidepressants in the forced swimming test: Are Wistar Kyoto rats an animal model of subsensitivity to antidepressants? Psychopharmacology. 1996;123:191–198. doi: 10.1007/BF02246177. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 18.Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. Journal of Neuroscience. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 20.McMurtry JP, Wright GL, Wexler BC. Spontaneous hypertension in cross-suckled rats. Science. 1981;211:1173–1175. doi: 10.1126/science.7466389. [DOI] [PubMed] [Google Scholar]
- 21.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001a;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 22.Meaney MJ. Nature, nurture, and the disunity of knowledge. Ann NY Acad Sci. 2001b;935:50–61. doi: 10.1111/j.1749-6632.2001.tb03470.x. [DOI] [PubMed] [Google Scholar]
- 23.Myers MM, Brunelli SA, Shair HN, Squire JM, Hofer MA. Relationships between maternal behavior of SHR and WKY dams and the adult blood pressures of cross-fostered F1 pups. Dev Psychobiol. 1989;22:55–67. doi: 10.1002/dev.420220105. [DOI] [PubMed] [Google Scholar]
- 24.Nadeau J, Singer J, Matin A, Lander E. Analysing complex genetic traits with chromosome substitution strains. Nature Genetics. 2000;24:221–225. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- 25.Pare WP. Behavioral despair test predicts stress ulcer in WKY rats. Physiol Behav. 1989;46:483–487. doi: 10.1016/0031-9384(89)90025-5. [DOI] [PubMed] [Google Scholar]
- 26.Pare WP, Redei E. Depressive behavior and stress ulcer in Wistar Kyoto rats. J Physiol Paris. 1993;87:229–328. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- 27.Parker G. Parental representations of patients with anxiety neurosis. Acta Psychiatr Scand. 1981;63:33–36. doi: 10.1111/j.1600-0447.1981.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 28.Rapp JP, Deng AY. Detection and positional cloning of blood pressure quantitative trait loci: Is it possible? Identifying the genes for genetic hypertension. Hypertension. 1995;25:1121–1128. doi: 10.1161/01.hyp.25.6.1121. [DOI] [PubMed] [Google Scholar]
- 29.Redei EE, Solberg LC, Kluczynski JM, Pare WP. Paradoxical hormonal and behavioral responses to hypothyroid and hyperthyroid states in the Wistar-Kyoto rat. Neuropsychopharmacology. 2001;24:632–639. doi: 10.1016/S0893-133X(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 30.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhees BK, Ernst CA, Miao CH, Atchley WR. Uterine and postnatal maternal effects in mice selected for differential rats of early development. Genetics. 1999;153:905–917. doi: 10.1093/genetics/153.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann NY Acad Sci. 1994;746:294–307. doi: 10.1111/j.1749-6632.1994.tb39247.x. [DOI] [PubMed] [Google Scholar]
- 34.Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 35.Treit D. Defensive burying: A pharmacolgocial animal model for specific fears? In: Soubrié P, Simon P, Widlocker D, editors. Anxiety, Depression, and Mania: Animal Models of Psychiatric Disorders. New York: Karger; 1991. pp. 1–19. [Google Scholar]
- 36.Treit D, Menard J. The shock-probe burying test. Neuroscience Protocols. 1993:9–17. [Google Scholar]
- 37.Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: A new paradigm for the study of anxiolytic agents. Phamacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- 38.Weaver IC, Szyf M, Meaney MJ. From maternal care to gene expression: DNA methylation and the maternal programming of stress responses. Endocr Res. 2002;28:699. doi: 10.1081/erc-120016989. [DOI] [PubMed] [Google Scholar]
- 39.Wigger A, Loerscher P, Weissenbacher P, Holsboer F, Landgraf R. Cross-fostering and cross-breeding of HAB and LAB rats: A genetic rat model of anxiety. Behav Genet. 2001;31:371–382. doi: 10.1023/a:1012222402346. [DOI] [PubMed] [Google Scholar]