Abstract
Quadrupeds and bipeds respond to horizontal perturbations of the support surface with muscular responses that are broadly tuned and directionally sensitive. Since the discovery of this directional sensitivity, interest has turned toward the critical sensory systems necessary to generate these responses. We hypothesize that cutaneous feedback affects the magnitude of muscle responses to postural perturbation but has little effect on the directionality of the muscle response. We developed a modified premammillary decerebrate cat preparation to evaluate the sensory mechanisms driving this directionally sensitive muscle activation in response to support surface perturbation. This preparation allows us the flexibility to isolate the proprioceptive (cutaneous and muscle receptors) system from other sensory influences. We found that loss of cutaneous feedback leads to a significant loss in background activity causing a smaller muscular response to horizontal perturbations. However, the directional properties of the muscular responses remained intact.
Keywords: Posture, Cutaneous, Decerebrate cat, Proprioception
Introduction
Studies utilizing the intact human (Henry et al. 1998), intact cat (Macpherson 1988b, a), and decerebrate cat (Honeycutt et al. 2009; Honeycutt and Nichols 2010) have revealed the neural strategy used to respond to horizontal, radial perturbations of the support surface. Muscular activity is quantified using tuning curves, in which the increases or decreases in muscle activity are compared and graphed against perturbation direction. In the decerebrate cat preparation, and in agreement with studies on human subjects and intact cats, the tuning curve for each muscle has a principal direction of activation and is generally active over about 25% of the perturbation space. Since the discovery of this directionally sensitive muscular activation, interest has turned toward the critical sensory systems necessary to generate these responses.
Sensory signals from the vestibular system, skin, and muscles have been implicated in mediating postural responses (Macpherson and Inglis 1993; Allum et al. 1998; Stapley et al. 2002; Beloozerova et al. 2003; Ting and Macpherson 2004; Everaert et al. 2005). The relative contributions of these systems to muscular activation patterns have yet to be determined; however, some progress has been made concerning the role of vestibular feedback. Vestibular loss leads to hypermetria, which destabilizes the animal (Macpherson and Inglis 1993). Nevertheless, the spatial tuning properties of the responses (direction and breadth) are little affected by the lesion, suggesting that feedback from either skin or muscle (or both) contributes in a major way to directional tuning of these responses.
Some have suggested that cutaneous feedback, specifically from the plantar surface of the foot, alone could be responsible for directional muscle activation (Bloem et al. 2000; Ting and Macpherson 2004). Evidence for an important role of cutaneous feedback comes from studies showing that cutaneous inputs can affect postural stability during unperturbed stance (Roll et al. 2002; Meyer et al. 2004). However, additional research has shown that feedback from muscle proprioceptors must be integrated with cutaneous inputs to achieve the observed spatial tuning (Kavounoudias et al. 2001). This notion is supported by work demonstrating that cutaneous stimulation during perturbed stance does not significantly alter muscular activation (Maurer et al. 2001) implicating a critical role for muscle proprioceptors. The extent to which these two sensory systems contribute to postural responses remains unresolved.
We hypothesize that loss of cutaneous feedback will affect the magnitude of the muscle response to postural perturbation but will have little effect on the directionality of the muscle response. We base this hypothesis on reports indicating that complete loss of cutaneous feedback does not alter the pattern of locomotion (Bouyer and Rossignol 2003). Rather, cutaneous feedback appears more important during challenging types of movement such as walking across a ladder or responding to limb perturbations during locomotion (Bouyer and Rossignol 2003; Bolton and Misiaszek 2007). Indeed, cutaneous denervation does not disrupt the response to perturbations during locomotion, but the response is diminished leading to increased postural instability (Bolton and Misiaszek 2007). Therefore, we suspect a similar result when cutaneous feedback is impaired during perturbations of postural tasks.
We developed the modified premammillary decerebrate cat preparation specifically to further evaluate the sensory mechanisms driving appropriate muscle activation in response to support surface perturbation. We have previously demonstrated that this preparation consistently generates muscle activation patterns and subsequent force trajectories typical of the intact animal (Honeycutt et al. 2009; Honeycutt and Nichols 2010). This preparation allows us the flexibility to isolate the proprioceptive (cutaneous and muscle receptors) system from other sensory influences. Specifically, fixation of the head largely precludes both visual and vestibular feedbacks. Therefore, the resulting muscle responses are generated mainly through feedback of skin, muscle, and joint receptors. Therefore, we disrupted the cutaneous system acutely and observed the system’s response without (1) the influence of the vestibular and visual systems and (2) the adaptation that likely would occur if completed chronically. We eliminated a major source of cutaneous feedback to the foot pads through tibial nerve crush at the ankle. We evaluated the muscular responses following this crush and found that indeed the loss of cutaneous feedback leads to a decrease in background activity and amplitude of the muscular response to postural perturbations. However, the latency and direction of the muscular activation remained intact.
Methods
Experimental protocol
Data from three cats, used in accordance with the issued standards of the National Institutes of Health and the Emory Institutional Animal Care and Use Committee, were utilized in these experiments. Data from these three animals were also represented in previous publications (Honeycutt et al. 2009; Honeycutt and Nichols 2010). Experiments are referred to by date (9/11, 10/8, and 6/8). Under isoflurane anesthesia, a tracheotomy was performed to monitor anesthesia levels, and an IV was inserted in the external jugular vein for hydration and drug delivery. Bipolar electrodes, constructed from teflon-coated braided wire, were inserted into the medial gastrocnemius (MG), lateral gastrocnemius (LG), soleus (Sol), vastus lateralis (VL), vastus medialis (VM), semitendinosis (ST), semimebranosis (SM), gracilis (Grac), iliopsoas (Ilio), anterior biceps femoris (aBF), posterior biceps femoris (pBF), and gluteus medius (Glut) muscles.
We used a modified premammillary decerebration technique, which has been previously described in detail (Honeycutt et al. 2009). In this technique, the premammillary decerebration is modified with a vertical transection near the subthalamic nucleus. This additional transection preserves postural tone and reactivity but significantly disrupts the locomotion patterns typically associated with the premammillary preparation.
After the initial surgery, the animal was positioned using a stereotaxic frame to support the head while a clamp at the base of the tail and a sling supported the body. The sling was detached after isoflurane was completely removed and weight bearing established. Anatomical measurements, collected on intact animals during standing, were used to position the hip height, head height, and paw spacing (both transverse and sagittal planes). The toe pads of all four limbs were fixed with glue and tape to four ATI force transducers. The force transducers were configured with right-handed coordinate systems, with the X direction pointing rightward. The natural turnout of the foot was used for each animal. The bipolar EMG electrodes were attached to a preamplifier, with a gain of 200 (overall gain of 1,000) and bandpass filter from 10 to 1,150 Hz. Electromyographic (EMG) data from the electrodes and three-dimensional force data from the transducers were collected using a Lab-View program designed specifically for this project.
Once the animal was removed from anesthesia, the support surface under all four limbs was translated in 16 different, horizontal directions using two motors: rotational and linear. The rotational motor positioned the linear motor for the perturbation. This technique ensured a linear perturbation. In experiments 9/11 and 6/8, the support surface was perturbed 8 cm over 400 ms with an acceleration of 0.5 (m/s2). In the third animal, the platform was moved 4 cm over 400 ms. The amplitude adjustment was made for purposes outside the range of this study; however previous reports indicate that the amplitude of the perturbation does not alter direction and breadth of muscular activation patterns (Diener et al. 1988). Therefore, this animal was included. The perturbation parameters were chosen based upon previous studies in intact cats (Macpherson 1988b, a) for comparison purposes. Unique to the decerebrate cat, the animals’ head and tail were in a fixed position. Thus, the limbs were perturbed, held at the extended position for 1,000 ms and then returned 4 or 8 cm over 400 ms to the initial position. In one animal (10/8), the hold position was only 500 ms.
Cutaneous denervation
Data were collected before and after cutaneous denervation of the foot pads. Cutaneous denervation was accomplished through crush of the tibial nerve at the ankle. This nerve innervates the foot pads and the intrinsic muscles of the foot (Crouch 1969). After collection of the control data, the medial side of the ankle was opened with a scalpel blade and the tibial nerve identified. The tibial nerve was then crushed proximally to the ankle. For two of the three experiments, a gauze pad with lidocaine was placed over the tibial nerve before crushing to reduce the noxious stimulus of the crush. After tibial nerve crush, perturbations were delivered in the same manner as during the control trials. Following data collection, the animal was euthanized using concentrated pentobarbital, and a pneumothorax was performed. In one experiment (6/8), the tibial nerve of the left hindlimb was additionally cut. Perturbations were delivered, and muscle activation evaluated with disruption of the tibial nerve in both hindlimbs.
EMG quantification
In order to quantify muscle activation, tuning curves were created. Raw EMG data were first notch filtered for 60-Hz noise, demeaned, high passed at 30 Hz (to remove movement artifact), and rectified. Changes in muscle activation were evaluated by calculating the mean EMG activity during the perturbation (400 ms) and subtracting the mean EMG activity during the 400 ms prior to perturbation. The tuning curve was created by graphing the resultant of this calculation against perturbation direction. Therefore, a single tuning curve is a compilation of data collected from all 16 directions of perturbation. We evaluated all trials (full set of 16 directions) where measurable EMG was present. EMG was considered measurable if it was above the level of noise in the system. Tuning curves were normalized to maximum mean activation across all 16 directions before quantification. Latencies were estimated by visual inspection. An increase in firing amplitude or firing rate greater than the maximum amplitude of firing rate during the background for a minimum of 5 ms was considered an active response.
In order to quantify the tuning curves, the breadth and principal direction were calculated for each muscle response. The breadth quantifies the responsiveness of a muscle to multiple directions. The breadth of the response was calculated using the area under the normalized excitatory tuning curves. The principal direction represents the maximum direction of activation. To determine the principal direction, each muscle response was converted to a vector, and x and y components averaged to find the primary vector or principal direction of the response. The principal direction represents the average direction of all directional muscle activity. In order to compare the tuning curves before and after tibial nerve crush, the principal directions obtained before crush were then plotted versus the principal directions obtained after nerve crush. If the data points lay along the unity line, then the principal direction was not altered substantially by the crush. Similar plots were constructed for breadth.
As principal directions and breadths were unique to each animal, these measures were calculated and compared in each muscle individually. Principal direction and breadth measures for each trial (full set of 16 directions) were averaged to find the mean and standard deviation of each measure in each muscle before (control) and after denervation (tibial crush). Principal directions and breadths were compared before and after denervation to determine whether they were “similar” or “not similar.” If the denervation measure was outside two standard deviations from the mean control measure, it was classified as “not similar”.
Results
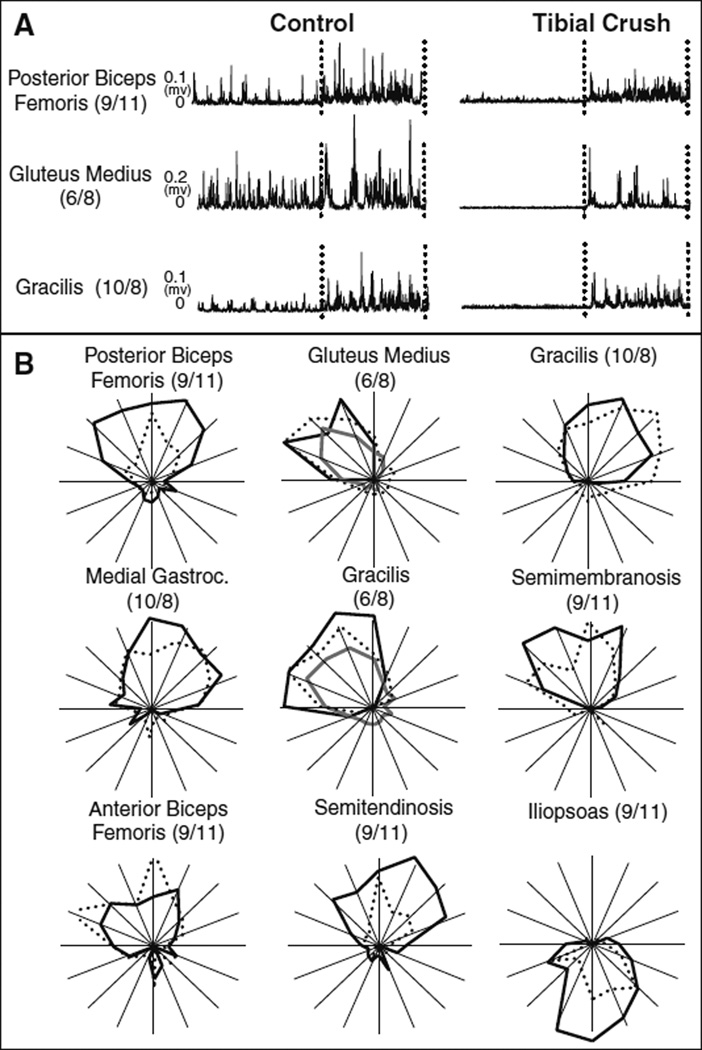
The excitatory response remained intact after tibial nerve crush; however background muscle activity was substantially reduced. Figure 1a depicts raw EMG traces (three trials averaged together) of one muscle from each of the three experiments before and after tibial nerve crush. The dashed lines demarcate the initiation and termination of the perturbation. All data are depicted on the same scale such that amplitude comparisons can be made between the control and “crush” conditions. Right tibial nerve crush almost extinguished the tonic background activity of each muscle in all three experiments. However, the excitatory response to the perturbation remained intact. This result was very consistent. Specifically all muscles studied (29 muscles across 3 animals), with one exception (VL: 9/11 experiment), produced an excitatory response following crush. Further, these excitatory responses occurred at similar latencies, between 20 and 30 ms. The loss of background firing remained present when both of the tibial nerves were crushed in the 6/8 experiment.
Fig. 1.
Comparison of raw EMG data and tuning curves before and after cutaneous denervation The raw EMG traces before and after tibial nerve crush are shown. (a). The background activity in all experiments was diminished, but a strong excitatory response remains intact. Also shown are the tuning curves for the intact (solid black), right tibial nerve crush (dashed) and both tibial nerves crushed (solid gray) conditions (b). Tuning curves remain intact in direction, but alterations in breadth and amplitude are present
Despite the loss of background firing, tuning curves remained intact after tibial nerve crush. Figure 1b shows the tuning curves (created by computing the difference between excitatory and background activity) before (solid black), after (dashed) right tibial nerve crush, and after both tibial nerves were crushed (solid gray). The first three tuning curves (pBF, Glut, and Grac) correspond to the raw EMG traces shown in Fig. 1a. While it was noted from the raw data that the excitatory response was smaller in amplitude, the tuning curves appeared similar in shape, direction, and magnitude after one or both tibial nerves were crushed. However, tuning curves following tibial crush were more variable in level of activation instead of the smooth and graded tuning curves seen during the control trials. As shown in Fig. 1b, for example, two peaks of activation were observed for SM and aBF. In the pBF, ST, and Ilio muscles, smaller changes in muscle activity were observed after denervation as demonstrated by the smaller magnitude of their tuning curves after denervation.
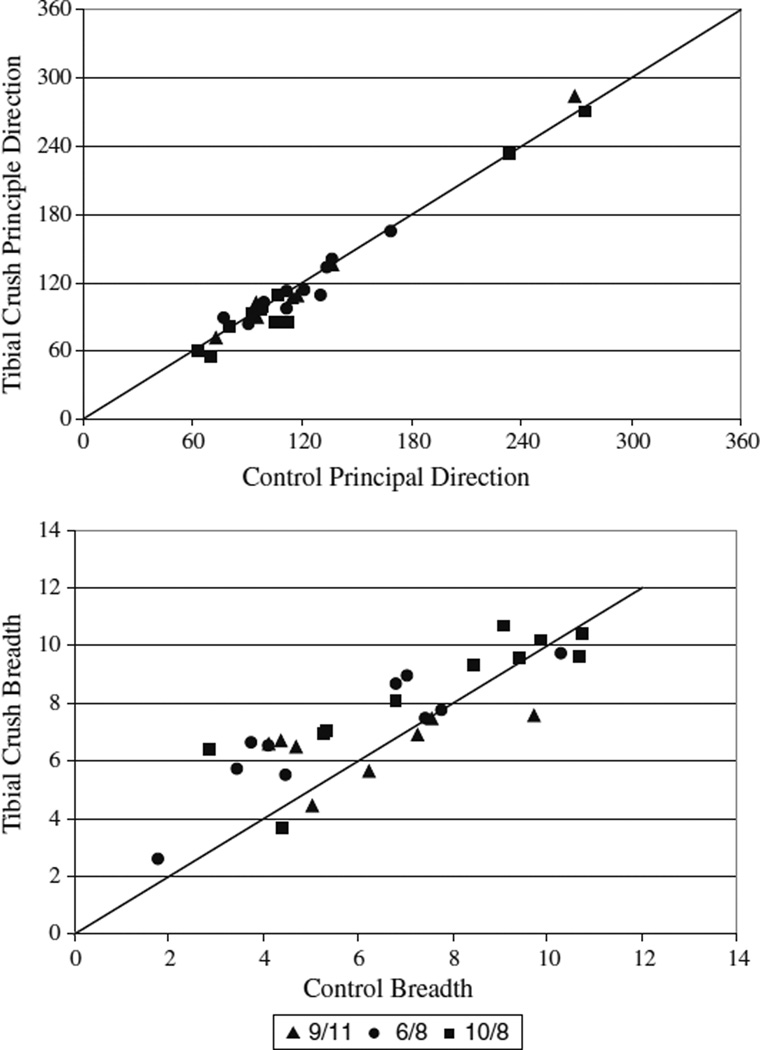
Principal direction was conserved after denervation. When only the right tibial nerve was crushed, all but 3 of 29 muscles surveyed were found to be “similar.” Fig. 2a shows the mean principal directions of all muscles plotted before versus after cutaneous denervation. The data points lie very close to the unity line indicating a strong similarity before and after denervation. The three individual muscles found to be “not similar” were IL (9/11), VM (6/8), and Grac (10/8). Grac (10/8) and IL (9/11). They are depicted in Fig. 1a. While the principal directions of these muscles were classified as “not similar,” they are still active over comparable directions. Further, all three of the muscles were tested in another experiment where their responses were found to be “similar.” Finally, there was an increase in principal direction variability after denervation with 68% of muscles showing larger standard deviations. When both the right and left tibial nerves were crushed in the 6/8 experiment, 9 of 10 muscles surveyed were found to have “similar” principal directions to the control condition.
Fig. 2.
Principal direction and breadth comparison before and after cutaneous denervation of the foot pads. Principal direction and breadth were quantified before and after the cutaneous intervention. The mean and standard deviation of the principal direction and breadth before and after intervention are depicted along with a unity line (representing a ratio of 1 between conditions) for comparison of the conditions. Principal directions were largely conserved (top) with most ratios lying near or on top of the unity line. Breadths were shown to be more variable with most muscles showing an increase in breadth after tibial crush (bottom)
Breadths were less conserved (Fig. 2b). When the right tibial nerve was crushed alone, only 11 of 29 muscles’ breadths were found to be “similar.” Of the muscles classified as “not similar,” most (15 out of 18) presented an increase in breadth. For most muscles (86%), we also observed increases in variability of breadth measures after denervation. When both the right and left tibial nerves were crushed in the 6/8 experiment, 3 out of 10 muscles were found to be “not similar” to the control condition.
Discussion
Crush of the tibial nerve diminished but did not eliminate directionally specific muscle activation in response to support surface perturbation. All muscles across all three subjects, with one exception, generated an excitatory responses following tibial nerve crush. The excitatory responses following crush of one or both tibial nerves remained directionally appropriate generating similar tuning curves to the control condition. However, tibial nerve crush did impact the scale of the muscle responses. Background firing was substantially reduced in all muscles leading to diminished excitatory response amplitudes to perturbation. This diminished amplitude was accompanied by increases in variability of the principal direction and breadth of the tuning curves.
The decrease in firing, both in background activity and in response to perturbation, was present in every animal, suggesting that it was due to the intervention. We continued to take data until the animal was no longer responsive. None of the animals surveyed ever regained background firing indicating that the denervation specifically affected muscle activity levels. While it is possible that some reduction was due to deterioration, the abrupt decrease in activity following denervation suggests that the loss of inputs from the tibial nerve was a major source of the reduction in response. In most cases, the differences between the excitatory responses and the corresponding background activities were unaltered as seen by the similarly sized tuning curves. Some muscles appeared less responsive in all the previous directions, exemplified by the semitendinosus and iliopsoas muscles of Fig. 1b.
Crush of the tibial nerve also led to alterations in breadth and increased variability in both tuning curve measures. The decreased amplitude of muscle activity was likely a strong contributor to the increased variability present after tibial nerve crush. The loss of signal decreases the signal-to-noise ratio leading to 68 and 86% of muscles showing increased standard deviations in principal direction and breadth, respectively. This increased variability also impacted the smoothness of the tuning curves. Generally, tuning curves were graded, with activation smoothly decreasing on either side of the principal direction. Activation is shown to slowly increase until a maximum is reached and then a slowly decrease until the muscle no longer responsive. After denervation, tuning curves were often less smooth. It follows that a loss of signal would lead to increase in breadth and variability, resulting in loss of consistent fine control of muscle activation and instability of the subject.
Feedback from the tibial nerve was not necessary for muscles to retain directionally specific activation patterns in response to support surface perturbation. Across all experiments and muscles surveyed, only three individual cases were found to have “not similar” principal directions. It is important to note, however, that even though the principal directions were “not similar”, each muscle retained directionally specific activation. Each of these “not similar” muscles was tested in another subject, and their directions found to be “similar.” Therefore, these differences do not appear to be considerable. Further, when both tibial nerves were crushed in the 6/8 experiment, 9 out of 10 muscles generated “similar” principal directions to control. A further illustration of the conservation of principal direction is shown by the close clustering to the line of identity observed in Fig. 2a. These results indicate that the primary direction of activation remained largely unaffected by tibial nerve crush.
Sensory mechanisms
This report gives us insight into the potential role of feedback from the tibial nerve regulating postural control. The tibial nerve is a mixed nerve supplying a major source of cutaneous feedback to the foot pads as well as motor innervation to the intrinsic muscles of the foot. Our data imply that these feedback sources scale muscle activation during postural tasks. Previous reports that various cutaneous nerves can excite or inhibit motor unit activity during cat fictive locomotion (Degtyarenko et al. 1996) demonstrate the feasibility that cutaneous feedback may be able to contribute to the scale of muscular activity. While it is noted that cutaneous reflexes possibly differ between the cat and human, similar cutaneous effects as those present in the cat have been observed in the intact human. A recent report described the firing patterns of individual cutaneous afferents of the foot isolated from the tibial nerve coupled with muscle activity during voluntary contraction in the human (Fallon et al. 2005). While all types of cutaneous receptors showed some reflex coupling, the fast adapting I type most consistently demonstrated association with EMG activity. Furthermore, this type of modulation appears present during motor tasks such as locomotion and posture. Cutaneous reflexes are known to impact the strength of muscular activation during locomotion in cats and humans (Duysens and Pearson 1976; Loeb 1993; Guertin et al. 1995; Schomburg et al. 1998; Zehr and Stein 1999). Moreover, loss of cutaneous inputs changes the amplitude of muscular responses to perturbation during walking but not the response itself (Bolton and Misiaszek 2009).
Although it has been noted that the strength of cutaneous responses may differ between premammillary and intact cats (Duysens 1977; Duysens and Loeb 1979), it should be pointed out that the postural responses of the decerebrate preparation used in the experiments described here (Honeycutt et al. 2009; Honeycutt et al. 2009) are similar to those observed in intact animals (Macpherson 1988b, a), supporting the relevance of the findings reported here to postural control in the intact animal.
The decreases in muscle activity reported here cannot necessarily be relegated to cutaneous sources. The tibial nerve also innervates some of the intrinsic muscles of the paw (Crouch 1969). While much information exists about the lumbrical muscles of the foot in relation to the intrinsic properties of these muscles and their spindles, little is known about their global neural connections (Solomonow and Krogsgaard 2001). However, there is growing evidence indicating that muscle spindles from the intrinsic foot muscles synapse onto the motor neuron pools of the leg and thigh muscles (Marque et al. 2001, 2005). It is reasonable to assume that these muscles would relay important information about the external environment and could play a role in the scaling of the muscular responses that occur after postural disturbances. More research is required to probe their influence during postural control.
While feedback from the foot pads and/or the lumbrical muscles may provide important scaling information, the absence of these sources does not alter the directionally specific muscle activation in response to support surface perturbations. As the visual and vestibular systems are largely prevented from contributing to these responses by fixation of the head, the resulting muscle activation patterns most probably result from joint, remaining cutaneous receptors, and/or muscle.
Joint receptors and other sources of cutaneous feedback play an important role in postural stability but are not likely candidates for providing specific information about direction. Joint receptors are less likely to contribute as they do not respond within the normal ranges of motion in the cat hindlimb (Burgess and Clark 1969). It is more difficult to rule out the other contribution of the cutaneous feedback from receptors in other limbs, non-foot pad sources, and partial feedback from the second and third digits of the foot innervated by the deep peroneal nerve (Bouyer and Rossignol 2003). The result obtained when both tibial nerves were crushed indicates that cutaneous feedback from the contralateral hindlimb is not necessary for directionally appropriate muscle activation. It is possible that the forelimbs, which were moved during this study, may be contributing to muscle activation. However, we have noted in other experiments where the forelimbs were placed on a nonmoving support, we still obtained directional tuning (unpublished observations). While some residual cutaneous feedback remains in the foot pads, our intervention eliminated the most substantial source of feedback. Based on these results, we suggest that other sources of cutaneous feedback are playing a similar scaling role in postural control.
Conversely, there is growing evidence for a role of muscle receptors, specifically muscle spindles, in driving the directional tuning of muscular responses. We have recorded from Group I and II muscle spindle afferents during horizontal translations of the right hindlimb. All muscle spindle afferents generated broad directionally specific activation patterns remarkably similar in direction and breadth to the muscle activation patterns shown in the intact and decerebrate cat (Honeycutt et al. 2007). This finding suggests that muscle spindle receptors are relaying important directional information about the support surface perturbation to the nervous system. Further, we have preliminary data that elimination of autogenic muscle receptor feedback abolishes directionally appropriate muscle activation (Honeycutt et al. 2008). We performed a reinnervation surgery, which eliminates autogenic feedback but preserves motor function (Cope et al. 1994; Abelew et al. 2000; Haftel et al. 2005). When we subjected these animals to the same perturbations as described in this report, we found that directionally appropriate muscle activation was abolished during perturbations of the one limb. Cutaneous feedback was unaffected by the intervention. This result indicates that cutaneous feedback is not sufficient to drive directional muscle activation. Although it cannot be excluded that cutaneous receptors could provide directional information, they are unlikely to be the primary contributors. Therefore, other sources of sensory feedback, such as muscle receptors, are probably playing an important role in driving the direction of muscular responses to postural perturbations.
Acknowledgments
The authors would like to thank Victoria Stahl and Dr. Jinger Gottschall, for their assistance in data collection and surgical expertise. The authors would also like to thank Bill Goolsby for the creation of the software necessary for data collection and Richard Kiser and Gene Schmitt for construction of major equipment. This work was supported by NIH grant NS20855 and HD32571.
Contributor Information
Claire F. Honeycutt, Email: c-honeycutt@northwestern.edu, Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, USA; Rehabilitation Institute of Chicago, 345 E. Superior St, SMPP: Suite 1406, Chicago, IL 60611, USA.
T. Richard Nichols, Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, USA; School of Applied Physiology, Georgia Institute of Technology, Atlanta, GA, USA.
References
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Allum JH, Bloem BR, Carpenter MG, Hulliger M, Hadders-Algra M. Proprioceptive control of posture: a review of new concepts. Gait Posture. 1998;8:214–242. doi: 10.1016/s0966-6362(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Allum JH, Carpenter MG, Honegger F. Is lower leg proprioception essential for triggering human automatic postural responses? Exp Brain Res. 2000;130:375–391. doi: 10.1007/s002219900259. [DOI] [PubMed] [Google Scholar]
- Bolton DAE, Misiaszek JE. Society for Neuroscience. San Diego: 2007. The role of hind paw cutaneous information in lateral stability during walking in the cat. [Google Scholar]
- Bolton DA, Misiaszek JE. Contribution of hindpaw cutaneous inputs to the control of lateral stability during walking in the cat. J Neurophysiol. 2009;102:1711–1724. doi: 10.1152/jn.00445.2009. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol. 2003;90:3625–3639. doi: 10.1152/jn.00496.2003. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Clark FJ. Characteristics of knee joint receptors in the cat. J Physiol. 1969;203:317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol. 1994;71:817–820. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Crouch JE. Text-atlas of cat anatomy. Philadelphia: Lea & Febiger; 1969. [Google Scholar]
- Degtyarenko AM, Simon ES, Burke RE. Differential modulation of disynaptic cutaneous inhibition and excitation in ankle flexor motoneurons during fictive locomotion. J Neurophysiol. 1996;76:2972–2985. doi: 10.1152/jn.1996.76.5.2972. [DOI] [PubMed] [Google Scholar]
- Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol. 1988;59:1888–1905. doi: 10.1152/jn.1988.59.6.1888. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Role of cutaneous afferents from distal hindlimb in regulation of step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Everaert DG, Ting LH, Stapley PJ, Chen K, Stein RB. Society for Neuroscience. Washington, DC: 2005. Postural responses to pitch and roll rotations in cats with vestibular loss. [Google Scholar]
- Fallon JB, Bent LR, McNulty PA, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol. 2005;94:3795–3804. doi: 10.1152/jn.00359.2005. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group-I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Phys London. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci. 2005;25:4733–4742. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol. 1998;80:1939–1950. doi: 10.1152/jn.1998.80.4.1939. [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Nichols TR. The decerebrate cat generates the essential features of the force constraint strategy. J Neurophysiol. 2010 doi: 10.1152/jn.00764.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Nardelli P, Cope TC, Nichols TR. Society for Neuroscience. San Deigo, CA: 2007. Autogenic spindle pathways mediate the postural response during horizontal support surface perturbations. [Google Scholar]
- Honeycutt CF, Stahl VA, Nichols TR. Society for Neuroscience. Washington, DC: 2008. Loss of proprioceptive feedback from muscle disrupts the excitatory postural response to support surface perturbations. [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol. 2009;101:2751–2761. doi: 10.1152/jn.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE. The distal hindlimb musculature of the cat—interanimal variability of locomotor-activity and cutaneous reflexes. Exp Brain Res. 1993;96:125–140. doi: 10.1007/BF00230446. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol. 1988a;60:204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol. 1988b;60:218–231. doi: 10.1152/jn.1988.60.1.218. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Inglis JT. Stance and balance following bilateral labyrinthectomy. Prog Brain Res. 1993;97:219–228. doi: 10.1016/s0079-6123(08)62281-5. [DOI] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Marchand-Pauvert V, Gautier J, Simonetta-Moreau M, Pierrot-Deseilligny E. Group I projections from intrinsic foot muscles to motoneurones of leg and thigh muscles in humans. J Phys London. 2001;536:313–327. doi: 10.1111/j.1469-7793.2001.t01-1-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Simonetta-Moreau M, Pierrot-Deseilligny E, Marchand-Pauvert V. Group II excitations from plantar foot muscles to human leg and thigh motoneurones. Exp Brain Res. 2005;161:486–501. doi: 10.1007/s00221-004-2096-6. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Bolha B, Hlavacka F. Human balance control during cutaneous stimulation of the plantar soles. Neurosci Lett. 2001;302:45–48. doi: 10.1016/s0304-3940(01)01655-x. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Roll R, Kavounoudias A, Roll JP. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport. 2002;13:1957–1961. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex efferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Krogsgaard M. Sensorimotor control of knee stability. A review. Scand J Med Sci Sports. 2001;11:64–80. doi: 10.1034/j.1600-0838.2001.011002064.x. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci. 2002;22:5803–5807. doi: 10.1523/JNEUROSCI.22-14-05803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol. 2004;92:808–823. doi: 10.1152/jn.00773.2003. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]