Abstract
Recent evidence suggests ghrelin may up-regulate the number of spine synapses. However, it is not completely understood whether an increased number of synapses are expressed on existing spines or accommodated in newly generated spines. We examined if ghrelin might have promoted the generation of new dendritic spines. Localization of polymerized actin (F-actin), highly expressed in dendritic spines, was assayed using phalloidin, a mushroom toxin that has a high affinity to F-actin. Alexa 488-conjugated phalloidin was visualized and relative changes in fluorescing puncta were quantified using a confocal microscope. Ghrelin was applied to cultured hippocampal slices for either 60 min or 23 h. Ghrelin increased the phalloidin fluorescent signals. The antagonist of the ghrelin receptor, D-Lys3-GHSR-6, blocked the ghrelin’s effect of increasing the phalloidin signal, suggesting that the ghrelin’s effect was mediated by the ghrelin receptor (GHSR1a). The ghrelin-mediated increase in phalloidin signals remained elevated while ghrelin was present in the culture media for 23 h. However, removal of ghrelin from culture media restored the phalloidin signal to control level. Our results suggest ghrelin may have a stimulating effect on the generation or remodeling of dendritic spines, and the spine change persists in the presence of ghrelin. The serum ghrelin level is high when the stomach is empty, and the ghrelin level remains high until metabolic demands are fulfilled. Thus, ghrelin may be involved in food-related and appetite-related learning in the hippocampus.
Keywords: Ghrelin receptor, Spine density, Phalloidin, CA1, Appetite-related learning
1. Introduction
Ghrelin is a 28-amino acid peptide and was first purified from the rat stomach. In the brain, ghrelin stimulates hypothalamic neurons by crossing the blood–brain barrier and initiates the release of growth hormone [15] and feeding behavior [3]. Although metabolic regulation has been a major role of ghrelin, evidence accumulates to suggest that ghrelin might be involved in cognitive functions and memories [7]. Indeed, this peripheral gut hormone was reported to affect the brain’s memory system at a neuronal level by promoting the formation of synapses at dendritic spines in the hippocampus [6]. The hippocampus is an important brain region for learning and memory. Dendritic spines are critical morphological target for the induction of synaptic plasticity. For example, long-term potentiation (LTP) in the hippocampus is maintained by structural changes that occur in dendritic spines. We previously reported that ghrelin stimulated phosphorylation of CREB (cAMP response element binding protein), a transcription factor, in the hippocampus [4]. CREB is a key molecule in the induction and maintenance of hippocampal synaptic plasticity such as LTP [18]. Therefore, a target protein of CREB-activation induced by ghrelin may include a component of dendritic spines. We examined whether ghrelin affected polymerized actin in the cytoskeleton, highly expressed in dendritic spines. We used a hippocampal slice culture preparation in the present study. This is because dendritic spines were reported to transiently increase up to 50% in response to decapitation [14]; thus, the use of acute hippocampal slices might not allow us to accurately assess the effect of ghrelin on spine changes.
2. Materials and methods
2.1. Slice preparation and pharmacological treatment
The hippocampal slice culture was prepared from P6 postnatal male pups of Sprague-Dawley rats as previously reported [11] according to a method described by Stoppini et al. [21]. All experimental protocols and animal use were approved by the Institutional Animal Care and Use Committee at the University of Texas Brownsville. The slices were used for the experiments after being cultured for 1 week in media consisted of: 50% MEM, 25% HBSS, 20% horse serum, 0.5% penicillin/streptomycin solution, 5% glucose solution, and 25 mM HEPES. Ghrelin was applied to the culture media with a concentration of 200 nM for 60 min or 23 h. In some experiments, the antagonist of the ghrelin receptor, L-Dys3-GHSR-6 in 100 µM (Phoenix pharmaceutical, Burlingame, CA) was applied 2 h before the application of ghrelin.
2.2. Application of phalloidin and quantification of fluorescent signals
In order to visualize dendritic spines, we used fluorochrome-conjugated phalloidin, a mushroom toxin that has high affinity to polymerized F-actin. First, slices were immersion-fixed at the end of experiments with 4% paraformaldehyde in 1 M PBS overnight, rinsed, and treated with 0.1% Triton X-100. The slices were then incubated in 6 µM of Alexa-488 phalloidin (Invitrogen, Carlsbad, CA) for 4 h to overnight. The slices were rinsed, mounted on glass slides, and cover-slipped with Vectashield (Vector Lab, Burlingame, CA).
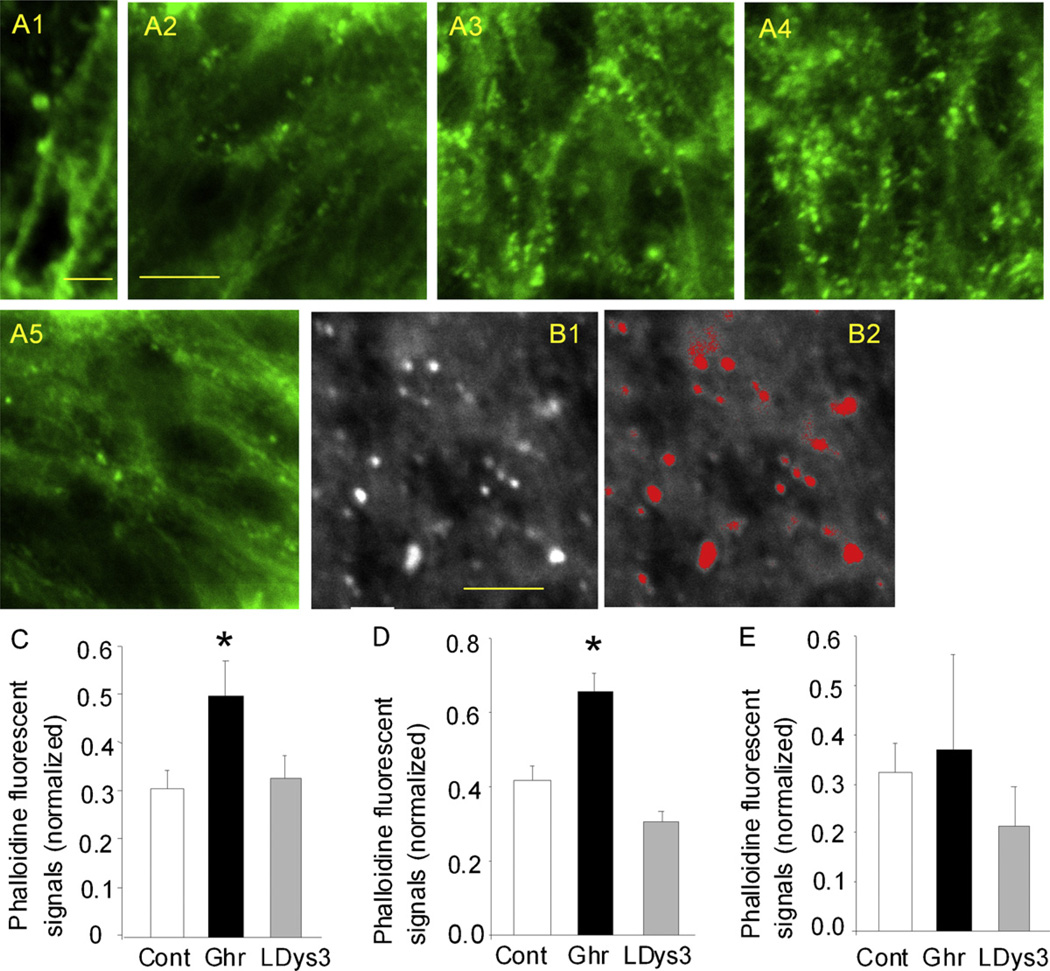
Results were imaged at a single cell resolution using a confocal microscope (Fluoview, Olympus, Center Valley, PA). The raw image taken by the confocal microscope (Fig. 1B1) was processed using an IPLab imaging software in order to select spines as ROIs (region of interest as red dots, Fig. 1B2). ROI was selected based on the fluorescence intensity. The threshold fluorescence to select ROIs was determined by the auto-segmentation logic termed Triangle in IPLab. This logic was used throughout the project, which allowed us to select ROIs using the identical fluorescence threshold across different slices and experiments.
Fig. 1.
Polymerized actin was visualized by the phalloidin-conjugated fluorescence in response to ghrelin. (A) Phalloidin signal was intense in dendritic regions of the CA1 stratum radiatum of the hippocampus. Strongly fluorescing puncta represent dendritic spines (A1). Phalloidin fluorescence signals in slices incubated in control media (A2), in ghrelin for 1 h (A3) and for 23 h (A4), and in control media for 22 h after 1 h of incubation in ghrelin (A5). Strongly fluorescing puncta (B1) were selected and quantified using an autosegmentation program provided by IPLab imaging software (B2). (C) Phalloidin signal in response to 60 min application of ghrelin and to co-application of ghrelin and the ghrelin receptor antagonist, L-Dys3-GHSR6. (D) Phalloidin signal in response to 23 h application of ghrelin. Co-application of the ghrelin receptor antagonist, L-Dys3-GHSR6 blocked the ghrelin’s effect. (E) Phalloidin signal was similar to control when ghrelin was applied for 1 h and removed from the media while the slices were continuously incubated in control media for additional 22 h. Calibrations: 2 µm in A1, 3 µm in B1, and 15 µm in A2. The calibration in A2 is shared with A3–5. The calibration in B1 is shared with B2. Asterisks indicate p < 0.001. Abbreviations: Control slices (Cnt), ghrelin-treated slices (Ghr), and D-Lys3-GHSR-6-pretreated and ghrelin-applied slices (LDys).
Because ROIs were created automatically when the fluorescence intensity exceeded the threshold intensity set by Triangle, ROIs could include non-spine objects. Therefore, we defined the “size” of ROI that is accepted as spine by selecting 50 clearly visible solitary spines (as shown in Fig. 1A1), and determined the minimum and maximum size of ROIs that were accepted for spine analysis. We then selected those ROIs whose size was within the maximum and the minimum.
In order to distinguish phalloidin-bound fluorescing spines from plain fluorescent puncta, we conducted a control experiment in which cultured slices were incubated in plain Alexa 488 (instead of phalloidin-conjugated Alexa 488). These control slices were processed identical to those slices received phalloidin-conjugated Alexa 488. We did not observe any fluorescence (fluorescent puncta) from these control slices, suggesting that our fluorescent puncta were indeed representing polymerized actin in dendrites and spines.
The magnitude of phalloidin-fluorescence was quantified by measuring fluorescing puncta of a defined size of ROIs in 3 non-overlapping locations in the Stratum Radiatum of the CA1 region in a given slice using a 40× objective with 3× zooming function and IPLab software (BD Bioscience, San Jose CA) (Fig. 1B1 and B2). We calculated the total area occupied by spines (ROIs) in the entire image, instead of actually counting the number of spines (ROIs). The advantage of analyzing the area of ROIs (instead of the number of ROIs) is that this method is sensitive to changes in (1) the number of spines and (2) the increase in the surface area of existing spines. Thus, the spine density was calculated as follows (per image):
This formula states that our definition of spine density is the area occupied by spines relative to the entire area imaged. The number of ROIs that were accepted for spine analysis varied from 5 to 22 per image among different experiments. Results were pooled for a given type of experiment, and a mean and a standard error of the mean (SEM) were calculated. Results among different types of experiments were tested for statistical significance using a student t-test. p < 0.05 was considered significant.
2.3. Immunohistochemistry and confocal detection of the ghrelin receptor
Localization of the ghrelin receptor was studied in a subset of cultured hippocampal slices that were used in ghrelin experiments. We used a primary antibody against the ghrelin receptor raised in the rabbit (Phoenix pharmaceutical, Burlingame, CA) and Alexa 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA). The corresponding region in the Stratum Radiatum of the CA1, used for the phalloidin analysis, was selected for analysis using a confocal microscope and quantified for the density of the ghrelin receptor using IPLab analysis packages (BD Bioscience, San Jose CA). No dual labeling was attempted because of technical limitations of identifying single individual spines and ghrelin receptors in the present study.
3. Results
Dendritic spines were observed as green fluorescing puncta (Fig. 1A1). Relative changes in the fluorescent signals were examined in response to local application of ghrelin and the ghrelin receptor antagonist. We wondered how long the “ghrelin-induced spine change” may last and whether the maintenance of the change required ghrelin. Ghrelin was applied in two differing durations as described below.
3.1. Short-term application of ghrelin and its effect on spines
Ghrelin was applied for 60 min (Fig. 1A3 and C). In control slices, the average spine density was 0.302/unit area ± 0.039 SEM (n = 30 images taken from 10 slices) (Fig. 1A2). Ghrelin (200 nM) increased the average spine density to 0.499/unit area ± 0.058 SEM (n = 30 images taken from 10 slices, p < 0.001) (Fig. 1A3). The effect of ghrelin was blocked by the ghrelin receptor antagonist, D-Lys3-GHSR-6 (100 µM) (0.333/unit area ± 0.041 SEM, n = 30 images taken from 10 slices), suggesting the involvement of the ghrelin receptor activation and possible downstream signaling cascades in the induction of ghrelin-mediated changes in dendritic spines.
3.2. Long-term application of ghrelin and its effect on spines
Ghrelin was applied for 23 h (Fig. 1A4 and D). Similar to the result of 60 min application, ghrelin-treated slices expressed a higher density of dendritic spines (0.618/unit area ± 0.043 SEM in 30 images taken from 10 slices, p < 0.001) (Fig. 1A4) compared with control slices (0.419/unit area ± 0.029 SEM in 30 images taken from 10 slices). Spine density remained elevated at the end of the 23 h application. Preincubation of slices in D-Lys3-GHSR-6 (100 µM) for 2 h before the application of ghrelin blocked the increase in spine density (0.322/unit area ± 0.024 SEM in 30 images taken from 10 slices).
3.3. Recovery of spines from ghrelin application
Our results show a 60 min-application of ghrelin was sufficient to increase the number of spines. Our results also show that a spine-increase was observed after 23 h of ghrelin application. In another series of experiments, we examined if the presence of ghrelin was required for continued elevation of spine density in the experiment of 23 h application. Ghrelin was applied for 60 min, the slices were removed from ghrelin-containing media and incubated in control media for additional 22 h. At the end of the 22 h incubation, the slices were fixed and processed for phalloidin binding. That is, we applied ghrelin for 60 min and waited for 22 h. Thus, in this experiment, the spine density was measured 22 h after the 60 min-application of ghrelin (Fig. 1A5 and E). The spine density was 0.370/unit area ± 0.193 SEM, and showed no significant changes compared with the control (0.314/unit area ± 0.057 SEM) or with the presence of the receptor antagonist (0.215/unit area ± 0.087 SEM). This result indicated that the spine density could increase in response to ghrelin within 60 min (Fig. 1C) and remain elevated in the presence of ghrelin for 23 h (Fig. 1D). However, when ghrelin is removed, spines appear to retract and the spine density recovers to control level (Fig. 1E). It may be that ghrelin is necessary to be present for this form of spine-generation and maintenance.
3.4. Effect of ghrelin on the ghrelin receptor
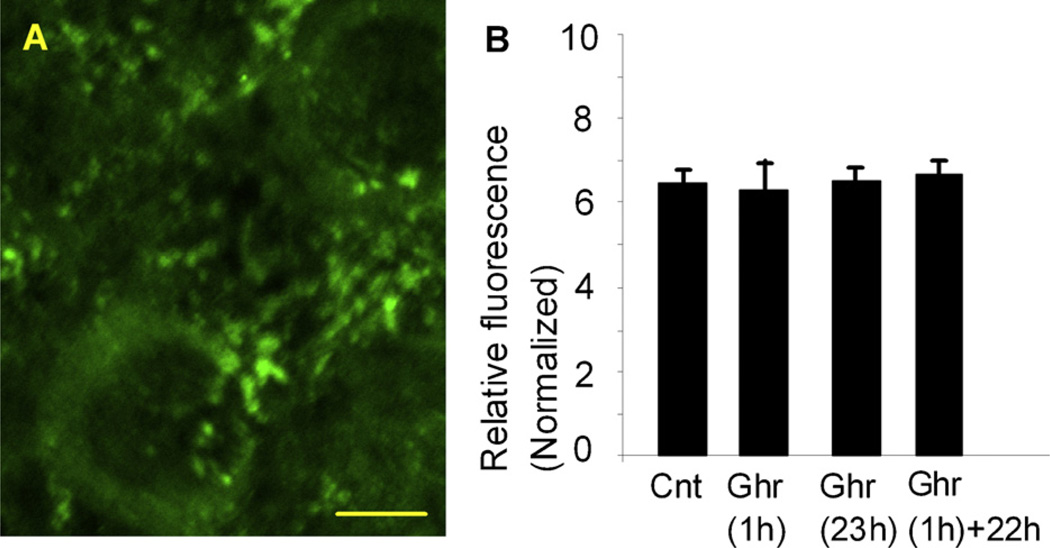
We studied whether the ghrelin receptor might have been affected during the application of ghrelin for 60 min or 23 h. We used a subset of cultured hippocampal slices that were used in ghrelin experiments above. Therefore, experimental paradigms and timing of fixation were identical to the phalloidin experiments discussed above. We used a primary antibody against the ghrelin receptor raised in the rabbit (Phoenix pharmaceutical, Burlingame, CA) and Alexa 488-conjugated secondary antibody (Invitrogen, Carlsbad, CA). The density of the ghrelin receptor was quantified with the IPLab analysis package of “mid-point” analysis and “triangle” analysis. The results were consistent between these two analyses. The ghrelin receptor was detected both on the cell somata and dendrites in the CA1 (Fig. 2A). Fluorescent intensities tended to be brighter in dendrites exhibiting many small puncta along dendritic shafts. We were not able to determine whether these puncta were dendritic spines. The density of ghrelin receptor was not different among experimental conditions, i.e. (1) in control, (2) in ghrelin for 1 h, (3) in ghrelin for 23 h, and (4) in ghrelin for 1 h followed by an additional 22 h-incubation (Fig. 2B). This finding suggested that the ghrelin receptor did not appear to be internalized by the agonist to a significant extent in the present study even with a long-term incubation of 23 h.
Fig. 2.
The ghrelin receptor was detected immunohistochemically (A). Density of the receptor was quantified in a normalized scale, which demonstrated that there were no significant changes in the receptor density among (1) control, (2) 1 h application of ghrelin, (3) 23 h application of ghrelin, and (4) 1 h application of ghrelin and additional 22 h incubation in control culture media (B). Calibration in A: 5 µm.
4. Discussion
The present study demonstrates ghrelin can reorganize dendritic spines of hippocampal CA1 neurons by promoting the generation of spines and maintaining the initiated changes. A removal of ghrelin from culture media reversed the effect of ghrelin on spines, suggesting that maintenance of ghrelin-induced increase in spine density may require continuous activation of the ghrelin receptor and downstream signaling mechanisms.
Spine generation was reported to occur rapidly within a few minutes [10,12] in the in vitro specimens of hippocampal slices and neurons. Microtubules are capable of entering dendritic spines in mature hippocampal neurons through dynamic polymerization in direct association with neuronal activities. Long-term stimulation by the application of BDNF was reported to increase spine density in hippocampal slices [22] with larger spine heads in mature neurons [13]. Therefore, it is not surprising that ghrelin increased spine density in 60 min in the present study. However, in the present study, it was not determined whether dynamic changes observed in spines correlated with changes in the postsynaptic molecular signaling induced by ghrelin and the activation of the ghrelin receptor. Future studies may provide direct evidence whether spines that were generated during the ghrelin application were the spines that expressed the ghrelin receptor.
The hippocampus is a critical brain region that contributes to food searching strategies while environment is explored. Specifically, ghrelin is thought to play a role in memory retention for the spatial localization of food sources [2]. Food search is typically initiated when metabolic demand increases, and it typically continues until the metabolic demand is fulfilled. During fasting, a serum concentration of ghrelin increases. The rate of ghrelin crossing the blood–brain-barrier also increases in a concentration-dependent manner [6]. In vivo experiments, fasting is reported to enhance synaptic plasticity and promotes learning consolidation [8]. Therefore, our findings of ghrelin promoting the generation of dendritic spines and maintaining the increased dendritic spine-density nicely complement the in vivo evidence that ghrelin-induced enhancement of synaptic function persists until metabolic demand is fulfilled.
Finally, the present finding suggests the ghrelin receptor may not desensitize easily. We found that the ghrelin receptor was functioning throughout the 23 h-application of ghrelin and contributed towards the maintenance of increased spine density. Indeed, the ghrelin receptor was reported to hardly desensitize with nM range of ghrelin [1], which we used in the present study. The ghrelin receptor is primarily linked to the Gq/11 type of G-protein [15] and elicits a rapid increase in PLC activity [9]. These signaling pathways are intimately associated with and influenced by cell membrane structure and dynamics including the production of membrane-associated lipids. An exposure of the ghrelin receptor to oleic acid has been reported to delay and inhibit ghrelin-induced desensitization and internalization of the ghrelin receptor [5], suggesting naturally existing unsaturated fatty acids may regulate the ghrelin receptor activity. The production of membrane lipids by the activation of the Gq/11-PLC pathways may have a negative feedback on the desensitization of the ghrelin receptor and maintain continual activation of the receptor until the serum/brain ghrelin level decreases.
5. Conclusions
Ghrelin increased dendritic spine density in the CA1 region of the hippocampus.
Ghrelin-induced increase in spine density was mediated by the ghrelin receptor.
Maintenance of ghrelin-induced increase in spine density may result from non-desensitizing property of the ghrelin receptor and/or a negative feedback given by downstream signaling of the receptor.
Acknowledgment
This work is supported by NIH grants R15DA021683 (to MI).
References
- 1.Camina JP, Carreira MC, Messari SE, Llorens-Cortes C, Smith RG, Casanuev FF. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue Receptor 1a. Endocrinology. 2004;145:930–940. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- 2.Cong W-N, Golden E, Pantaleo N, White CM, Maudsley S, Martin B. Ghrelin receptor signaling: a promising therapeutic target for metabolic syndrome and cognitive dysfunction. CNS & Neurological Disorders - Drug Targets. 2010;9:557–563. doi: 10.2174/187152710793361513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowley MC, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman LM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 4.Cuellar JN, Isokawa M. Ghrelin-induced activation of cAMP signal transduction and its negative regulation by endocannabinoids in the hippocampus. Neuropharmacology. 2011;60:842–851. doi: 10.1016/j.neuropharm.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delhanty PJ, van Kerkwijk A, Huisman M, van de Zande B, Verhoef-Post M, Gauna C, Hofland L, Themmen AP, van der Lely AJ. Unsaturated fatty acids prevent desensitization of the human growth hormone secretagogue receptor by blocking its internalization. American Journal of Physiology-Endocrinology and Metabolism. 2010;299:E497–E505. doi: 10.1152/ajpendo.00414.2009. [DOI] [PubMed] [Google Scholar]
- 6.Diano S, Farr SA, Benoit SC, McNay WC, da Silvo I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 7.Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Current Neuropharmacology. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, Delgado-Garcia JM. Carrion AM Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. Journal of Neuroscience. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu K, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, De-Martino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, Dent EW. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. Journal of Neuroscience. 2011;31:15597–15603. doi: 10.1523/JNEUROSCI.2445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isokawa M. Time-dependent induction of CREB phosphorylation in the hippocampus by the endogenous cannabinoid. Neuroscience Letters. 2009;457:53–57. doi: 10.1016/j.neulet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nature Neuroscience. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. Journal of Neuroscience. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima M. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 18.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation dependent factor CREB. Nature Reviews Molecular Cell Biology. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 21.Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. Journal of Neuroscience Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 22.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. Journal of Neuroscience. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]