Abstract
This is a summary of the published literature on the urinary 2/16 estrogen metabolite ratio in human populations, and a report the observed range of normal values in healthy women. Original research studies that included the measurement of urinary estrogen metabolites in human subjects were identified through an extensive Medline search; 43 distinct studies were indentified, including a total of 6802 healthy women. The range of mean values of the 2/16 ratio measured with the ELISA method varied from 0.98 to 1.74; in studies of pre-menopausal women the range of mean values was 1.5 to 2.74, in studies of post-menopausal women mean values ranged from 1.15 to 2.25. The heterogeneity across studies was highly significant (p-value Q test: <0.0001). In multivariable analyses, only race confirmed its role as an independent predictor of 2/16 ratio (F value: 7.95; p value: 0.009), after adjustment for age and menopausal status. There appears to be a large body of data on the 2/16 urinary ratio in healthy women. However, summary estimates are difficult to perform due to the high variability of the published study-specific values. The data suggests that race may be a contributor to 2/16 urinary ratio levels.
Introduction
Estrogen metabolism is a complex process that starts with a reversible chemical reaction, the conversion of estradiol to estrone in the C17 position. After this step, estrone undergoes hydroxylation at positions C2, C4 or C16 [1, 2], with the production of several metabolites, including 2-hydroxyestrone (2-OHE1), 4-hydroxyestrone (4-OHE), 16-alpha-hydroxyestrone (16α-OHE1) or estriol (1); the 2-hydroxylation represents the prevalent metabolic pathway and takes place mostly in the liver [3]. Both the 2-OHE1 and the 16α-OHE1 metabolites have estrogenic properties, but with different activity; the 16α-OHE1 metabolite binds to the estrogen receptor and has similar properties to those of estradiol and thus, has been classified as an estrogen-like compound. The 2-OHE1 metabolite has nearly no affinity to the estrogen receptor; therefore it is virtually devoid of estrogen activity. Furthermore, the metabolic pathway produces either the 2-OHE1 or 16α-OHE1 in a mutually exclusive way [1], thus their ratio may be a useful measure of a woman’s exposure to estrogen-like metabolites.
Some constitutional factors such as genetic polymorphisms and family history of breast cancer are associated with the levels of the 2/16 ratio measured in urine [4]. Environmental and behavioral factors, including diet, physical activity, body weight, hormone therapy, smoking and alcohol consumption have been examined as potential modifiers of the 2/16 ratio. Exercise may increase 2-OHE1 production [5], while higher body weight may favor the 16 pathway [6]. Similarly, a diet rich in cruciferous vegetables is suggested to modify the 2/16 ratio by increasing the 2-hydroxylation pathway, as previously reviewed [7]. Hormone replacement therapy seems to increase 2-OHE1 production more than 16α-OHE1 [8].
It has been hypothesized that estrogen metabolites are mutagens through the production of depurinating DNA adducts [9].
Urinary estrogen metabolites have been also studied in relation to breast [studies summarized in [10], endometrial [11] and prostate cancer [12]. However, prior to establishing associations between the metabolites and cancer, it is crucial to assess the range of the urinary estrogen metabolite levels in healthy women. Although several studies have reported on this topic, the expected range of the urinary 2/16 ratio among healthy women remains unknown. Aspects that also need to be addressed are the variation of the 2/16 ratio in healthy women with regards to ethnicity, menstrual cycle, and menopausal status, as well as individual characteristics, such as age and lifestyle factors. Additional variability could be introduced by the sensitivity/specificity of the laboratory kit used for each metabolite, as well as variability across laboratory kits.
This review aims at summarizing the published literature on the urinary 2/16 estrogen metabolite ratio in human populations, with the objective of reporting the observed range of normal values in healthy women.
Materials and Methods
Study Identification and Selection Criteria
Origin al research studies that included the measurement of urinary estrogen metabolites in human subjects were identified by searching the National Library of Medicine and National Institutes of Health Pubmed database. The search strategy involved the following keyword search terms: estrogen metabolites, 2-OHE1, 16α-OHE1, 2-hydroxyestrone, 16α-hydroxyestrone, hormone metabolites; additional limits were females and English language. This search is current as of December 1, 2009. Each of the 1,854 citations and abstracts were reviewed, and articles were considered eligible for inclusion if they met the following pre-determined inclusion criteria: (1) an original research study, (2) inclusion of healthy women, (3) inclusion of the ratio of 2-hydroxyestrone (2-OHE1) over 16-alpha-hydroxyestrone (16α-OHE1), and (4) urine as the sample source. A total of 87 articles were identified as potentially containing the measurement of the 2/16 urinary ratio in healthy women; after reviewing the details of the publications, thirty-two articles were further excluded for the following reasons: (1) methodological articles including 1 or 2 subjects [13-15], (2) did not provide the 2/16 ratio, only the individual metabolites [16-33]; in this case, a possible option would have been to calculate the ratio using the average 2/average 16, but this would have not taken into account the sample variance of the measurement, (3) in vitro studies [34, 35], (4) the ratio was calculated but not reported [36-38], (5) the metabolites were measured in plasma or serum [39-43] and (6) the population was treated with HRT [44]. Reference lists from retrieved articles were also reviewed in order to identify additional eligible articles; no additional studies were identified.
The number of articles that met the criteria for inclusion was 56; however, study populations from 13 publications partially overlapped with each other and thus, the final number of distinct studies was 43.
Criteria for inclusion of data from each study
If the parent study was a case-control study, only information on controls were extracted; if it was a randomized clinical trial or an intervention study, only baseline information of non-treated women were used. Follow-up measurements were not included. Some of the included studies reported on controls with and without hormone therapy or oral contraceptive intake; in this case, only data on women without treatment were included.
Laboratory methods
Earlier studies utilized the gas chromatography-mass spectrometry technique [45], a labor intensive method [46]. In 1994, Klug et al. developed an Enzyme-Linked Immunosorbent Assay (ELISA) technique [47], which was subsequently validated in its modified version against gas chromatography-mass spectrometry [48]. The original ELISA kit did not have the appropriate limit of detection when used to measure 2-OHE1 and 16α-OHE1 in urine samples from postmenopausal women [14]; a modified version of the kit was developed with an increased sensitivity level of 0.625 ng/ml [49] and adjustments applied to the antibody concentrations, enzyme concentrations and standards.
Data extraction and tabulation
For each eligible study the following information was extracted and tabulated: the main objective of the study, the number of women included, the type of laboratory method used to measure the estrogen metabolites, the number of samples collected per study subject, the time of the day/menstrual cycle (if available), method used to report the ratio (mean, median, range), and the main analyses reported in the original published paper.
Statistical Analyses
Comparisons across studies were performed based on mean values. For studies where the median and the full range were reported, the mean was calculated according to Hozo et al. as follows: [a+2m+b]/4+[a−2m+b]/4n, where m is the median, a and b are the extremes of the range, n is the sample size. For studies including SD of the mean, SE was calculated as SE=SD/√n [50]. When 95% Confidence Intervals were presented, they were used as an approximation of the SD. For studies where the range of values were reported, the variance was calculated as 1/12[(a−2m+b)2 /4+(a−b)2] for sample sizes between 1 and 15, as range/4 for sample sizes between 16 and 70, as range/6 for sample sizes greater than 70. Studies were grouped based on the method of determining hormone levels, either ELISA or GC-MS studies. An attempt to summarize the evidence was performed separately for each of these groups. Before calculating summary estimates in each group, the Q test for heterogeneity [51] was performed to assess the degree of heterogeneity across studies. In the case of statistically significant heterogeneity, summary estimates were not calculated.
For the studies using the ELISA method, univariate analyses were performed to compare the 2/16 ratio in studies conducted on pre-, post- or both pre- and postmenopausal women. Additional comparisons were performed on the 2/16 ratio according to race and type of urine collection (spot, morning or 12 or more hours). The non-parametric Kruskal-Wallis test was used for these statistical comparisons of means. Linear regression analysis was applied to identify possible predictors of the ratio, after the assumption of normality was checked. In this model, the dependent variable was the 2/16 ratio, the independent variables were categorical, and included menopausal status (classified as studies on premenopausal women, on postmenopausal women, on both pre and post menopausal women), race (classified as: White, Asian, Black, multiracial or Hispanic, unknown) and type of sample collection (classified as: spot urine, morning urine, 12 hours or more urine collection).
Results
Description of the studies
The 43 distinct studies included a total of 6802 healthy women (Table 1). The vast majority of the data sets (36/43) measured the 2/16 ratio using the ELISA method [4-6, 49, 52-95] while 6 studies used GC-MS [96-103], and 1 study [104] did not report the laboratory method. Self-defined ethnicity was reported in 30/43 data sets. Of these, 14 [6, 53, 59-63, 104, 65, 66, 82, 83, 86-89, 93-97] studies reported results on the 2/16 ratio separately for white women, 7 [5, 60, 64, 68, 76, 87, 90, 91, 99] reported separately for Asian women, and 4 [6, 60, 87-89, 104] for African Americans. The remaining studies included multiracial populations for which no breakdown of the results according to race was reported. Thirteen studies involved exclusively pre-menopausal women [58-62, 67, 71, 73, 85, 92-94, 96, 100-102], 15 included only post menopausal women [52, 53, 56, 57, 65, 66, 72, 74, 75, 80-84, 86, 90, 91, 98, 99, 103], and the remaining 15 included a mixture of both pre and post menopausal women, of which 6 studies presented the results stratified by menopausal status.
Table 1. Studies of the Urinary 2/16 Estrogen Metabolite Ratio in Healthy Populations of Women.
| Study reference |
Main Topic of the study |
N£ | Race | Age (years) |
Menopausal Status |
Assay | # Measures; methods of urine collection |
2/16 ratio | Statistics presented |
Main analyses in the original paper |
|---|---|---|---|---|---|---|---|---|---|---|
| 96, 97^ | Dietary fiber/multiethnic |
23 | White | 31.7/34.6 | Pre | GC-MS | 2; 72 hrs, mid follicular. |
2.0 | GM, SE | Diet groups |
| 52 | HRT | 34 | N/A | N/A | Post | ELISA | 1; spot | 2.71 ± 0.84 | Mean, SD | HRT & overall |
| 53 | HRT | 174 | White | Median: 60 | Post | ELISA | 1; 24 hrs | 2.74 ± 0.24 | Mean, SE | HRT |
| 54, 55 | Equol excretion | 61 | White/Asian/AA | 25-59 | Pre, Post | ELISA | 1; overnight | 1.45 (0.4-5.6) | GM, range |
Overall |
| 56, 57 | Physical activity intervention |
173 | 86% White | 50-75 | Post | ELISA | 3; spot | 1.15 (1.03-1.29) | GM, range |
Baseline, after intervention |
| 58 | Soy intervention | 187 | Mostly whites | Median: 42 | Pre | ELISA | 1; morning, day 5-9 of cycle |
1.42 (1.3-1.6) | GM, 95% CI |
Baseline,after soy load |
| 59 | Physical activity | 77 | White | 18-51 | Pre | ELISA | 1; first morning |
1.82 ± 0.13 | Mean, SE | Overall |
| 98 | Smoking, HRT treatment |
16 | N/A | N/A | Post | GS MS | 2; 24 hrs | NS: 1.23 ± 0.32; SMK:1.62 ±0.45 |
Mean, SD | Smoking |
| 60 | Methodology on volunteers |
511 | Multiracial | 17-35 | Pre | ELISA | 1; morning., Phase of cycle recorded |
White: 1.67 AA: 1.69 Asian: 1.25 Indian: 1.51 |
Median, interquarti le range+ |
urine versus plasma |
| 49 | Assay validation | 214 | N/A | N/A | Pre, Post | ELISA | Several, morning spot follicular phase |
Pre: 2.3±0.6 Post: 1.5± 0.4 |
Mean, SD | Menopausal |
| 61, 62 | Aerobic fitness | 30& | White | 20-42 | Pre | ELISA | 2; first morning, luteal |
1.86$ | Mean, 95% CI |
Fitness, Menstrual cycle |
| 63 | Methods | 10 | White | 23-58 | Pre, Post | ELISA | 8; first morning, days 23-31of cycle |
2.03 ± 0.32$ | Individual values |
|
| 104 | Case/control | 58 | White, AA | N/A | Pre, Post | N/A | N/A | W: 2.2 ± 1.3 AA: 1.8 ± 1.1 |
Mean, SD | |
| 64 | Descriptive multi- geographic Asian populations |
511 | Asian | 25-55 | Pre, Post | ELISA | 1; overnight, day of menstrual cycle recorded |
Premeno: China 1.89; Japan: 1.67; Philippines:1.31 Postmeno: China 1.71; Japan 1.68; Philippines 1.8 |
GM | Menopausal status |
| 65, 66* | Dietary factors; Brassica trial |
37 | White | >45 | Post | ELISA | 2; 24 hrs | 2.35 ± 1.33 | Mean, SD | Overall and by intervention |
| 4 | Family history of breast cancer |
64 | 91% white | Median: 50 | Pre, Post | ELISA | 1; spot | 2.13$ (Pre: 2.1 ± 0.8; Post: 2.2 ± 0.7) |
Mean, SD | Menopause & age |
| 67 | Flaxseed | 15 | mostly white | 20-38 | Pre | ELISA | 3; 24 hrs, mid- luteal |
2.26 ± 0.19 | Mean, SE | Diet group |
| 68 | Case/control | 36 | Asian | Median: 54.8 | Pre, post | ELISA | 1; overnight | 2.0 ± 0.3 | Mean, SE | |
| 69 | Case/control | 64 | Multiracial | Median: 54.2 | Pre, post | ELISA | 1;spot | 1.72 ± 0.66 | Mean, SD | Menopausal |
| 70 | Case control | 326 | White, AA | N/A | Pre, post | ELISA | 1;spot | Pre: 1.9 ±1.0; post: 2.0 ± 1.0 |
Mean, SD | |
| 99# | Osteopenia, Bone density |
59 | Korean | 55-60 | Post | GC-MS | 1; overnight | 0.17; 0.1# | Mean, SE | Bone density |
| 71 | Soy, isoflavones | 85 | Mostly Asian | Median: 33 | Pre | ELISA | Daily; 12 hrs | 2.0 ± 0.32 | Mean, SE | Diet |
| 72** | Bone density | 71 | N/A | 47-59 | Post | ELISA | 1; 24 hrs | 2.85 ± 1.73 | Mean, SD | Bone density |
| 73 | Soy intake | 16 | N/A | 18-40 | Pre | ELISA | 2; 48 hrs, mid luteal & mid follicular |
2.32 ± 1.11 | Mean, SD | Diet & OC use |
| 74 | Blood pressure | 54 | White/AA/Latino | 50-67 | Post | ELISA | 1; overnight | 1.5 | Mean | HRT status |
| 75 | Flax, genes | 132 | 97% white | 45-75 | Post | ELISA | 2; morning spot |
1.54 ± 0.75 | Mean, SD | Overall |
| 5, 76* | Physical activity | 146 | Chinese | 47.1 | Pre, Post | ELISA | 2; overnight | 1.2 ± 1.0 | Mean, SD | Race |
| 77 | Cancer incidence | 296 | N/A | >35 | Pre, Post | ELISA | 1; spot | Pre: 2.1; Post: 1.7 |
Median | Menopausal |
| 78, 79# | OC & HRT | 67, 63 | N/A | Median: Pre= 26; Pos=55 |
Pre, Post | ELISA | 2; 8 hrs overnight |
Pre: 1.6 ± 0.12; 1.5 ± 0.1 Post: 2.1 ± 0.18; 1.67 ± 0.13 |
Mean, 95% CI |
Pre & Post HRT or OC |
| 80, 81# | Various hormonal treatment groups |
74 | N/A | Median: 54 | Post | ELISA | 2; 8hrs overnight |
1.15 ± 0.14; 1.5 ± 0.22; 1.2 ± 0.1; 2.0 ± 0.04 |
Mean, 95%CI |
HRT |
| 82, 83** | Genes/ bone density | 156 | White | Median: 63.5 | Post | ELISA | 1; 24 hrs | 2.1 ± 0.13 | Mean, SE | Genotype |
| 84 | Soy intake in cancer survivors |
20 | N/A | Median: 56.2 | Post | ELISA | 4; 24 hrs | 1.92 (1.61-2.29) | GM, 95% CI |
Overall, survivors, controls |
| 85 | Low fat diet, physical activity |
174 | Mostly White | 44-50 | Pre | ELISA | 2; spot | 2.3 ± 1.1 | Mean, SD | Treatment group |
| 86 | Breast density | 140 | White | 40-65 | Post | ELISA | 1; N/A | 0.98 (0.59-1.28) | Mean, range |
Breast density |
| 100 | Sedentary and Physical Activity |
15 | N/A | Median: 29 | Pre | GS MS | 3; 24 hrs | 0.93 ± 0.62 | Mean, SD | Intervention |
| 87, 6 | Diet &lifestyle | 1881 | AA/White/Asian/ Hispanic |
45-54 | Pre, Peri | ELISA | 1; overnight, mostly follicular |
AA: 1.65 ± 0.05 White:2.1 ± 0.04 China:1.9 ± 0.12 Hisp.:1.95±0.14 Japan:1.92± 0.09 |
Mean, SE | Race |
| 88, 89 | Multiethnic | 33 | White/AA | 18-73 | Pre, Post | ELISA | 1; morning | White:2.25±0.89 AA: 1.42 ± 0.61 |
Mean, SD | Race |
| 90, 91 | Multiethnic (pilot) |
125 23 |
Chinese, AA, White |
45-75 55-64 |
Post | ELISA | 1; morning | Chinese:1.63 (1.41-1.89); White+AA: 1.48 (1.27-1.84); 1.58 ± 0.2 |
GM 95% CI Mean, SE |
Race Breast cancer |
| 92 | Family history of breast cancer |
97 | White | 20-50 | Pre | ELISA | 1; morning, 5- 9 days of cycle |
1.82 (1.49-2.15) | GM 95% CI |
Family history |
| 93, 94 | Diet & exercise | 24 | N/A | Median: 31.5 | Pre | ELISA | 5; morning, mid follicular and mid luteal |
1.95 ± 0.18 | Mean, SE | Menstrual phase |
| 95 | Case/control | 426 | White | 50-64 | Pre, Post | ELISA | 1; spot | 1.6 (0.3-0.5); | Median + (5-95%) |
HRT |
| 101, 102 | Menstrual cycle, soy isoflavones |
12 | N/A | Median: 26 | Pre | GC-MS | 3; 24 hrs | 18 ± 3.92 | Mean, SD | Diet, menstrual cycle |
| 103 | Soy diets | 18 | N/A | Median: 56.9 | Post | GC-MS | 2; 24 hrs | 1.33 ± 0.08 | Mean, SE | Overall & diet |
Abbreviations: GC-MS=Gas chromatography mass spectrometry, ELISA=Enzyme -linked immunosorbent assay, RIA=Radiometric ImmunoAssay, N/A=not indicated in publication, AA=African American, HRT=hormone replacement therapy, OC=oral contraceptive, NS=non-smokers, SMK=smokers; GM=geometric mean, SD=standard deviation, SE=standard error, CI=confidence intervals
Number of subjects in control or non-treatment groups
ratio reported only on a subset of the various populations included
Partial overlap, 26 Whites were included in ref 83; (new data: 45)
mean was calculated from median; SD from ranges when full ranges were available
16/2 is reported in the original paper
(3 on OC, 1 on HRT)
overall mean over 4 menstrual cycles was re-calculated
The number of urine measurements performed varied greatly across studies, from 1 to 8 or more. The methods for urine collection always involved the addition of ascorbic acid for preservation purposes, but the time of urine collection varied: a spot urine collection was used in 10 studies [4, 49, 52, 56, 57, 69, 70, 75, 77, 85, 95], while 18 studies [5, 6, 54, 55, 58-64, 68, 74, 76, 78-81, 87-94, 99] reported on overnight urine collection. The remaining studies included 12, 24, 48 or 72 urine collection protocols. The information was not available for one study [86].
The time of the menstrual cycle during which urine was collected from premenopausal women was also variable: while the majority of the studies collected urine regardless of time of the cycle, 13 of the publications reported collecting urine at a specific time during the menstrual cycle, or kept record of the day of the cycle when urine was collected, in order to adjust for it in the analysis [6, 49, 58-64, 67, 73, 87, 92-94, 96, 97]. The statistical approach to data presentation also varied: means were reported by most of the publications (32/43), geometric means by 8 studies [54-58, 64, 84, 90-92, 96, 97], medians by 3 studies [60, 77, 95].
Summary estimates
An attempt to summarize the evidence was performed separately for the 36 ELISA studies and the 6 GC-MS studies. Twenty eight out of the 36 studies using ELISA presented the data as means, 7 as geometric means; 5 of the studies using GC-MS presented the results as means. A visual description of the means and SE for each study in the group using ELISA as laboratory method is presented in figure 1. The range of mean values of the 2/16 ratio varied from 0.98 to 1.74; in studies of pre-menopausal and postmenopausal women, the mean values ranged from 1.50 to 2.74 and 1.15 to 2.25, respectively. The heterogeneity across studies was highly significant (p-value Q test: <0.0001), therefore a summary estimate was not calculated.
Figure 1. Distribution of 2/16 ratio in studies where the ELISA was used. Data are presented as Mean ± SE.
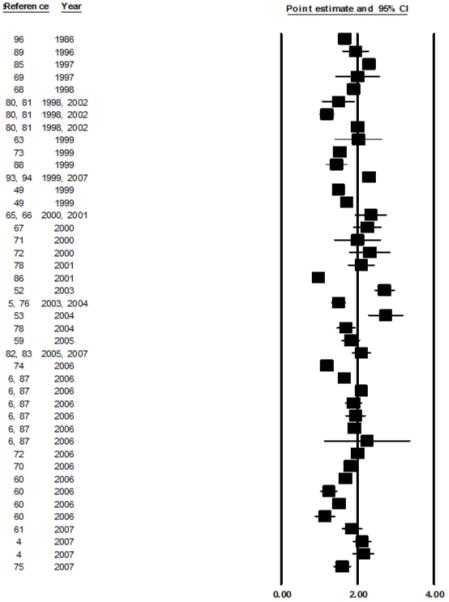
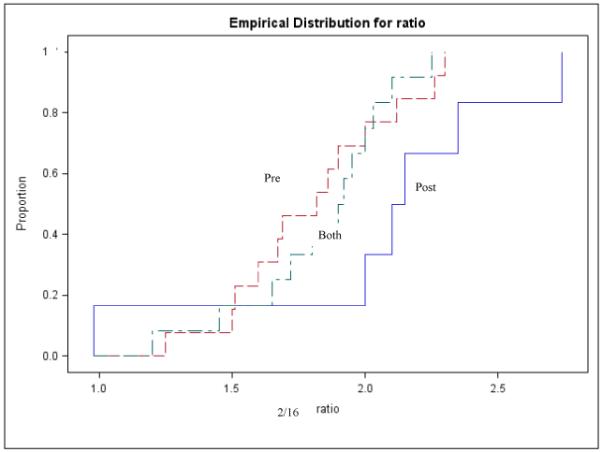
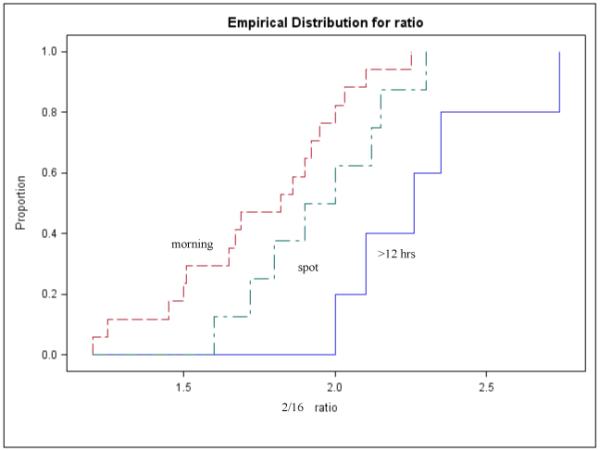
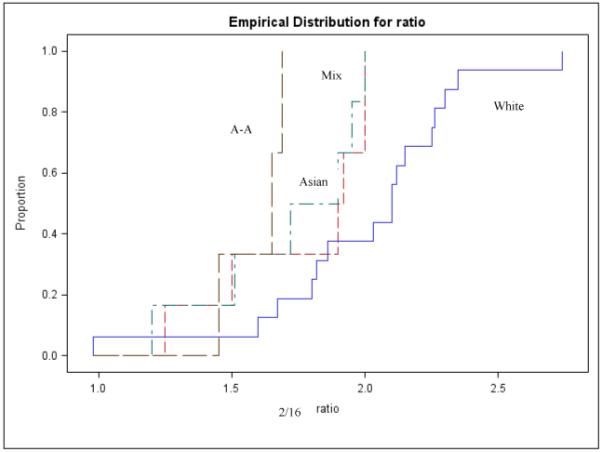
Few variables available from the published papers could be analyzed as predictors of the 2/16 ratio. Stratified analyses by menopausal status, race and type of urine collection still showed statistically significant heterogeneity; therefore, summary estimates were not calculated. Menopausal status was not associated with the 2/16 urinary ratio (Kruskal-Wallis p-value=0.9) (figure 2); studies that collected urine over a period of 12 or more hours consistently reported higher 2/16 ratios than studies collecting a morning or a spot sample (Kruskal-Wallis p-value=0.006) (figure 3). When race was considered, White women showed significantly higher mean values of 2/16 metabolite ratios than Asians, African Americans or mixed populations (Kruskal-Wallis p-value=0.05) (figure 4). In multivariable analyses that used the 2/16 ratio as dependent variable, only race confirmed its role as an independent predictor of 2/16 ratio (F value: 7.95; p value: 0.009), after adjustment for age and menopausal status.
Figure 2. Distribution of 2/16 mean values in healthy women according to menopausal status.
Kruskal-Wallis p value: 0.9
Figure 3. Distribution of 2/16 mean values in healthy women according to type of urine collection.
Kruskal-Wallis p value: 0.006
Figure 4. Distribution of 2/16 mean values in healthy women according race.
Kruskal-Wallis p value: 0.05
Other co-factors
Some of the studies included in Table 1 reported on cofactors that could potentially modify the 2/16 ratio. More specifically, studies which assessed the role of dietary factors on estrogen metabolite levels included the examination of flaxseed consumption [67, 75, 6], brassica vegetable consumption [65], fat and fibers intake [67], soya diet [58, 71, 73, 84, 101-103], dietary fibers [96, 97], low fat/low calories diet [85, 93] and indole-3-carbinol supplementation [27]. Almost all these studies suggest that a low calorie/low fat diet as well as the intake of cruciferous vegetables, indole-3-carbinole, flax and soy, increase 2-hydroxyestrone levels and therefore favorably modify the 2/16 ratio. Two publications did not show any differences in the 2/16 ratio with soy intake [73] or with a low fat diet [85]. Additional studies have evaluated the effects of lifestyle factors such as physical activity on estrogen metabolite levels [5, 6, 56, 59, 61, 85, 93, 100]. While moderate exercise seems not to influence the 2/16 ratio [56, 85], vigorous exercise, especially if associated with a low BMI [5, 6] and an increase in lean body mass [61, 100] may modify the ratio in a favorable way.
One study addressed the role of family history of breast cancer [92] on the 2/16 levels and reported no association; a recent investigation restricted to women with a positive family history of breast cancer [4] suggests that metabolic gene polymorphisms may be responsible for differences in 2/16 ratio within this group. Out of the studies that addressed postmenopausal hormone usage, three did not report a significant impact on the 2/16 ratio [52, 78, 79] while another study observed significant changes in the ratio in smokers only [98].
Smoking was analyzed in very few studies [98, 69, 60]; current smoking was associated with a slightly higher 2/16 ratio than non smokers in two studies [98, 60], but not in another [69].
DISCUSSION
This study presents a review of the literature on urinary estrogen metabolite levels in healthy women. Several findings emerge from this analysis: over the years, more than 6,000 healthy women underwent urinary 2/16 ratio measurements as part of epidemiologic studies on cancer etiology or potential determinants of estrogen metabolism, including dietary and behavioral factors. Despite the availability of the 2/16 ratio on such a large population of women, and the fact that only two main laboratory methods are in use by the investigators, several methodological differences in study design, sample collection and data analysis are present. This greatly limits the possibility of conducting a combined analysis to determine the average urinary values in the healthy population of women as a whole and according to menopausal status and race. A separate analysis of studies which used the ELISA method shows that some differences in average 2/16 levels may exist according to race, with lower levels of the urinary 2/16 ratio observed in Asian and African-American populations in comparison to White women. This difference seems to hold after adjustment for type of sample collection and menopausal status. Out of the individual studies that have addressed and evaluated potential racial differences in estrogen metabolites [6, 60, 87-89, 104], few reported data separately for Black women, and only one [60] did not confirm the result observed in the present meta-analysis.
Because of the limitations of any published data, a comprehensive re-analysis of cofactors that could explain the observed differences in 2/16 urinary levels with race was not possible. Individual studies suggest that dietary components, obesity, physical activity, smoking and a combination of all these factors are possible determinants of the 2/16 ratio in healthy women. A summary estimate of these factors could not be performed due to methodological differences across studies.
The data presented here refer to the 2/16 ratio measured in urine; most of the studies have focused on the measurement of these metabolites in urine since the 2-OHE1 and 16α-OHE1 metabolites are present in low concentrations in the blood. With the introduction of new technologies involving mass spectrometry, studies on serum and plasma levels will become more common. Information on the correspondence between urinary and plasma measurements is very limited, as well as between urine and breast tissue [60].
The studies presented in this review relied mostly on 1 measurement; studies with a follow-up after intervention reported a subsequent measure. Very few studies addressed within-person variability and the stability of estrogen metabolite levels over time. Chen et al. conducted a study to assess the within-person variability of the ratios of urinary 2-OHE1 to 16α-OHE1 [63] over a two-month period; the 2-OHE1 and 16α-OHE1 metabolites measured at any one time point correlated with the average ratio over the eight week study period (mean correlation coefficient: 0.85). Findings from a longitudinal study of five samples [105] collected over a year (n=34), suggests that a single measure of the estrogen metabolites may not have the ability to adequately account for at least 50% of the true variance. Therefore, questions still remain on the within-person variation of estrogen metabolites over time, the validity of a single measure over multiple measures as well as additional methodological issues concerning the measurement of estrogen metabolites.
Another aspect that needs to be addressed is the time of the menstrual cycle when the measure should be performed in pre-menopausal women. Currently, investigators have chosen to collect urine with different strategies including the following: sample collection only in a certain phase of the cycle, performing the measures two or more times in a cycle, or only recording the time of the menstrual cycle in which urine was collected and then adjusting for this factor in the analysis. Although the 2/16 OHE ratio is reported to be reproducible during the day and during the menstrual cycle [93, 94], a more uniform and standardized criterion in sample collection would likely help reduce variability and increase precision of the measurement. Ongoing collaborative projects aimed at standardization and improvement of steroid hormone measurements should be extended to include estrogen metabolism studies [106].
In addition to variability in the laboratory methods, variations across the ELISA kits used by the various investigators could also contribute to the observed heterogeneity across studies. A recent study comparing the ELISA method to both the radioimmunoassay and the mass spectrometry methods indicates that the ELISA method has higher coefficient of variation (< 14.2%) than the spectrometry method (< 9.4%) [107].
In the present review, the large heterogeneity across studies could not be explained by the available information on study design, sample collection, sample storage and laboratory methods; this may have implications for future pooling of data to address a possible association between estrogen metabolites and cancer. As previously suggested, study-specific quantiles or percentage increases of biomarkers values in relation to cancer risk may have to be employed [108].
In conclusion, there appears to be a large body of data on the 2/16 urinary ratio in healthy women. However, summary estimates are difficult to perform due to the high variability of the data published. This review suggests that race may be a contributor to 2/16 urinary ratio levels, however this hypothesis could not be studied in more depth because of the large variability of the available data, and the lack of available information on covariates that could affect the ratio and be associated with race.
Acknowledgments
CMD was partially supported by a training grant R25 CA 57703
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1).Sepkovic DW, Bradlow HL. Estrogen hydroxylation--the good and the bad. Ann N Y Acad Sci. 2009;1155:57–67. doi: 10.1111/j.1749-6632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 2).Lippert TH, Seeger H, Mueck AO. The impact of endogenous estradiol metabolites on carcinogensis. Steroids. 2000;65:357–369. doi: 10.1016/s0039-128x(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 3).Kerlan V, Dreano Y, Bercovici JP, et al. Nature of cytochrome P450 involved in the 2-/4-hydroxylations of estradiol human liver microsomes. Biochem Pharmacol. 1992;44:1745–1756. doi: 10.1016/0006-2952(92)90068-t. [DOI] [PubMed] [Google Scholar]
- 4).Greenlee H, Chen Y, Kabat GC, Wang Q, Kibriya MG, Gurvich I, et al. Variants in estrogen metabolism and biosynthesis genes and urinary estrogen metabolites in women with a family history of breast cancer. Breast Cancer Res Treat. 2007;102:111–7. doi: 10.1007/s10549-006-9308-7. [DOI] [PubMed] [Google Scholar]
- 5).Matthews CE, Fowke JH, Dai Q, Bradlow HL, Jin F, Shu XO, et al. Physical activity, body size, and estrogen metabolism in women. Cancer Causes Control. 2004;15:473–81. doi: 10.1023/B:CACO.0000036445.04238.87. [DOI] [PubMed] [Google Scholar]
- 6).Sowers MR, Crawford S, McConnell DS, Randolph JF, Jr, Gold EB, Wilkin MK, Lasley B. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136:1588–95. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 7).Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: Rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7:119–129. [PubMed] [Google Scholar]
- 8).Modugno F, Kip KE, Cochrane B, Kuller L, Klug TL, Rohan TE, Chlebowski RT, Lasser N, Stefanick ML. Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1292–301. doi: 10.1002/ijc.21487. [DOI] [PubMed] [Google Scholar]
- 9).Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Im A, Vogel VG, Ahrendt G, Lloyd S, Ragin C, Garte S, Taioli E. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30:1532–5. doi: 10.1093/carcin/bgp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Fishman J, Schneider J, Hershcopf, et al. Increased estrogen-16α-hydroxylase activity in women with breast and endometrial cancer. J Steroid Biochem. 1984;20:1077–1081. doi: 10.1016/0022-4731(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 12).Markushin Y, Gaikwad, Zhang H, et al. Potential biomarker for early risk assessment of prostate cancer. The Prostate. 2006;66:1565–1571. doi: 10.1002/pros.20484. [DOI] [PubMed] [Google Scholar]
- 13).Chen C, Malone KE, Prunty J, Daling JR. Measurement of urinary estrogen metabolites using a monoclonal enzyme-linkedmimmunoassay kit: assay performance and feasibility for epidemiological studies. Cancer Epidemiol Biomarkers Prev. 1997;6:145–7. [PubMed] [Google Scholar]
- 14).Ziegler RG, Rossi SC, Fears TR, Bradlow HL, Adlercreutz H, Sepkovic D, et al. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ Health Perspect. 1997;105:607–14. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Parl FF. Estrogen Metabolism and Breast Cancer A risk Model. Steroid Enzymes and cancer: Ann. N.Y. Acad. Sci. 2009;1155:68–75. doi: 10.1111/j.1749-6632.2008.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Berg FD, Kuss E. Urinary excretion of catecholestrogens, 2-methoxy-estrogens and “classical estrogens” throughout the normal menstrual cycle. Arch Gynecol Obstet. 1991;249:201–7. doi: 10.1007/BF02390388. [DOI] [PubMed] [Google Scholar]
- 17).Gerhardt K, Ludwig-K√∂hn H, Henning HV, Remberg G, Zeeck A. Identification of oestrogen metabolites in human urine by capillary gas chromatography and mass spectrometry. Biomed Environ Mass Spectrom. 1989;18:87–95. doi: 10.1002/bms.1200180202. [DOI] [PubMed] [Google Scholar]
- 18).Michnovicz JJ. Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int J Obes Relat Metab Disord. 1998;22:227–9. doi: 10.1038/sj.ijo.0800573. [DOI] [PubMed] [Google Scholar]
- 19).Bradley Vn Voorhis J. The relationship of Bleeding Patterns to Daily Reproductive Hormones in Women Approaching menopause. Ostet Gynecol. 2008;112:101–108. doi: 10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Skurnick JH, Weiss G, Goldsmith LT, Santoro N, Crawford S. Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil Steril. 2009;91:1127–34. doi: 10.1016/j.fertnstert.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 21).Gold EB. Relation of Daily Urinary Hormone Patterns to Vasomotor Symptoms in a Racially/Ethnically Diverse Sample of Midlife Women: Study of Women’s Health Across the Nation. Reproductive Sciences. 2007 Dec;14(8) doi: 10.1177/1933719107308613. [DOI] [PubMed] [Google Scholar]
- 22).Salih S, Xu X, Veenstra TD, Duleba AJ, Fouad H, Nagamani M, Al-Hendy A. Lower levels of urinary 2-hydroxyestrogens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:3285–91. doi: 10.1210/jc.2006-2719. [DOI] [PubMed] [Google Scholar]
- 23).Carruba G, Granata OM, Pala V, Campisi I, Agostara B, Cusimano R, et al. A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutr Cancer. 2006;56:253–9. doi: 10.1207/s15327914nc5602_18. [DOI] [PubMed] [Google Scholar]
- 24).Falk RT, Gail MH, Fears TR, Rossi SC, Stanczyk F, Adlercreutz H, et al. Reproducibility and validity of radioimmunoassays for urinary hormones and metabolites in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1999;8:567–77. [PubMed] [Google Scholar]
- 25).Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schünemann HJ, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 26).Michnovicz JJ, Naganuma H, Hershcopf RJ, Bradlow HL, Fishman J. Increased urinary catechol estrogen excretion in female smokers. Steroids. 1988;52:69–83. doi: 10.1016/0039-128x(88)90218-8. [DOI] [PubMed] [Google Scholar]
- 27).Michnovicz JJ, Adlercreutz H, Bradlow HL. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89:718–23. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- 28).Michnovicz JJ, Bradlow HL. Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer. 1991;16:59–66. doi: 10.1080/01635589109514141. [DOI] [PubMed] [Google Scholar]
- 29).Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette s moking. N Engl J Med. 1986;315:1305–9. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 30).Longcope C, Gorbach S, Goldin B, Woods M, Dwyer J, Morrill A, Warram J. The effect of a low fat diet on estrogen metabolism. J Clin Endocrinol Metab. 1987;64:1246. doi: 10.1210/jcem-64-6-1246. [DOI] [PubMed] [Google Scholar]
- 31).Bulbrook RD, Swain MC, Wang DY, Hayward JL, et al. Breast cancer in Britain and Japan: Plasma Oestradiol. Europ J Cancer. 1976;12:725. doi: 10.1016/0014-2964(76)90023-2. [DOI] [PubMed] [Google Scholar]
- 32).Aldercreutz H, Gorbach SL, Goldin BR, et al. Estrogen metabolism and excretion in oriental and Caucasian women. J Natl. Cancer Inst. 1994;86:1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 33).Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, Wong E, Thompson LU. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79:318–25. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- 34).Salama SA, Kamel M, Awad M, Nasser AH, Al-Hendy A, Botting S, Arrastia C. Catecholestrogens induce oxidative stress and malignant transformation in human endometrial glandular cells: Protective effect of catechol-O-methyltransferase. Int. J. Cancer. 2008;123:1246–1254. doi: 10.1002/ijc.23653. [DOI] [PubMed] [Google Scholar]
- 35).Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Laird NM, Khuhaprema T, et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int. J. Cancer. 2009;125:837–843. doi: 10.1002/ijc.24434. [DOI] [PubMed] [Google Scholar]
- 36).Williams MC, Goldzieher JW. Chromatographic patterns of urinary ethynyl estrogen metabolites in various populations. Steroids. 1980;36:255–82. doi: 10.1016/0039-128x(80)90001-x. [DOI] [PubMed] [Google Scholar]
- 37).Cohen LA, Crespin JS, Wolper C, Zang EA, Pittman B, Zhao Z, Holt PR. Soy isoflavone intake and estrogen excretion patterns in young women: effect of probiotic administration. In Vivo. 2007;21:507–12. [PubMed] [Google Scholar]
- 38).Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini Hill A, Ross RK, Pike MC. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:1067–72. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 39).Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy and 16-alpha hydroxyl estrone levels and risk of breats cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA. Estradiol and its metabolites and their association with knee osteoarthritis. Arthritis Rheum. 2006;54:2481–7. doi: 10.1002/art.22005. [DOI] [PubMed] [Google Scholar]
- 41).Jernström H, Klug TL, Sepkovic DW, Bradlow HL, Narod SA. Predictors of the plasma ratio of 2-hydroxyestrone to 16alpha-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24:991–1005. doi: 10.1093/carcin/bgg047. [DOI] [PubMed] [Google Scholar]
- 42).Spierto FW, Gardner F, Smith SJ. Evaluation of an EIA method for measuring serum levels of he estrogen metabolite 2-hydroxyestrone in adults. Steroids. 2001;66:59–62. doi: 10.1016/s0039-128x(00)00139-2. [DOI] [PubMed] [Google Scholar]
- 43).Jernstrom H, Klug TL, Sepkovic DW, Bradlow HL, Narod SA. Predictors of the plasma ratio of 2-hydroxyestrone to 16α-hydroxyestrne among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24:991–1005. doi: 10.1093/carcin/bgg047. [DOI] [PubMed] [Google Scholar]
- 44).Hsu IP, Jou HJ, Huang CW, Wang TA, Wu WH. The effects of soygerm extracts on blood lipoproteins, antioxidative capacity and urinary estrogen metabolites in postmenopausal women on hormone therapy. Int J Gynaecol Obstet. 2007;98:29–33. doi: 10.1016/j.ijgo.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 45).Aldercreutz H, Martin F, Wahlroos O, Soini E. Mass spectrometric and mass fragmentographic determination of natural and synthetic steroids in biological fluids. Steroid Biochem Molec Biol. 1975;6:247–259. doi: 10.1016/0022-4731(75)90140-5. [DOI] [PubMed] [Google Scholar]
- 46).Fotsis T, Aldercreutz H. The multicomponent analysis of estrogens in urine by ion exchange chromatography and GC-MS I. Quantitation of estrogens after initial hydrolosis of conjugates. J Steroid Biochem. 1987;28:203–218. doi: 10.1016/0022-4731(87)90379-7. [DOI] [PubMed] [Google Scholar]
- 47).Klug T, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16α-hydroxyestrone in urine. Steroids. 1994;59:648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 48).Falk RT, Rossi SC, Fears TR, Sepkovic DW, Migella A, Adlercreutz H, et al. A new ELISA Kit for Measuring Urinary 2-Hydroxyestrone, 16α-Hydroxyestrone, and their ratio: reproducibility, validity, and assay performance after freeze-thaw cyling and preservation by boric acid. Cancer Epidemiol Biomarkers Prev. 2000;9:81–87. [PubMed] [Google Scholar]
- 49).Bradlow HL, Sepkovic DW, Klug T, Osborne MP. Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 1998;63:406–13. doi: 10.1016/s0039-128x(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 50).Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:5–13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 52).Alvarez-Vasquez RB, Axelrod D, Frenkel K, Newman MC, Sepkovic DW, Bradlow HL, Zumoff B. Influence of postmenopausal hormone replacement therapy on an estrogen metabolite biomarker of risk for breast cancer. Horm Metab Res. 2003;35:358–61. doi: 10.1055/s-2003-41357. [DOI] [PubMed] [Google Scholar]
- 53).Armamento-Villareal RC, Napoli N, Klug T, Civitelli R. The oxidative metabolism of estrogen modulates response to ERT/HRT in postmenopausal women. Bone. 2004;35:682–8. doi: 10.1016/j.bone.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 54).Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wähälä K, Schwartz SM, Lampe JW. Urinary equol excretion in relation to 2-hydroxyestrone and 16alpha-hydroxyestrone concentrations: an observational study of young to middle-aged women. J Steroid Biochem Mol Biol. 2003;86:71–7. doi: 10.1016/s0960-0760(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 55).Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wähälä K, Schwartz SM, Lampe JW. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol Biomarkers Prev. 2002;11:253–60. [PubMed] [Google Scholar]
- 56).Atkinson C, Lampe JW, Tworoger SS, Ulrich CM, Bowen D, Irwin ML, Schwartz RS, Rajan BK, Yasui Y, Potter JD, McTiernan A. Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:868–74. [PubMed] [Google Scholar]
- 57).Frankenfeld CL, McTiernan A, Tworoger SS, Atkinson C, Thomas WK, Stanczyk FZ, et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J Steroid Biochem Mol Biol. 2004;88:399–408. doi: 10.1016/j.jsbmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 58).Atkinson C, Newton KM, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control. 2008;19:1085–93. doi: 10.1007/s10552-008-9172-3. [DOI] [PubMed] [Google Scholar]
- 59).Bentz AT, Schneider CM, Westerlind KC. The relationship between physical activity and 2-hydroxyestrone, 16alpha-hydroxyestrone, and the 2/16 ratio in premenopausal women (United States) Cancer Causes Control. 2005;16:455–61. doi: 10.1007/s10552-004-6256-6. [DOI] [PubMed] [Google Scholar]
- 60).Bradlow HL, Jernström H, Sepkovic DW, Klug TL, Narod SA. Comparison of plasma and urinary levels of 2-hydroxyestrogen and 16 alpha-hydroxyestrogen metabolites. Mol Genet Metab. 2006;87(2):135–46. doi: 10.1016/j.ymgme.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 61).Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2007;16:731–9. doi: 10.1158/1055-9965.EPI-06-0784. [DOI] [PubMed] [Google Scholar]
- 62).Campbell KL, Westerlind KC, Harber VJ, Friedenreich CM, Courneya KS. Associations between aerobic fitness and estrogen metabolites in premenopausal women. Med Sci Sports Exerc. 2005;37:585–92. doi: 10.1249/01.mss.0000158185.23595.24. [DOI] [PubMed] [Google Scholar]
- 63).Chen Z, Zheng W, Dunning LM, Anderson KG, Parrish RS, Holtzman JL. Within-person variability of the ratios of urinary 2-hydroxyestrone to 16alpha-hydroxyestrone in Caucasian women. Steroids. 1999;64:856–9. doi: 10.1016/s0039-128x(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 64).Falk RT, Fears TR, Xu X, Hoover RN, Pike MC, Wu AH, et al. Urinary estrogen metabolites and their ratio among Asian American women. Cancer Epidemiol Biomarkers Prev. 2005;14:221–6. [PubMed] [Google Scholar]
- 65).Fowke JH, Longcope C, Hebert JR. Brassica vegetable consumption shifts estrogen metabolism in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:773–9. [PubMed] [Google Scholar]
- 66).Fowke JH, Longcope C, Hebert JR. Macronutrient intake and estrogen metabolism in healthy postmenopausal women. Breast Cancer Res Treat. 2001;65:1–10. doi: 10.1023/a:1006429920719. [DOI] [PubMed] [Google Scholar]
- 67).Haggans CJ, Travelli EJ, Thomas W, Martini MC, Slavin JL. The effect of flaxseed and wheat bran consumption on urinary estrogen metabolites in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:719–25. [PubMed] [Google Scholar]
- 68).Ho GH, Luo XW, Ji CY, Foo SC, Ng EH. Urinary 2/16 alpha-hydroxyestrone ratio: correlation with serum insulin-like growth factor binding protein-3 and a potential biomarker of breast cancer risk. Ann Acad Med Singapore. 1998;27:294–9. [PubMed] [Google Scholar]
- 69).Kabat GC, Chang CJ, Sparano JA, Sepkovic DW, Hu XP, Khalil A, Rosenblatt R, Bradlow HL. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:505–9. [PubMed] [Google Scholar]
- 70).Kabat GC, O’Leary ES, Gammon MD, Sepkovic DW, Teitelbaum SL, Britton JA, Terry MB, Neugut AI, Bradlow HL. Estrogen metabolism and breast cancer. Epidemiology. 2006;17:80–8. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 71).Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000;60:1299–305. [PubMed] [Google Scholar]
- 72).Leelawattana R, Ziambaras K, Roodman-Weiss J, Lyss C, Wagner D, Klug T, et al. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J Bone Miner Res. 2000;15:2513–20. doi: 10.1359/jbmr.2000.15.12.2513. [DOI] [PubMed] [Google Scholar]
- 73).Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer. 1999;34:133–9. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- 74).Masi CM, Hawkley LC, Berry JD, Cacioppo JT. Estrogen metabolites and systolic blood pressure in a population-based sample of postmenopausal women. J Clin Endocrinol Metab. 2006;91:1015–20. doi: 10.1210/jc.2005-2339. [DOI] [PubMed] [Google Scholar]
- 75).McCann SE, Wactawski-Wende J, Kufel K, Olson J, Ovando B, Kadlubar SN, Davis W, Carter L, Muti P, Shields PG, Freudenheim JL. Changes in 2-hydroxyestrone and 16alpha-hydroxyestrone metabolism with flaxseed consumption: modification by COMT and CYP1B1 genotype. Cancer Epidemiol Biomarkers Prev. 2007;16:256–62. doi: 10.1158/1055-9965.EPI-06-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Fowke JH, Qi D, Bradlow HL, Shu XO, Gao YT, Cheng JR, Jin F, Zheng W. Urinary estrogen metabolites and breast cancer: differential pattern of risk found with pre- versus post-treatment collection. Steroids. 2003;68:65–72. doi: 10.1016/s0039-128x(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 77).Meilahn EN, De Stavola B, Allen DS, Fentiman I, Bradlow HL, Sepkovic DW, Kuller LH. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78:1250–5. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Mueck AO, Seeger H, Gräser T, Oettel M, Lippert TH. The effects of postmenopausal hormone replacement therapy and oral contraceptives on the endogenous estradiol metabolism. Horm Metab Res. 2001;33:744–7. doi: 10.1055/s-2001-19139. [DOI] [PubMed] [Google Scholar]
- 79).Mueck AO, Seeger H, Wallwiener D. Endogenous estradiol metabolism during treatment with oral contraceptives. Int J Clin Pharmacol Ther. 2004;42:160–4. doi: 10.5414/cpp42160. [DOI] [PubMed] [Google Scholar]
- 80).Mueck AO, Seeger H, Wallwiener D. Impact of hormone replacement therapy on endogenous estradiol metabolism in postmenopausal women. Maturitas. 2002;43:87–93. doi: 10.1016/s0378-5122(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 81).Lippert TH, Seeger H, Mueck AO. Estradiol metabolism during oral and transdermal estradiol replacement therapy in postmenopausal women. Horm Metab Res. 1998;30:598–600. doi: 10.1055/s-2007-978940. [DOI] [PubMed] [Google Scholar]
- 82).Napoli N, Villareal DT, Mumm S, Halstead L, Sheikh S, Cagaanan M, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20:232–9. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Napoli N, Thompson J, Civitelli R, Armamento-Villareal RC. Effects of dietary calcium compared with calcium supplements on estrogen metabolism and bone mineral density. Am J Clin Nutr. 2007;85:1428–33. doi: 10.1093/ajcn/85.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135:603–8. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 85).Pasagian-Macaulay A, Meilahn EN, Bradlow HL, Sepkovic DW, Buhari AM, Simkin-Silverman L, et al. Urinary markers of estrogen metabolism 2-and 16 alpha-hydroxylation in premenopausal women. Steroids. 1996;61:461–7. doi: 10.1016/0039-128x(96)00089-x. [DOI] [PubMed] [Google Scholar]
- 86).Riza E, dos Santos Silva I, De Stavola B, Bradlow HL, Sepkovic DW, Linos D, Linos A. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10:627–34. [PubMed] [Google Scholar]
- 87).Sowers MR, Wilson AL, Kardia SR, Chu J, McConnell DS. CYP1A1 and CYP1B1 polymorphisms and their association with estradiol and estrogen metabolites in women who are premenopausal and perimenopausal. Am J Med. 2006;119:S44–51. doi: 10.1016/j.amjmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 88).Taioli E, Bradlow HL, Garbers SV, Sepkovic DW, Osborne MP, Trachman J, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev. 1999;23:232–7. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 89).Taioli E, Garte SJ, Trachman J, Garbers S, Sepkovic DW, Osborne MP, et al. Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1996;88:617. doi: 10.1093/jnci/88.9.617. [DOI] [PubMed] [Google Scholar]
- 90).Ursin G, Wilson M, Henderson BE, Kolonel LN, Monroe K, Lee HP, et al. Do urinary estrogen metabolites reflect the differences in breast cancer risk between Singapore Chinese and United States African-American and white women? Cancer Res. 2001;61:3326–9. [PubMed] [Google Scholar]
- 91).Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, Pike MC. A pilot study of urinary estrogen metabolites (16alpha-OHE1 and 2-OHE1) in postmenopausal women with and without breast cancer. Environ Health Perspect. 1997;105:601–5. doi: 10.1289/ehp.97105s3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Ursin G, London S, Yang D, Tseng CC, Pike MC, Bernstein L, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and family history of breast cancer in premenopausal women. Breast Cancer Res Treat. 2002;72:139–43. doi: 10.1023/a:1014896417653. [DOI] [PubMed] [Google Scholar]
- 93).Westerlind KC, Williams NI. Effect of energy deficiency on estrogen metabolism in premenopausal women. Med Sci Sports Exerc. 2007;39:1090–7. doi: 10.1097/mss.0b013e3180485727. [DOI] [PubMed] [Google Scholar]
- 94).Westerlind KC, Gibson KJ, Wolfe P. The effect of diurnal and menstrual cyclicity and menopausal status on estrogen metabolites: implications for disease-risk assessment. Steroids. 1999;64:233–43. doi: 10.1016/s0039-128x(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 95).Wellejus A, Olsen A, Tjonneland A, Thomsen BL, Overvad K, Loft S. Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:2137–42. doi: 10.1158/1055-9965.EPI-04-0934. [DOI] [PubMed] [Google Scholar]
- 96).Adlercreutz H, Fotsis T, Bannwart C, Hämäläinen E, Bloigu S, Ollus A. Urinary estrogen profile determination in young Finnish vegetarian and omnivorous women. J Steroid Biochem. 1986;24:289–96. doi: 10.1016/0022-4731(86)90067-1. [DOI] [PubMed] [Google Scholar]
- 97).Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hämäläinen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994;86:1076–82. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 98).Berstein LM, Tsyrlina EV, Kolesnik OS, Gamajunova VB, Adlercreutz H. Catecholestrogens excretion in smoking and non-smoking postmenopausal women receiving estrogen replacement therapy. J Steroid Biochem Mol Biol. 2000;72:143–7. doi: 10.1016/s0960-0760(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 99).Lim SK, Won YJ, Lee JH, Kwon SH, Lee EJ, Kim KR, et al. Altered hydroxylation of estrogen in patients with postmenopausal osteopenia. J Clin Endocrinol Metab. 1997;82:1001–6. doi: 10.1210/jcem.82.4.3875. [DOI] [PubMed] [Google Scholar]
- 100).Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer Epidemiol Biomarkers Prev. 2008;17:220–3. doi: 10.1158/1055-9965.EPI-07-0058. [DOI] [PubMed] [Google Scholar]
- 101).Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:1101–8. [PubMed] [Google Scholar]
- 102).Xu X, Duncan AM, Merz-Demlow BE, Phipps WR, Kurzer MS. Menstrual cycle effects on urinary estrogen metabolites. J Clin Endocrinol Metab. 1999;84:3914–8. doi: 10.1210/jcem.84.11.6134. [DOI] [PubMed] [Google Scholar]
- 103).Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:781–6. [PubMed] [Google Scholar]
- 104).Coker AL, Crane MM, Sticca RP, Sepkovic DW. Re: Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1997;89:89–90. doi: 10.1093/jnci/89.1.89. [DOI] [PubMed] [Google Scholar]
- 105).Williams AE, Maskarinec G, Franke AA, Stanczyk FZ. The temporal reliability of serum estrogens, progesterone, gonadotropins, SHBG and urinary estrogen and progesterone metabolites in premenopausal women. BMC Womens Health. 2002;2:13. doi: 10.1186/1472-6874-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Vesper HW, Botelho JC, Shacklady C, Smith A, Myers GL. CDC project on standardizing steroid hormone measurements. Steroids. 2008;73:1286–92. doi: 10.1016/j.steroids.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 107).Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol. Biom. Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Key TJ, Appleby PN, Allen NE, Reeves GK. Pooling Biomarker Data from Different Studies of Disease Risk, with a Focus on Endogenous Hormones. Cancer Epidemiol. Biom. Prev. 2010;19:960–5. doi: 10.1158/1055-9965.EPI-10-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]


