Abstract
Our study compares the outcomes of men and women with early stage colon cancer by analyzing the ACCENT database. Overall, men experienced inferior prognoses when compared with women for time to recurrence after adjusting for age, stage, and treatment. Sex was not a predictive factor of treatment efficacy. In exploratory analyses, worse outcomes in men were more prominent in older patients, but the stage of disease and type of adjuvant regimen did not modify the prognostic value of sex.
Purpose
To compare long-term outcomes between men and women in a large cohort of clinical trial participants with early-stage colon cancer, specifically by examining whether the prognostic effect of sex varies based on age, stage of disease, and type of adjuvant therapy received.
Methods
A pooled analysis of individual patient data from 33,345 patients with colon cancer enrolled in 24 phase III studies of various adjuvant systemic therapies was conducted. Chemotherapy consisted of (1) fluorouracil (5-FU), (2) 5-FU variations, (3) 5-FU plus oxaliplatin, (4) 5-FU plus irinotecan, or (5) oral fluoropyrimidine-based regimens. The primary endpoint was disease-free survival; secondary endpoints included overall survival and time to recurrence. Stratified Cox models were used to assess the effect of sex on outcomes. Multivariate models were used to assess adjusted effects and to explore the interaction among sex and other factors.
Results
A total of 18,244 (55%) men and 15,101 (45%) women were included. In the entire cohort, the median age was 61 years; 91% (24,868) were white; 31% (10,347) and 69% (22,964) had stage I/II and III disease, respectively. Overall, men had inferior prognoses when compared with women for time to recurrence (hazard ratio [HR] 1.05 [95% CI, 1.01–1.09]) and other endpoints after adjusting for age, stage, and treatment. Sex was not a predictive factor of treatment efficacy (P for interaction between sex and treatment when adjusting for age and stage were .40, .67, and .77 for disease-free survival, overall survival, and time to recurrence, respectively). In exploratory analyses, worse outcomes in men were more prominent in the older patients when adjusting for stage and treatment (HR 1.08 in age ≤ 65 years vs. HR 1.18 in age > 65 years; interaction P = .016 for disease-free survival). The stage of disease and type of adjuvant regimen did not modify the prognostic value of sex.
Conclusions
Sex is a modest independent prognostic marker for patients with early-stage colon cancer, particularly in older patients.
Keywords: Sex, Colon cancer, Survival, Outcomes
Introduction
Despite preventive health measures and advances in diagnostic strategies, colon cancer continues to be a serious global health problem and remains one of the leading causes of cancer-related mortality worldwide.1,2 In the United States alone, there are an estimated 150,000 incident cases of colon cancer per year and approximately one-third of these patients are expected to die of their disease.1,2 The introduction of novel cytotoxic chemotherapy agents as well as the incorporation of molecularly targeted drugs into the treatment paradigms for colon cancer has contributed to significant improvements in patient outcomes.3–7 Recent research efforts have examined new predictive and prognostic markers that can better enrich patient selection and further optimize the treatment success. To this end, sex has been identified as an independent prognostic factor for survival from colon cancer, with women surviving longer than men, although the results have been inconsistent and focused primarily on surgical outcomes.8–13 It also is currently unclear if any potential sex differences in outcomes are due to specific lifestyle patterns among the female population, possible genetic and molecular mechanisms that predispose women to more favorable outcomes, or differential efficacy of adjuvant treatment between the sexes.
Because many of these prior studies were limited to either small sample sizes or single institutions, such sex differences have not been consistently observed. Importantly, most of these analyses were conducted before the widespread use of contemporary agents, eg, oxaliplatin, in the treatment of colon cancer. Analysis of emerging data highlights the existence of sex-specific pharmacokinetics for both fluoropyrimidines and platinum agents, which are frequently used in colon cancer management.14–18 To our knowledge, the role of sex on outcomes has not been examined in detail within the context of oxaliplatin-based therapies. On the basis of these observations, we performed a prospectively planned, pooled analysis of patients participating in randomized phase III clinical trials based on the Adjuvant Colon Cancer Endpoint (ACCENT) group database. The main purpose of this analysis was to evaluate the prognostic effect of sex for survival in early-stage colon cancer. We also examined whether the effect of sex on outcomes varies based on age, stage of disease, study time period, and type of adjuvant therapy received. It is our hope that the findings from this analysis will be useful for improving therapy of colon cancer between men and women as well as for designing future clinical trials that involve new cytotoxic and molecularly targeted agents.
Patients and Methods
The ACCENT Database
ACCENT is an international collaboration of colon cancer researchers who have assembled a large database that consists of individual patient-level data from 24 large randomized phase III clinical trials that enrolled, between 1978 and 2003, patients with resected stage I to III colon cancer. The investigated adjuvant treatments included the following: (1) fluorouracil (5-FU) based regimens, (2) variations of 5-FU–based regimens, (3) combinations of 5-FU with oxaliplatin, (4) combinations of 5-FU with irinotecan, and (5) oral fluoropyrimidine chemotherapy. Data from a total of 55 treatment arms (46 active treatment arms and 9 surgery-only arms) and more than 33,000 patients with colon cancer have been collected. All clinical trials included in this data set were performed after obtaining approval from local institutional review committees, in accordance with the Declaration of Helsinki. Clinical trial participants also were required to provide written informed consent at the time that the studies were initially conducted. This sex-based, pooled analysis was approved by the Mayo Clinic Institutional Review Board.
Clinical Trials and Data Included
For this analysis, we included all clinical trials from the ACCENT database. In addition to sex information, we collected demographic, clinical, and pathologic factors, such as age, race, performance status, stage, grade of disease, and number of lymph nodes examined. Long-term clinical endpoints included the following: overall survival (OS), defined as the time from randomization to death due to all causes; disease-free survival (DFS), defined as the time from randomization to the first occurrence of disease recurrence or death due to all causes; and time to recurrence (TTR), defined as the time from randomizatio to the first documented disease recurrence in which deaths without recurrence are censored for the TTR endpoint at the time of death. Data on subsequent treatment in the event of recurrent disease and on salvage therapy were beyond the scope of these studies and were not collected by the study protocols. Data on treatment toxicities and comorbid conditions also were largely unavailable in this database. We analyzed data from 33,345 individual patients who participated in 24 adjuvant phase III randomized controlled clinical trials for early-stage colon cancer. Details about the adjuvant ACCENT trials included in this analysis are summarized in Table 1.
Table 1.
Treatment and Total Sample Size of Clinical Trials Included
| Study Group | Clinical Trial | Control Arm | Experimental Arm | Total Sample Size |
|---|---|---|---|---|
| 5-FU vs. surgery | NCCTG-78-48-52a | Surgery | 5-FU + LEV | 247 |
| NCCTG-87-46-51a | Surgery | 5-FU + LV | 408 | |
| INT-0035a | Surgery | 5-FU + LEV | 926 | |
| FFCDa | Surgery | 5-FU + CF | 256 | |
| NCICa | Surgery | 5-FU + CF | 359 | |
| SIENAa | Surgery | 5-FU + CF | 239 | |
| GIVIOa | Surgery | 5-FU + CF | 846 | |
| C01 | Surgery | MOF | 724 | |
| C02 | Surgery | PVI of 5-FU | 896 | |
| 5-FU vs. 5-FU variations | NCCTG-89-46-51 | 5-FU + LV × 1 y | 5-FU + LEV × 6 mo; 5-FU + LEV + LV × 1 y; 5-FU + LEV + LV × 6 mo | 915 |
| NCCTG-91-46-53 | Standard dose; LEV + 5-FU + CF | High dose; LEV + 5-FU + CF | 878 | |
| S9415 | 5-FU + LV + LEV | CI 5-FU + LV | 939 | |
| C03 | MOF | 5-FU + LV | 1042 | |
| C04 | 5-FU + LV | 5-FU + LV + LEV; 5-FU + LEV | 2083 | |
| C05 | 5-FU + LV | 5-FU + LV + interferon | 2136 | |
| GERCOR | LV5FU2 | FUFOL | 902 | |
| INT-0089 | LEV | HDLV; LDLV; LEV + LDLV | 3363 | |
| QUASAR | 5-FU + 175 mg L-folinic acid + placebo | 5-FU + 25 mg L-folinic acid + placebo; 5-FU + 25 mg L-folinic acid + levamisole; 5-FU + 175 mg L-folinic acid + levamisole | 3517 | |
| 5-FU vs. 5-FU + oxaliplatin | MOSAICa | 5-FU + LV | FOLFOX4 | 2241 |
| C07a | 5-FU + LV | FOLFOX | 2434 | |
| 5-FU vs. 5-FU + irinotecan | C89803 | 5-FU + LV | IFL | 1264 |
| PETACC3 | 5-FU + LV | FOLFIRI | 3188 | |
| 5-FU vs. oral | XACT | 5-FU + LV | Capecitabine | 1986 |
| C06 | 5-FU + LV | Uracil/tegafur | 1557 |
Abbreviations: 5-FU = fluorouracil; CF = cisplatin/5-FU; CI 5-FU = continuous infusion 5-FU; FOLFOX = 5-FU infusion/oxaliplatin/leucovorin; FUFOL = 5-FU bolus plus leucovorin; HDLV = high-dose leucovorin; IFL = 5-FU bolus/oxaliplatin/leucovorin; LDLV = low-dose leucovorin; LEV = levamisole; LV = leucovorin; MOF = semustine/vincristine/5-FU; PVI = protracted venous infusion.
The significant treatment effect differences were shown among the arms in original publications based on individual trial data.
Statistical Methods
Baseline characteristics were summarized with descriptive statistics, such as medians (ranges) and frequencies (percentages). The distributions of demographic and clinical characteristics between men and women were compared with the 2-sample t test (or Wilcoxon rank sum test) and the χ2 test for continuous and categorical factors, respectively. The distributions of time-to-event endpoints were estimated by Kaplan-Meier methods. The log-rank test, stratified by study, was used to compare these endpoints between men and women. Multivariate Cox proportional hazards models that stratified by study and controlled for potential confounding variables (eg, age, stage, and treatment) were constructed to estimate hazard ratios (HR) and associated 95% confidence intervals (CI) for the risk of recurrence and/or death between men and women. In addition to evaluating the prognostic value of sex, we assessed its predictive value on treatment outcomes. For trials that demonstrated a significant difference in treatment effect between the study arms in the original primary efficacy analysis, the interaction between sex and treatment on outcomes was tested by including an interaction term in the multivariate Cox models. Finally, we carried out exploratory analyses stratified by age (≤65 vs. > 65 years) and stage (II vs. III) to determine whether the prognostic impact of sex was modified by these parameters. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Characteristics of the Patient Population
Of the 33,345 patients with early-stage colon cancer included in this analysis, 18,244 (55%) were men and 15,101 (45%) were women. Distributions of patient and tumor characteristics by sex are detailed in Table 2. Overall, men were slightly older than women (median age, 62 years [range, 15–91 years] vs. 61 years [range, 15–90 years], respectively; P < .01) and more likely to be white (91% vs. 90%, respectively; P < .01) but less likely to have stage III disease (68% vs. 70%, respectively; P < .01) and right-sided cancers (43% vs. 45%, respectively; P < .01). In general, slightly higher percentages of male patients were enrolled across the different clinical trials that examined various types of adjuvant chemotherapy regimens because of the consistently lower rates of female study participation. However, treatments were well balanced between men and women within the individual clinical trials that were included in this analysis.
Table 2.
Baseline Patient Characteristics by Sex
| Characteristics | No. Trials | Overall (n = 33,345) | Men (n = 18,244) | Women (n = 15,101) | P Value |
|---|---|---|---|---|---|
| Age, years | 24 | <.0001a | |||
| n | 33,345 | 18,244 | 15,101 | ||
| Median (range) age, years | 61.0 (15.0–91.0) | 62.0 (15.0–90.0) | 61.0 (15.0–91.0) | ||
| Total no. age group | .0006b | ||||
| ≤65 years | 22,092 (66.3) | 11,939 (65.4) | 10,153 (67.2) | ||
| >65 years | 11,253 (33.7) | 6305 (34.6) | 4948 (32.8) | ||
| Total no. race | 20 | <.0001b | |||
| n | 27,282 | 14,832 | 12,450 | ||
| White | 24,868 (91.2) | 13,621 (91.8) | 11,247 (90.3) | ||
| African American | 1546 (5.7) | 729 (4.9) | 817 (6.6) | ||
| Asian | 294 (1.1) | 161 (1.1) | 133 (1.1) | ||
| Other | 574 (2.1) | 321 (2.2) | 253 (2.0) | ||
| Total no. ECOG PS | 20 | .3081b | |||
| n | 27,221 | 14,805 | 12,416 | ||
| 0–1 | 26,928 (98.9) | 14,637 (98.9) | 12,291 (99.0) | ||
| 2 | 293 (1.1) | 168 (1.1) | 125 (1.0) | ||
| Total no. stage | 24 | .0011b | |||
| n | 33,311 | 18,226 | 15,085 | ||
| I–II | 10,347 (31.1) | 5798 (31.8) | 4549 (30.2) | ||
| III | 22,964 (68.9) | 12,428 (68.2) | 10,536 (69.8) | ||
| Total no. tumor grade | 16 | .2988b | |||
| n | 19,531 | 10,625 | 8906 | ||
| 1/2 | 15,782 (80.8) | 8614 (81.1) | 7168 (80.5) | ||
| 3/4 | 3749 (19.2) | 2011 (18.9) | 1738 (19.5) | ||
| LNs examined | 21 | ||||
| n | 26,511 | 14,436 | 12,075 | ||
| No. LN assessed, median (range) | 12.0 (0.0–116.0) | 12.0 (0.0–116.0) | 12.0 (0.0–99.0) | .0036c | |
| LN assessed, binary, no. (%) | .0039b | ||||
| <12 | 13,031 (49.1) | 7213 (50.0) | 5818 (48.2) | ||
| ≥12 | 13,483 (50.9) | 7225 (50.0) | 6258 (51.8) | ||
| Positive LN, countsd | 21 | .0030c | |||
| n | 26,820 | 14,608 | 12,212 | ||
| Median (range) | 1.0 (0.0–44.0) | 1.0 (0.0–42.0) | 1.0 (0.0–44.0) |
Abbreviations: ECOG PS = Eastern Cooperative Oncology Group Performance Status Score; LN = lymph node.
Two-sample t test.
The χ2 test.
The Wilcoxon rank sum test.
Among patients with stage III disease.
Association of Sex with Outcomes
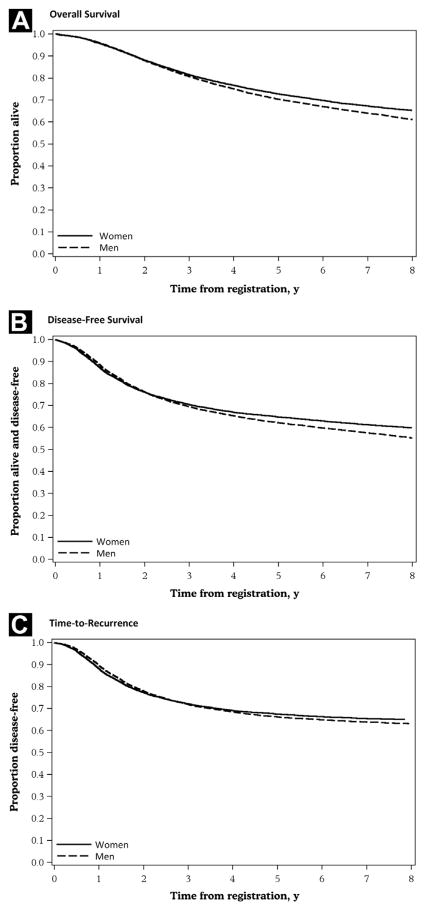
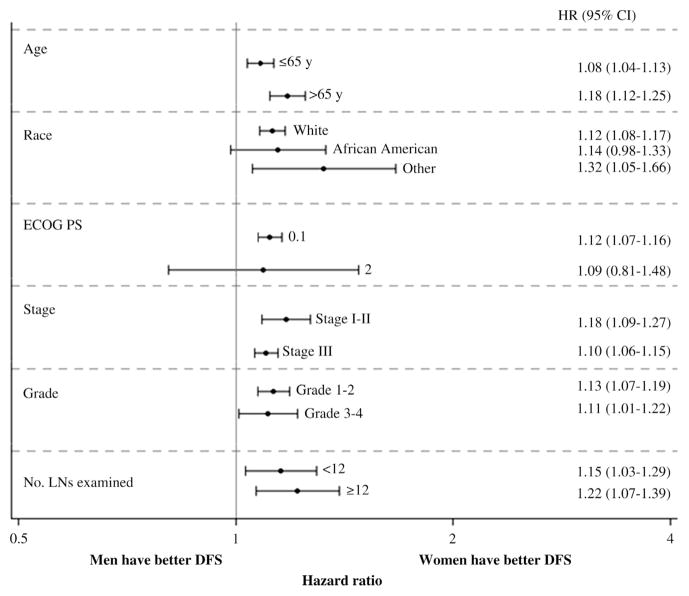
Men consistently had shorter event-free times for DFS, OS, and TTR when compared with women (Table 3). The unadjusted 5-year DFS, OS, and recurrence-free rates were 62% vs. 65% (P < .01), 70% vs. 73% (P < .01), and 66% vs. 67% (P = .04) for men vs. women, respectively (Figure 1). When adjusting for age, overall stage, and treatment, sex differences in colon cancer outcomes persisted and continued to favor women. This effect was more pronounced for DFS (adjusted HR 1.12 [95% CI, 1.08–1.16]; P < .0001) and OS (adjusted HR 1.13 [95% CI, 1.09–1.17]; P < .0001) and less apparent for TTR in which the effect size was smaller (adjusted HR 1.05 [95% CI, 1.01–1.09]; P = .0073). A similar effect was observed in a separate model in which T and N stages were controlled separately (adjusted HR for DFS 1.13 [95% CI, 1.08–1.17], P < .0001; adjusted HR for OS 1.15 [95% CI, 1.10–1.20], P < .0001; and adjusted HR for TTR 1.06 [95% CI, 1.01–1.10], P = .0183). The prognostic value of sex was seen across all treatment types and patient subgroups for DFS (Figure 2) as well as for OS and TTR (data not shown).
Table 3.
Comparing Clinical Outcomes Between Men and Womena
| Sex | No. Patients | No. Events | 5-Y Event rate (95% CI) | Adjusted Hazard Ratio (95% CI)b | P Valuec |
|---|---|---|---|---|---|
| DFS | |||||
| Men | 18,237 | 7634 | 0.62 (0.61–0.63) | 1.12 (1.08–1.16) | <.0001 |
| Women | 15,092 | 5735 | 0.65 (0.64–0.66) | Reference | |
| OS | |||||
| Men | 18,245 | 6550 | 0.70 (0.70–0.71) | 1.13 (1.09–1.17) | <.0001 |
| Women | 15,101 | 4893 | 0.73 (0.72–0.73) | Reference | |
| TTR | |||||
| Men | 18,225 | 6271 | 0.66 (0.65–0.67) | 1.05 (1.01–1.09) | .0073 |
| Women | 15,085 | 5005 | 0.67 (0.67–0.68) | Reference | |
Abbreviations: DFS = disease-free survival; OS = overall survival; TTR = time to recurrence.
All analyses were stratified by study.
Adjusting for age, stage, and treatment.
Likelihood ratio test of Cox proportional hazard model.
Figure 1.
Kaplan-Meier Curves for Outcomes. (A) Overall Survival. (B) Disease-Free Survival. (C) Time to Recurrence
Figure 2. Prognostic Impacts of Sex on DFS Stratified by Patient Characteristics.
HRs (95% CI) (which compared DFS in men with women) were estimated by Cox model adjusted for age, stage, and treatment when it was applicable and stratified by the study. HRs for subgroups classified by the number of LNs examined were only based on patients with stage II disease.
Abbreviations: DFS = disease-free survival; HR = hazard ratio; LN = lymph node.
Effect Modification
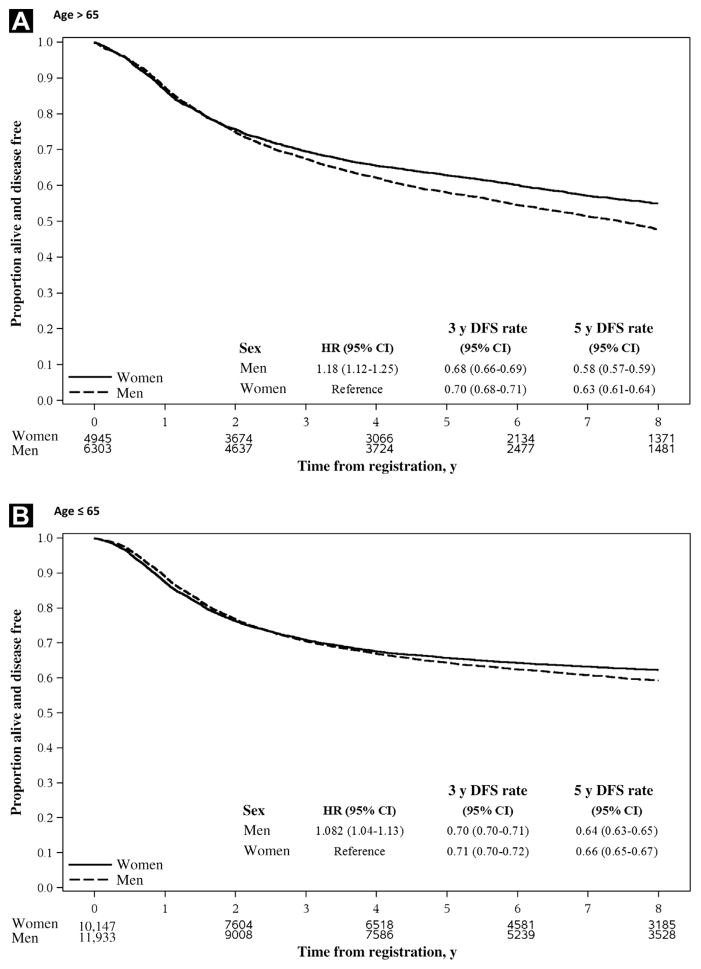
Among studies with significant treatment differences between the experimental arm and the control arm, the prognostic impact of sex on clinical outcomes was not modified by treatment assignment (Pinteraction = .41, .67, and .77 for DFS, OS, and TTR, respectively). Furthermore, subset analyses stratified by age and stage of disease were conducted to determine if the prognostic effect of sex was modified by these variables (Table 4). Our findings showed that age was an effect modifier of DFS because sex disparities in outcomes were significantly different between younger and older patients (Figure 3). Although the favorable prognostic impact of female sex persisted across age groups, it was more evident in the elderly subset of the population, whereby the adjusted HR was 1.08 (95% CI, 1.04–1.13) in patients aged ≤ 65 years, whereas the adjusted HR was 1.18 (95% CI, 1.12–1.25) in patients aged > 65 years (Pinteraction = .02). Age also modified the effect of sex on OS, albeit less strongly (Pinteraction = .09), but it did not consistently modify the effect of sex on TTR (Pinteractions = .36). In contrast, the stage of disease did not alter the prognostic influence of sex on DFS, OS, and TTR (all Pinteraction > .05).
Table 4.
Testing Interaction Effects Between Sex and Key Factors on Outcomesa
| Key Factor | Disease-free Survival | Overall Survival | Time to Recurrence |
|---|---|---|---|
| Treatment (experimental arm vs. control arm)b | .41 | .67 | .77 |
| Stage (I/II vs. III) | .13 | .20 | .94 |
| Age (>65 vs. ≤65 y) | .016 | .086 | .36 |
The table presents P values based on the likelihood-ratio test for testing the interaction terms included in the Cox models, stratified by study; all the models were adjusted for treatment, stage, and age when it was applicable.
Only studies with significant treatment effects were included for assessing the interaction effect between sex and treatment.
Figure 3. Kaplan-Meier Curves for DFS Stratified by Sex and Age Group. (A) Age > 65 y. (B) Age ≤ 65 y.
HRs (95% CI) were estimated by Cox model adjusted for stage and treatment and were stratified by study. The numbers under the graph are the number of patients at risk at randomization, 2, 4, and 8 y after randomization.
Abbreviations: DFS = disease-free survival; ECOG PS = Eastern Cooperative Oncology Group Performance Status Scale; HR = hazard ratio; LN = lymph node.
Discussion
Female sex as a favorable prognostic factor in colon cancer has previously been described, but the results have been very inconsistent.8–13 Moreover, the majority of this research involved small numbers of patients, recruited subjects from only several local institutions, and almost all were conducted before the widespread use of oxaliplatin in the adjuvant treatment of colon cancer. Consistent with some of these prior studies, our analysis of a large representative cohort of more than 30,000 clinical trial participants from 24 studies who received various adjuvant treatments after their curative resections, indicated that women with early-stage colon cancer experienced a statistically significant but very modest survival advantage when compared with men. This pattern was observed for DFS, OS, and TTR. Furthermore, we demonstrated that this survival benefit for women persisted across all ages, stages, and treatment types.
Of note, the sex differences in colon cancer survival seen in this study were less pronounced than those reported in previous series. For example, Watanabe et al13 reported in a study of adjuvant 5-FU–based chemotherapy in colon cancer that the 5-year OS rate among women was 10% higher than that for men. In contrast, we only showed a 3% difference in 5-year OS rates between sexes that favored women. A potential factor for why the survival benefit for women was lower in this analysis may be related to our primary focus on clinical trial participants, most of whom were highly functional, with a good performance status at the time of study entry (99% of patients were Eastern Cooperative Oncology Group Performance Status Scale 0 or 1). This could have minimized any sex differences that would otherwise be evident in routine practice settings in which functional status may be more disparate. Results of some studies also indicate that there may be differences in colorectal screening uptake between sexes that lead to earlier disease detection among women and hence better outcomes.19,20 In our cohort of patients, however, men were slightly more likely to be diagnosed with earlier-stage colon cancer, which may have further minimized any sex differences in survival. Nonetheless, the men included in this analysis were slightly older than the women, which may have contributed in part to the differences observed in specific survival endpoints, such as DFS and OS.
To date, the causes for these sex differences in colon cancer outcomes have remained unclear. However, similar differences have been noted in both small- and non–small-cell lung cancers whereby women consistently fared better than men,21–23 which suggests that there may be broad underlying hormonal, genetic, or molecular mechanisms that predispose women in general to more favorable prognoses that span across cancer sites. It is also possible that the survival advantage among women that is described for these cancers is purely a consequence of the longer natural life expectancy of women, as supported by recently published data that confirmed a reduced 10-year risk of death from all causes for women.1 The lesser difference between men and women in the disease-specific endpoint TTR than DFS or OS could be partly explained by the higher mortality from non–colon cancer causes in men than in women. A possible contributor to the difference in TTR between men and women is the higher proportion of women than men with good prognostic features, such as defects in the DNA mismatch repair gene.24
One of the strengths of the current analysis is its reliable ascertainment of 3 outcome measurements: DFS, OS, and TTR. This is possible because of the standard, uniform follow-up protocols mandated by most clinical trials. Given that the primary aim of adjuvant therapy for colon cancer is to prolong patient survival by delaying or preventing recurrence, TTR is the most-sensitive measurement of the intended effect of adjuvant therapy. Unlike DFS and OS, TTR is less affected by disparities in treatment of recurrent disease, management of comorbid conditions, and differential rates of death from competing causes that are unrelated to colon cancer. In this study, the magnitude of the sex effect was lowest for TTR (HR 1.05, a 5-year difference of 1% between men and women), which suggests that any differential response to adjuvant therapy must be small. This is supported by our analysis of the predictive value of sex in which there was no evidence of significant interaction between sex and adjuvant treatment on any of the outcomes (all Pinteractions > .05). Conversely, the effect sizes for DFS and OS were larger than that for TTR, which highlights that the observed sex disparities in colon cancer outcomes were less likely to be related to adjuvant treatment and more likely to be reflections of the wider phenomenon of differences in comorbidities and life expectancies between men and women.
An interesting study result was that the survival benefit for women was more apparent in patients aged > 65 years (HR 1.18 for DFS in men [95% CI, 1.12–1.25]) than in patients aged ≤ 65 years (HR 1.08 for DFS in men [95% CI, 1.04–1.13) (Pinteraction < .01). Previous studies have proposed estrogen as a putative mechanism for the better cancer outcomes in women through its protective effect on carcinogenesis and metastases,25,26 but our finding of a larger survival advantage in older (postmenopausal) women, in whom estrogen levels would be lower than younger women, cannot be easily explained by this postulate. Rather, there likely are a number of possible alternative reasons for the sex differences, including later stage at diagnosis in men, differences in access to and delivery of treatment, and the possibility that men may have a biologically more-aggressive disease or a poorer response to adjuvant therapies. By using the ACCENT clinical trial database, we attempted to begin exploring these hypotheses and found that stage of disease neither explained nor modified the sex effect on colon cancer outcomes (Pinteractions > .05). Further studies are warranted to investigate some of the remaining alternative hypotheses.
Despite its large size, this study has several limitations. First, treatment data that consist of chemotherapy dose intensities, delays, and toxicities were not available for analyses even though these treatment parameters could have a significant impact on survival. Future studies should try to account for such treatment-related data, especially because results of early research show that there may be sex differences in the metabolism of commonly used chemotherapy agents as well as sex differences in toxicity.14–18 Second, although we were able to collect performance status data for the majority of individuals, other important baseline prognostic factors, such as pretreatment laboratory values and comorbid conditions, were not considered. Likewise, data about certain lifestyle patterns and behaviors, including concurrent medications as well as smoking and alcohol history, that can also affect outcomes were not captured. Concomitant medications may differ between sexes, and the potential influence of these drugs on chemotherapy efficacy and the measured outcomes is unknown.27 The interplay among patient’s sex, lifestyle, and behavioral factors, and outcomes is clearly an area that requires further research. Third, this was a pooled analysis of a very heterogeneous group of clinical trials that span 2 decades. However, it would seem unlikely that any tumor biology differences between men and women would vary significantly over time. Finally, the role of predictive and prognostic biomarkers and how they are differentially distributed between sexes are beyond the scope of this analysis, and remain to be studied.
Conclusion
This analysis of an extensive cohort of clinical trial patients demonstrates only modest differences in outcomes, including DFS, OS, and TTR, between men and women who received various adjuvant chemotherapies for resected colon cancer, particularly among patients aged > 65 years. Additional studies are needed to better elucidate the processes that contribute to these sex-specific effects and to understand the interplay between such demographic determinants and novel molecular biomarkers. Of note, sex did not predict for adjuvant treatment benefit, which indicates that both men and women derived a similar magnitude of benefit from chemotherapy. Thus, future clinical trials of colon cancer in the adjuvant setting may not need to be stratified by sex and decisions regarding adjuvant chemotherapy should not be altered based on the sex of the patient alone.
Clinical Practice Points.
Female sex as a favorable prognostic factor in colon cancer has been previously described, but the results have been very inconsistent.
The majority of this research involved small numbers of patients, recruited subjects from only several local institutions, and almost all were conducted before the widespread use of oxaliplatin in the adjuvant treatment of colon cancer.
Our analysis of a large representative cohort of more than 30,000 clinical trial participants from 24 studies who received various adjuvant treatments after their curative resections indicated that women with early-stage colon cancer experienced a statistically significant but a very modest survival advantage when compared with men.
This pattern was observed for disease-free survival, overall survival, and time to tumor recurrence.
We also demonstrated that this survival benefit for women, albeit minor, persisted across all ages, stages, and adjuvant treatment types.
Given the modest survival difference between sexes, future clinical trials of colon cancer in the adjuvant setting may not need to be stratified by sex and decisions regarding adjuvant chemotherapy should not be altered based on the sex of the patient alone.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–74. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 5.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 7.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 8.Paulson EC, Wirtalla C, Armstrong K, et al. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–91. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 9.Koo JH, Jalaludin B, Wong SK, et al. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488–95. doi: 10.1111/j.1572-0241.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen BL, Pahlman L, Gunnarsson U, et al. The effect of age and gender on outcome after treatment for colon carcinoma. A population-based study in the Uppsala and Stockholm region. Crit Rev Oncol Hematol. 2008;67:229–36. doi: 10.1016/j.critrevonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.McArdle CS, McMillan DC, Hole DJ. Male gender adversely affects survival following surgery for colorectal cancer. Br J Surg. 2003;90:711–5. doi: 10.1002/bjs.4098. [DOI] [PubMed] [Google Scholar]
- 12.Wichmann MW, Muller C, Hornung HM, et al. Gender differences in long-term survival of patients with colorectal cancer. Br J Surg. 2001;88:1092–8. doi: 10.1046/j.0007-1323.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan JA, Goldberg RM, Sargent DJ, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20:1491–8. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 15.Sloan JA, Loprinzi CL, Novotny PJ, et al. Sex differences in fluorouracil-induced stomatitis. J Clin Oncol. 2000;18:412–20. doi: 10.1200/JCO.2000.18.2.412. [DOI] [PubMed] [Google Scholar]
- 16.Cascinu S, Barni S, Labianca R, et al. Evaluation of factors influencing 5-fluorouracil-induced diarrhea in colorectal cancer patients. An Italian Group for the Study of Digestive Tract Cancer (GISCAD) study. Support Care Cancer. 1997;5:314–7. doi: 10.1007/s005200050079. [DOI] [PubMed] [Google Scholar]
- 17.Lagrange JL, Medecin B, Etienne MC, et al. Cisplatin nephrotoxicity: a multivariate analysis of potential predisposing factors. Pharmacotherapy. 1997;17:1246–53. [PubMed] [Google Scholar]
- 18.Milano G, Etienne MC, Cassuto-Viguier E, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol. 1992;10:1171–5. doi: 10.1200/JCO.1992.10.7.1171. [DOI] [PubMed] [Google Scholar]
- 19.Meissner HI, Breen N, Klabunde CN, et al. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 20.Wardle J, Miles A, Atkin W. Gender differences in utilization of colorectal cancer screening. J Med Screen. 2005;12:20–7. doi: 10.1258/0969141053279158. [DOI] [PubMed] [Google Scholar]
- 21.Wheatley-Price P, Blackhall F, Lee SM, et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: a pooled analysis of five randomized trials. Ann Oncol. 2010;21:2023–8. doi: 10.1093/annonc/mdq067. [DOI] [PubMed] [Google Scholar]
- 22.Wheatley-Price P, Le Maitre A, Ding K, et al. The influence of sex on efficacy, adverse events, quality of life, and delivery of treatment in national cancer institute of canada clinical trials group non-small cell lung cancer chemotherapy trials. J Thorac Oncol. 2010;5:640–8. doi: 10.1097/JTO.0b013e3181d40a1b. [DOI] [PubMed] [Google Scholar]
- 23.Singh S, Parulekar W, Murray N, et al. Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J Clin Oncol. 2005;23:850–6. doi: 10.1200/JCO.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 24.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–70. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 25.Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–7. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JA, Meyerhardt JA, Chan AT, et al. Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:5680–6. doi: 10.1200/JCO.2006.08.0580. [DOI] [PubMed] [Google Scholar]
- 27.Venturini CD, Engroff P, Ely LS, et al. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics (Sao Paulo) 2011;66:1867–72. doi: 10.1590/S1807-59322011001100004. [DOI] [PMC free article] [PubMed] [Google Scholar]