Abstract
Toll like receptors (TLRs) sense microbial products and initiate adaptive immune responses by activating dendritic cells (DCs). Since pathogens may contain several TLR agonists we asked whether different TLRs synergize in DC activation. In human and mouse DCs TLR3 or TLR4 potently synergized with TLR7, TLR8 and TLR9 in the induction of a selected set of genes. Synergic TLR stimulation increased IL-12 and IL-23 production and the Delta-4/Jagged-1 ratio leading to DCs with enhanced and sustained TH1 polarizing capacity. Global gene transcriptional analysis showed that TLR synergy boosted only approximately 1% of the transcripts induced by single TLR agonists. These results identify a combinatorial code by which DCs discriminate pathogens and suggest new strategies to promote TH1 responses.
Introduction
Toll like receptors (TLRs) are innate receptors that sense microbial products and trigger dendritic cell (DC) maturation and cytokine production thus effectively bridging innate and adaptive immunity1. Several aspects of TLR biology contribute to the efficient microbial detection by immune cells. First, each TLR is triggered by a distinct set of microbial compounds2. For instance TLR4 is triggered by lipopolysaccharide (LPS)3,4, TLR9 by unmethylated oligonucleotides (CpG)5 and TLR3 by double-stranded RNA [mimicked by poly(I:C)]6. Second, TLRs are differently coupled to signal transduction pathways7. With the exception of TLR3, all TLRs are coupled to the MyD88 adaptor, while TLR3 and TLR4 also couple to the adaptor TRIF8 (also known as TICAM-19), that triggers interferon-β (IFN-β) transcription. Other adaptors are differentially recruited by TLR2, TLR3 and TLR4, but their relative function is less clear10–14. Third, TLRs are localized in different compartments. While most TLRs are present on the cell surface, TLR7, TLR8 and TLR9 are found in the endosomal compartment15,16 and TLR3 is present intracellularly although its exact location has not yet been defined. Finally, TLRs are expressed, in a constitutive or induced fashion, in different cell types determining their capacity to detect microbial products17–19.
DCs express the broadest repertoire of TLRs through which they can recognize a large plethora of microbial compounds. Upon challenge with microbial or inflammatory stimuli immature DCs undergo a complex process of maturation, resulting in their migration from tissues to secondary lymphoid organs and upregulation of major histocompatibility complex (MHC) and costimulatory molecules that are essential for T cell priming20. In addition it is well appreciated that DCs represent a critical source of interleukin 12 (IL-12), a cytokine that plays a key role in innate responses and drives TH1 polarization21. Several studies have shown that IL-12 production by DCs is tightly controlled since it requires a first priming signal provided by microbial products or IFN-γ followed by an amplifying signal provided by T cells through CD40L22–24. Thus, DCs are capable of integrating signals from pathogens, cytokines and T cells leading to the generation of an adaptive immune response of the appropriate class25.
Besides IL-12, additional cues driving T cell differentiation have been recently described. One such factor is IL-23, which shares with IL-12 the p40 chain but pairs with a unique p19 chain26. IL-23 drives the differentiation of inflammatory T cells capable of secreting high amounts of tumor necrosis factor (TNF) and IL-17 that mediate tissue damage in autoimmune diseases27,28. Furthermore, two Notch ligands, Delta-4 and Jagged-1 can be expressed by DCs and are capable of selectively inducing TH1 or TH2 differentiation, respectively29. The regulation of Notch ligands in human DCs has not been investigated.
Most studies thus far analyzed DC activation induced by single microbial compounds. However, pathogens express several TLR agonists that may engage different TLRs at different times and in distinct cellular compartments. A modest synergistic effects of TLR agonists on the production of inflammatory mediators, mainly TNF, by macrophage cell lines has been reported, but the impact on T cell priming has not been analyzed30–32. In the present study we asked whether agonists triggering different TLRs may synergize in DC activation. We report that in human DCs agonists of TLR3 and TLR4 potently synergize with agonist of TLR8 in inducing IL-12, IL-23 and Delta-4 in amounts that are 50–100 fold higher than those induced by optimal concentrations of single agonists leading to enhanced and sustained TH1 polarizing capacity. These results identify a combinatorial code by which DCs discriminate pathogens and may provide a rationale to design adjuvants for TH1 responses.
Results
Selected TLR agonists synergize in IL-12p70 induction
Previous studies showed that IL-12p70 is not efficiently elicited in human or mouse DCs by microbial stimuli alone and that a second signal, provided by natural killer (NK) cells through IFN-γ or by T helper cells through CD40L, is required for optimal induction22,23. In this study we asked whether distinct TLRs may synergize in the induction of IL-12p70. As agonists we used poly(I:C), LPS and the synthetic imidazoquinoline resiquimod (R848) that trigger TLR3, TLR4 and TLR833, respectively. When added alone to human immature monocyte-derived DCs (MoDCs), the three agonists induced full DC maturation as assessed by upregulation of HLA-DR, B7.1 (also known as CD80) and B7.2 (CD86), as well as the production of low amounts of IL-12p70 (Fig. 1a and Supplementary Fig. 1a). Remarkably, the simultaneous addition of R848 and LPS or R848 and poly(I:C), but not LPS and poly(I:C), induced 20–50 fold higher amounts of IL-12p70 than single agonists without further increasing HLA-DR and B7 expression. The same synergistic combinations of TLR agonists were also capable of inducing the production of high amounts of IL-12p70 in primary DCs isolated from human peripheral blood, which produced no or low amounts of IL-12p70 in response to single agonists (Fig. 1b).
Figure 1. Synergistic stimulation of IL-12p70 production by combinations of TLR agonists.
Human MoDCs (a), human DCs isolated from peripheral blood (b), mouse bone marrow derived DCs (BMDCs) (c) were cultured with 10 ng/ml LPS, 20 µg/ml poly(I:C), 2.5 µg R848 and 2.5 µg CpG 1826 alone or in combination. IL-12p70 was measured in the 48 h culture supernatant. Data are presented as mean ± SD of three replicates of one representative experiment out of five. (d,e) MoDCs were stimulated with LPS (filled diamonds), R848 (filled squares), poly(I:C) (filled triangles) or combinations of LPS plus R848 (empty squares) or poly(I:C) plus R848 (empty triangles). The TLR agonists were added at the indicated concentrations. IL-12p70 was measured in the 48 h culture supernatant. Data are presented as mean of duplicates of one representative experiment out of four.
Mouse bone marrow derived DCs do not express TLR8, but express the homologous receptors TLR7 and TLR934, which are triggered by R848 and CpG respectively. When mouse DCs were challenged by TLR agonists, alone or in combination, a strong synergy was observed with combinations of LPS or poly(I:C) and CpG or R848, while other combinations were ineffective (Fig. 1c).
We investigated the dose requirements for synergistic TLR stimulation by challenging human MoDCs with a range of concentrations of LPS, R848 and poly(I:C) (Fig. 1d). Combinations of suboptimal doses of TLR agonists, that were unable to induce IL-12p70 release when given alone, induced IL-12p70 production at concentrations that were higher than those induced by optimal doses of single agonist, consistent with a synergistic mode of cytokine induction. In addition combinations of non stimulatory concentrations of TLR agonists were able to induce B7.2 expression, but the levels did not reach those induced by an optimal dose of the single agonists (Supplementary Fig. 1b). These results indicate that TLRs may have additive effects in the induction of costimulatory molecules that may increase sensitivity of detection, while they potently synergize in the induction of IL-12.
Requirements for TLR synergy
The above results indicate that in both human and mouse DCs the simultaneous activation of a TRIF-coupled receptor (TLR3 or TLR4) together with an endosomal receptor (TLR8 in humans and TLR7 or TLR9 in mice) leads to a potent synergistic activation of IL-12p70 production. We considered the possibility that the synergistic effect might be mediated by production of IFN-β, which is rapidly and selectively induced by triggering TRIF-dependent TLRs8. However, addition of exogenous IFN-β caused only a modest increase in IL-12 production and this increase was observed in all conditions tested, irrespective of the nature of the stimulus and of the concentration of IFN-β added (Supplementary Fig. 2). Although we cannot exclude a contribution of IFN-β or of IFN-β-induced genes in TLR synergy we conclude that this pathway is unlikely to be the basis of TLR synergy.
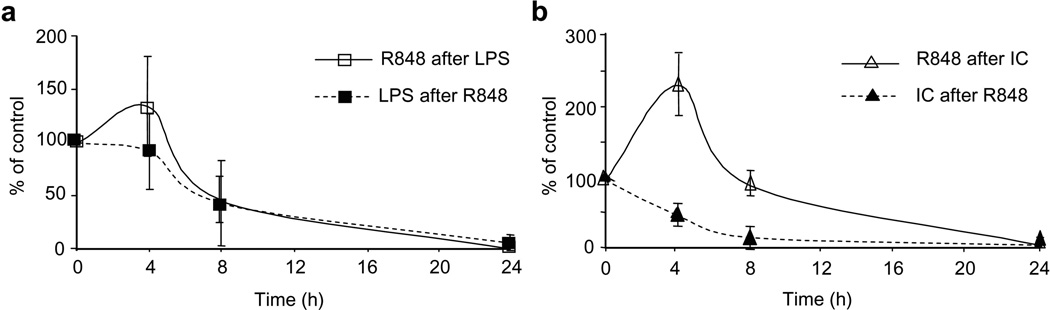
The fact that TLR3, TLR4 and TLR8 are present in different cellular compartments suggests that they may be triggered with different kinetics15,16. In addition, upon initial TLR stimulation DCs become refractory to subsequent stimulation by the same or other TLR agonists35,36. We therefore investigated whether the timing and the order of addition of TLR agonists would affect the extent of synergy in the induction of IL-12p70. In the case of LPS and R848 synergy was maximal when the agonists were given within a 4 hour window and irrespective of the order of addition (Fig. 2a). In contrast, in the case of poly(I:C) and R848, synergy was maximal when poly(I:C) was added 4 hours before R848 and was substantially decreased when poly(I:C) was added after R848 (Fig. 2b). In all cases no synergy was observed when the two stimuli were given 24 hours apart. Since the different localization of the TLRs involved in synergistic activation we considered the possibility that synergism may be related to a redistribution of endosomal TLR8 at the cell surface induced by TLR3 or TLR4 engagement. The inhibitor of endosomal acidification Bafilomycin A116,37 abolished the effect of R848 both when the latter was used as single agonist and when given 5 hours after LPS or poly(I:C) (Supplementary Fig. 3 and data not shown), indicating that TLR8 activation requires endosomal acidification also after TLR3 and TLR4 stimulation.
Figure 2. Temporal requirements for TLR synergy.
(a,b) MoDCs were stimulated with LPS (10 ng/ml) and R848 (2.5 µg/ml) (a) or poly(I:C) (20 µg/ml) and R848 (b). The stimuli were given simultaneously (time 0) or sequentially (dashed line: LPS or poly(I:C) after R848; solid line: R848 after LPS or poly(I:C)) at the indicated interval. IL-12p70 was measured in the 48 h culture supernatant. The data are expressed as percent of the value obtained when the agonists were added simultaneously. Data represent the mean ± SD of three independent experiments.
Superinduction of IL-12 by multiple synergizing agonists
The above results indicate that synergistic TLR stimulation can directly trigger high amounts of IL-12p70 even in the absence of amplifying signals provided by IFN-γ or CD40L22,23. It was therefore important to establish whether the IL-12p70 production induced by synergistic TLR stimulation had already reached a maximal ceiling or could be further boosted by IFN-γ or CD40L. Human DCs were stimulated with TLR agonists alone or in combination in the absence or in the presence of IFN-γ or CD40L (Fig. 3). As previously reported22,23, IFN-γ and CD40L potently boosted IL-12p70 production induced by single TLR agonists. Notably, the amount of IL-12p70 elicited by a combination of single TLR agonists and CD40L was comparable to that elicited by synergizing TLR agonists in the absence of IFN-γ or CD40L. In addition, IFN-γ and CD40L were able to further increase the production of IL-12p70 induced by synergizing TLR combinations. Indeed, irrespective of the type of TLR stimulation, the amount of IL-12p70 produced was increased approximately 5 fold by IFN-γ and approximately 50 fold by CD40L. In certain experiments, under maximal stimulatory conditions (synergistic TLR stimulation plus CD40L), 106 DCs produced as much as 2 µg of IL-12p70. Taken together these findings indicate that DCs have a very high capacity to produce IL-12p70, which is deployed only when they are triggered by multiple synergizing stimuli. While IFN-γ and CD40L can potently boost cytokine production, the final extent is set by the nature and combination of microbial stimuli.
Figure 3. IFN-γ and CD40L further amplify IL-12p70 production induced by synergistic combinations of TLR agonists.
MoDCs were stimulated with 10 ng/ml LPS, 2.5 µg/ml R848, 20 µg/ml poly(I:C) alone or in combinations in the absence (grey histograms), or in the presence of 5 ng/ml IFN-γ (empty histograms) or 2 µg/ml CD40L (filled histograms). IL-12p70 was measured in the 48 h culture supernatant. Data represent the mean ± SD of three replicates of one representative experiment out of three.
TLR synergy increases IL-12, IL-23 and Delta-4 mRNAs
IL-12 is a heterodimeric cytokine consisting of IL-12p40 chain paired with IL-12p35. In addition IL-12p40 can pair with IL-23p19 to form bioactive IL-2326. It was therefore relevant to analyze the effect of single or synergistic stimulation on the expression of the corresponding mRNAs. Stimulation of human DCs with synergistic combinations of TLR agonists [LPS plus R848 or poly(I:C) plus R848] led to a 4–6 fold increase in IL-12p40 mRNA and to a 40–60 fold increase in IL-12p35 and IL-23p19 mRNAs (Fig. 4a). In addition transcript abundance was more sustained following synergistic TLR stimulation. These findings indicate that the simultaneous triggering of two specific TLRs is particularly critical for inducing and sustaining IL-12p35 and IL-23p19 mRNAs which are limiting for IL-12 and IL-23 production.
Figure 4. Synergistic TLR stimulation is required for maximal upregulation of IL-12p35, IL-23p19 and Delta-4.
MoDCs were stimulated with 10 ng/ml ultra pure LPS (filled diamonds), 2.5 µg/ml R848 (filled squares), 20µg/ml poly(I:C) (filled triangles), LPS+R848 (empty squares) or poly(I:C)+R848 (empty triangles). The amounts of IL-12p40, IL-12p35, IL-23p19 (a), and of Delta-4 and Jagged-1 (b) mRNAs relative to 18S rRNA were determined by quantitative real time RT-PCR at the indicated time points. One representative experiment out of three. AU, arbitrary units of the indicated mRNA/18S rRNA x 10−3.
We next investigated whether TLR synergy might also modulate the expression of the two Notch ligands Delta-4 and Jagged-1 that have been recently shown to promote TH1 and TH2 differentiation, respectively29. Delta-4 mRNA was undetectable in immature DCs and relatively few transcripts were induced by LPS, R848 or poly(I:C). Remarkably, synergistic combinations [LPS plus R848 or poly(I:C) plus R848] increased Delta-4 mRNA more than 100 fold (Fig. 4b). In contrast, Jagged-1 mRNA was constitutively expressed in immature DCs and was transiently increased above the basal expression by all stimuli. However synergistic combinations of TLR agonists resulted in approximately one-tenth less abundant Jagged-1 mRNA at late time points (Fig. 4b). Thus, synergistic stimulation of TLRs dramatically increases the Delta-4 to Jagged-1 ratio of expression in DCs.
TLR synergy enhances TH1 polarizing capacity of DCs
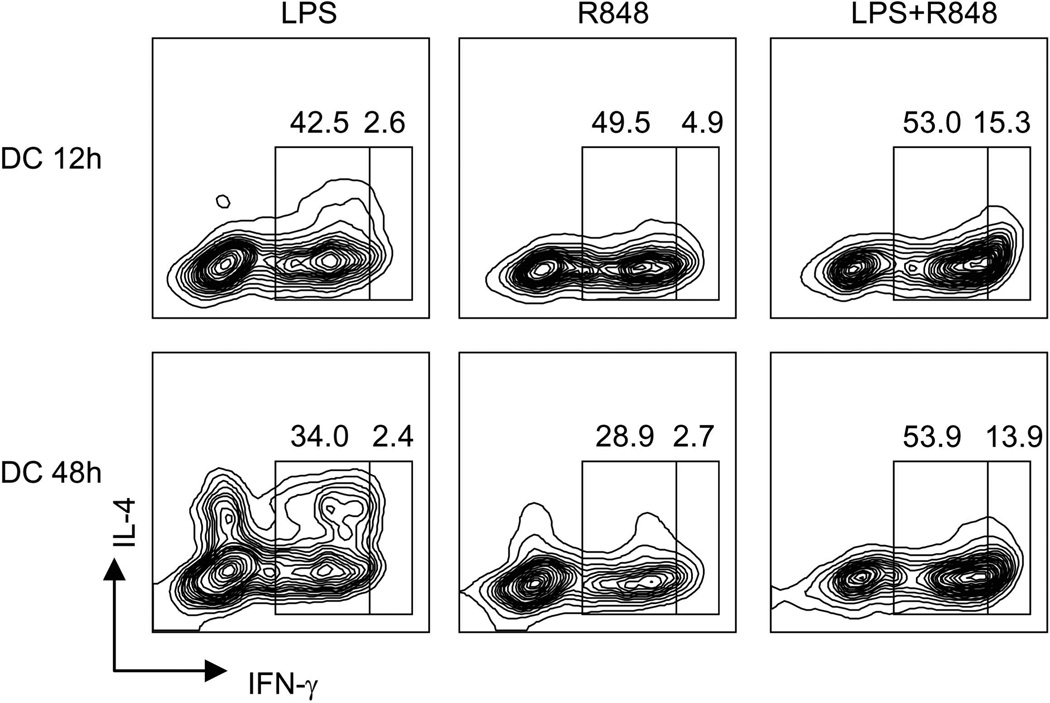
Previous studies revealed that the capacity of mature DCs to prime naïve T cells is determined by the expression of MHC and costimulatory molecules, yet the TH1 polarizing capacity is dependent on the actual secretion of IL-12 that is exhausted by 24 hours after LPS stimulation35. To evaluate the effect of TLR synergy on TH1 priming, DCs were stimulated with single or synergistic combinations of TLR agonists for 12 or 48 hours, washed and tested for their capacity to prime naive allogeneic CD4+ T cells. T cell proliferation was comparable in all conditions tested, consistent with the comparable expression of MHC class II molecules, B7.1 and B7.2 (not shown). In striking contrast, DCs stimulated by LPS plus R848 induced much higher TH1 polarization than DCs stimulated by single agonists (both in terms of percentage of positive cells and of amount of IFN-γ produced per cell). Furthermore, DCs stimulated by LPS and R848 for 48 hours showed persistent TH1 polarizing capacity (Fig. 5). We conclude that synergistic TLR stimulation both increases and sustains the TH1 polarizing capacity of mature DCs.
Figure 5. TLR synergy enhances and sustains the TH1 polarizing capacity of DCs.
MoDCs were stimulated with 10 ng/ml LPS, 2.5 µg/ml R848 or LPS+R848 for 12 or 48 h, washed and cultured with allogeneic naive CD4+ T cells. After 8 days the proliferating T cells were tested for their capacity to produce IFN-γ and IL-4 following stimulation with PMA and Ionomycin. Numbers indicate percent of cells producing intermediate and high amounts of IFN-γ. One representative experiment out of five.
TLR synergy affects cytokine production and release
TLR-activated DCs produce a large variety of inflammatory cytokines and chemokines, and upregulate the chemokine receptor CCR7 which drives them to the lymph node35. Following synergistic TLR stimulation the mRNAs of the inflammatory mediators cyclooxygenase-2 (COX-2), TNF and IL-6 were increased and sustained (Fig. 6a). As a consequence there was an increase in the amount of TNF and IL-6 released in the culture supernatant (Supplementary Fig. 4).
Figure 6. Selective effect of TLR synergy on DC gene expression and on IL-1β release.
MoDCs were stimulated with 10 ng/ml ultra pure LPS (filled diamonds), 2.5 µg/ml R848 (filled squares), 20 µg/ml poly(I:C) (filled triangles), LPS+R848 (empty squares) or poly(I:C)+R848 (empty triangles). The amounts of TNF, IL-6, IL-10, COX-2, IFN-β, CXCL10, CCL3, CCR7 (a) and of IL-1β (b) mRNAs relative to 18S rRNA were determined by quantitative real time RT-PCR. AU, arbitrary units of the indicated mRNA/18S rRNA x 10−3 (c) IL-1β release was measured in the 48 h culture supernatant and data are presented as mean ± SD of three replicates of one out of three independent experiments.
In the same stimulatory conditions IFN-β mRNA expression was increased, whereas mRNAs encoding the IFN-β–dependent chemokines were either unaffected (CXCL10), or showed only a modest increase (CCL5 and CCL22) (Fig. 6a and data not shown). TLR agonists combinations had no synergistic effect on the expression of CCL3 mRNA, which peaks at early time points, nor on the expression of CCR7 mRNA, which was induced progressively and showed maximal expression at late time points (Fig. 6a). Finally TLR agonists combinations boosted the expression and release in the supernatant of the immuno-modulatory cytokine IL-10 (Fig. 6a and Supplementary Fig. 4).
Notably we found that, while IL-1β mRNA was induced by single agonists and only moderately increased by synergistic TLR stimulation (Fig. 6b), release of IL-1β protein in the supernatant was not observed upon stimulation with highly purified LPS36 or R848 but was efficiently induced following synergistic stimulation (Fig. 6c). This finding suggests that synergistic TLR stimulation provides signals necessary to trigger the inflammasome37.
TLR synergy regulates a small set of genes
To evaluate the impact of TLR synergy on global gene expression we performed a microarray analysis of MoDCs stimulated with LPS, R848 or LPS plus R848 for 2 and 8 hours. A large number of genes (8,828 out of the 14,550 expressed) were upregulated or downregulated by maturation stimuli in at least one of the conditions tested. Figure 7 shows for each of the 8,828 regulated transcripts the correlation between the fold change induced by LPS plus R848 and the fold change induced by the best of the single agonists. No significant differences were found in the gene expression pattern 2 hours after stimulation (Fig. 7a). In contrast, at the 8 hour time point a striking change in gene expression was observed. In synergistically stimulated DCs most of the upregulated transcripts (including TNF, IL-6, IL-10, IL-12p40 and COX-2) were induced 2–5 fold more compared to DCs stimulated by single agonists and, reciprocally, most of the downregulated genes were further suppressed. Remarkably, however, the expression of only approximately 1% of the genes induced by single TLR agonists was increased more than 5 fold upon synergistic TLR stimulation (Fig 7b and Table 1). Out of these transcripts most, including IL-12p35 (IL12B), and IL-23p19 (IL23A) were “late genes” (expression peak at 8 h), while a few (IL29, EGR3, OSR2, DUSP2, LOC400581) were “early genes” (expression peak at 2 h) whose mRNA expression was sustained in synergistically stimulated DCs. This microarray analysis failed to reveal Jagged-1 and Delta-4, as genes regulated by synergic stimulation. However Jagged-1 downregulation (Fig. 4) was evident only at late time points (24 h) which are not present in the Affymetrix analysis, while the Delta-4 specific Affymetrix probes recognized a 3’-untranslated region present in only one of the two splice variants recognized by the TaqMan probe utilized. We conclude that TLR synergy affects global gene expression only at late time points and that it selectively boost a small number of genes of high relevance for the immune response.
Figure 7. Effect of TLR synergy on global gene expression is observed only at late time point.
MoDCs from three different donors were left untreated (control) or stimulated with 10 ng/ml ultra pure LPS, 2.5 µg/ml R848 or LPS+R848 for 2 or 8 h. Global transcriptional analysis was performed using the Affymetrix methodology. (a,b) Correlation between fold change (relative to control) induced by LPS+R848 and fold change induced by the best agonist (either LPS or R848). The data are from the 2 h (a) and 8 h time points (b). The dashed lines mark five fold changes.
Table 1.
Highly regulated genes in DCs stimulated by synergistic TLR combinations
| Gene | LPS + R848 2h |
Fold-increase over single agonist2 |
LPS + R848 8h |
Fold-increase over single agonist2 |
|---|---|---|---|---|
| IL23A | 28.81 | 1.0 | 905.8 | 29.0 |
| IL29 | 158.1 | 1.1 | 144.5 | 25.9 |
| CSF3 | 1.5 | 1.1 | 148.2 | 24.5 |
| C10orf116 | 1.8 | 2.2 | 165.8 | 22.3 |
| TFPI2 | 1.8 | 1.9 | 569.2 | 21.2 |
| CSF2 | 4.5 | 1.1 | 605.3 | 12.6 |
| EGR3 | 21.1 | 1.1 | 8.6 | 12.4 |
| LOC91614 | 0.4 | 1.0 | 16.1 | 11.4 |
| MMP10 | 4.3 | 1.1 | 446.2 | 11.2 |
| LIF | 2.2 | 1.5 | 15.8 | 10.8 |
| IL1F9 | 16.6 | 1.3 | 375.2 | 9.1 |
| TPBG | 2.8 | 1.3 | 8.7 | 7.6 |
| LOC387763 | 16.1 | 2.0 | 56.5 | 7.4 |
| LAMC1 | 1.0 | 1.6 | 12.7 | 6.8 |
| CITED4 | 0.7 | 1.1 | 34.7 | 6.7 |
| OSR2 | 19.3 | 1.1 | 22.4 | 6.6 |
| RHCG | 1.4 | 1.3 | 196.3 | 6.5 |
| PERP | 2.3 | 1.4 | 101.3 | 6.4 |
| CDKN2B | 79.1 | 2.1 | 148.4 | 6.3 |
| LOC400581 | 28.0 | 1.7 | 21.3 | 6.3 |
| IL12A | 7.1 | 2.1 | 1495.0 | 6.3 |
| TDH | 18.1 | 1.3 | 46.1 | 6.2 |
| GNG2 | 2.1 | 1.2 | 16.2 | 6.2 |
| GFPT2 | 5.6 | 1.8 | 49.6 | 6.0 |
| OXTR | 2.6 | 1.6 | 15.2 | 5.9 |
| GSTA4 | 1.0 | 1.7 | 24.3 | 5.9 |
| AREG | 1.3 | 1.1 | 50.6 | 5.9 |
| FOXF1 | 4.8 | 1.4 | 29.9 | 5.8 |
| RGS16 | 5.4 | 1.1 | 78.5 | 5.7 |
| DUSP2 | 21.1 | 1.3 | 36.0 | 5.6 |
| MGC16121 | 1.9 | 1.4 | 11.5 | 5.4 |
| TSLP | 9.5 | 1.1 | 62.9 | 5.4 |
| CLC | 26.5 | 1.7 | 64.5 | 5.4 |
| GPR54 | 1.2 | 1.1 | 10.9 | 5.1 |
| LOC256021 | 0.4 | 3.7 | 359.2 | 5.0 |
Fold change over unstimulated control samples
Ratio between fold change induced by synergistic TLR agonist combination (LPS + R848) and fold change induced by the best single TLR agonist (either LPS or R848).
TLR synergy sustains signaling
IκB-ζ is a transcription factor that is transiently induced immediately after TLR triggering and is required for driving the expression of several genes that are induced at late time points 41. Since most of the genes affected by TLR synergy have been shown to be regulated by IκB-ζ41, we asked whether this effect might correlate with increased IκB-ζ expression. IκB-ζ mRNA was induced within one hour and to a similar extent by all stimuli. However when DCs were stimulated by synergistic TLR combinations, the peak of IκB-ζ mRNA expression was wider, consistent with a higher availability of this essential transcription factor (Fig. 8a).
Figure 8. TLR synergy sustains IκB-ζ expression and c-Jun phosphorylation.
(a) MoDCs were stimulated with 10 ng/ml LPS (filled diamonds), 2.5 µg/ml R848 (filled squares), 20 µg/ml poly(I:C) (filled triangles), LPS+R848 (empty squares) or poly(I:C)+R848 (empty triangles). IκB-ζ mRNA was determined by quantitative real time RT-PCR at the indicated time points. One representative experiment out of three. (b) Cell lysates of MoDCs stimulated with the indicated agonists were prepared at different time points, separated by SDS electrophoresis and blotted with antibodies to phosphorylated c-Jun (p-Jun), IκB-α, phosphorylated p38 (p-p38) and total p38. L, LPS; R, R848; I, poly(I:C). One representative experiment out of three.
Since synergy was more evident at late time points we investigated whether synergistic TLR stimulation might sustain signaling in maturing DCs (Fig. 8b). The kinetics of p38 phosphorylation and IκB-α degradation and resynthesis were comparable in DCs activated by single TLR agonists or synergistic combinations. However, in the presence of a synergistic combination of R848 plus LPS or R848 plus poly(I:C), phosphorylation of c-Jun was enhanced and sustained up to 12 hours, which is consistent with the late impact of TLR synergism in transcription.
Discussion
In this study we show that in DCs TRIF-coupled TLRs (TLR3 and TLR4) potently synergize with endosomal TLRs (TLR8 in humans and TLR7 and TLR9 in mice) in the induction of IL-12p70 and IL-23. Synergistic TLR stimulation preferentially affected the rate limiting components, IL-12p35 and IL-23p19, whose mRNA increased up to 50 fold over that induced by single agonists, while IL-12p40 mRNA was increased approximately 5 fold. Addition of CD40L (and to a lower extent IFN-γ) further boosted IL-12p70 production promoted by synergistic TLR stimulation up to amounts as high as 2 µg/106 cells. These results indicate that DCs have very high capacity to produce IL-12 that is deployed only in response to multiple synergizing stimuli. In addition synergistic stimulation upregulated approximately 100 fold the TH1 promoting ligand Delta-4, while downregulating approximately 10 fold the TH2 ligand Jagged-1. Consistent with these findings DCs stimulated by synergizing TLR agonists showed increased and more sustained capacity to prime TH1 responses.
In contrast to the superinduction of TH1 polarizing signals, upregulation of MHC and costimulatory molecules was not increased by TLR synergy over the expression induced by optimal concentrations of single agonists, indicating that the two programs have different induction thresholds. This finding makes biological sense since antigen presentation and costimulation are required for T cell activation and expansion, while IL-12, IL-23 and Delta-4 promote the generation of potentially harmful effector cells and therefore need to be kept under strict control. Thus, the synergistic TLR stimulation may represent a combinatorial “security code” (two microbial products in different cellular compartments) that ensures that powerful effectors will be generated only in response to invading pathogens.
Synergistic TLR stimulation is probably the rule in real life since pathogens may contain several TLR agonists that trigger TLRs in different cellular compartments. Our results show that the first contact with a TLR agonist opens a temporal window for stimulation by another TLR that intensifies, complements and sustains the DC activation process. The integration of multiple stimuli over a defined temporal window might allow a more effective response to invading pathogens as compared to soluble microbial products. For instance dsRNA released by virus infected cells may trigger TLR3 and prime DCs for a subsequent triggering of TLR8 by virus particles in the endosomal compartment. This mechanism may ensure that the polarizing signals will be delivered by those DCs that carry the pathogen in the endosomal compartment and therefore can present the relevant antigens.
A detailed kinetics of several cytokine transcripts by real time PCR combined with a global transcriptional analysis at 2 and 8 hours following DC stimulation confirmed that only a limited number of genes is controlled by TLR synergy. Interestingly most of these genes play an important role in the immune response. These include IL-29, granulocyte-macrophage colony-stimulating factor (GM-CSF or CSF2), granulocyte colony-stimulating factor (G-CSF or CSF3) and leukemia inhibitory factor (LIF). In addition dual TLR engagement, albeit modestly increasing IL-1β mRNA, potently triggered IL-1β release, a fact that extends the effect of synergy beyond transcription.
The fact that synergy involves a TRIF-dependent and an endosomal TLR would be consistent with the possibility that IFN-β, which is rapidly produced in response to triggering of TRIF-dependent TLRs8, can selectively enhance the response to triggering of endosomal TLRs. This mechanism is unlikely since exogenous IFN-β had only a modest and variable effect on IL-12 production that was observed irrespective of the nature of the stimulus. Another possibility is that the synergy is mediated by a redistribution of the endosomal TLRs induced by triggered TLR3 and TLR4. However this possibility is also unlikely since we found that the effect of R848 was dependent on endosomal acidification both when R848 was used as single agonist or when added after LPS or poly(I:C). The mechanism by which selected TLR pairs synergize in the induction of a small number of genes remains to be established. Besides the different TRIF usage and cellular localization, the signaling capabilities of synergizing TLRs are thought to overlap entirely. In particular no distinct signaling pathways have been associated with endosomal TLRs. Nonetheless it is possible that the potent synergy observed in the induction of a selected set of genes might depend on the induction of complementary signaling pathways still to be discovered.
A distinct possibility is that synergy results from sustained signaling provided by dual TLR engagement. This hypothesis is supported by two observations. The first is that synergistic TLR stimulation affects gene expression mainly at late time points. Indeed the transcripts that are controlled by TLR synergy are either late genes or early genes whose expression is sustained in time. The second related observation is that in DCs stimulated by synergizing TLR agonists c-jun phosphorylation is sustained at late time points that coincide with the peak of mRNA expression of target genes, consistent with the pivotal role of the JNK pathway and of the transcription factor AP-1 in controlling cytokine expression upon TLR stimulation42–44. It remains to be established whether the sustained phosphorylation is due to an increased activity of JNK, a block of phosphatase activity or a more sustained signaling from engaged TLRs. It is tempting to speculate that the signals emanating from the synergizing TLRs may be integrated kinetically resulting in a more sustained signaling at late time points that is required to activate transcription of late genes such as IL-12 and IL-23 or to sustain the expression of early genes.
Our findings might be relevant for the design of adjuvants capable of priming strong TH1 and inflammatory responses. While single agonists may provide suboptimal stimulation, synergistic pairs could fully deploy the TH1 polarizing capacity of DCs. This may be particularly relevant in newborns that have low capacity to produce CD40L. Synergistic TLR stimulation could also be relevant for immunotherapy with ex vivo treated DCs and for the induction of high systemic amounts of IL-12 for cancer therapy.
Methods
Isolation and stimulation of DCs
Blood samples for transfusion were from the Swiss Blood Center. Permission to carry experiments with human primary cells was obtained from the Federal Office of Public Health (BBW no A000197/2 to F.S.). Circulating myeloid DCs were isolated using a combination of magnetic and FACS sorting as described18. MoDCs were generated by culturing human monocytes isolated from buffy coats using CD14 microbeads (Miltenyi) and culture in medium supplemented with GM-CSF and IL-4 as described45. All DC preparations analyzed were >98% pure. Bone marrow derived DCs were generated from BALB/C bone marrow cultured in GM-CSF (R&D Systems) as described46. DCs were challenged with one or more of the following TLR agonists: 1-0.01 µg/ml LPS (E. coli 055:B5 LPS, Sigma, or E. coli 0111:B4 LPS Ultra-Pure, Invivogen), 2.5 µg/ml of R848 (GLSynthesis), 20 µg/ml poly(I:C) (Amersham), 2.5µg/ml CpG oligodeoxynucleotide 1826 (5’-CCATGACGTTCCTGACGTT-3’).
Sorting and priming of naive T cells
Naive CD4+ CD45RA+ T cells were isolated from cord blood using a combination of magnetic and FACS sorting. MoDCs were stimulated for 12 or 48 h with TLR agonists, washed and plated with allogeneic naïve T cells at a 1:10 ratio in flat bottom 96 well plates. After 5 days, proliferating cells were expanded with IL-2 and analyzed on day 8.
Surface markers and cytokine production
Cell surface staining was performed using directly conjugated mAb (Immunotech). For detection of intracellular cytokines T cells were stimulated with PMA plus ionomycin for 5 h. Brefeldin A (10 µg/ml, Sigma) was added during the last 3 h. Cells were fixed with 2% paraformaldehyde, permeabilized with PBS containing 2% FCS and 0.5% saponin and stained with fluorescein isothiocyanate (FITC)-labeled IFN-γ-specific and phycoerythrin (PE)-labeled IL-4– specific monoclonal antibodies (PharMingen). Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson). For intracellular staining of IL-12p40 and IL-12p70 in MoDCs, cells were stimulated first with LPS and after 5 h with R848. Bafilomycin A1 (0.5 µM, Biomol) was added 2 h before R848 stimulation, Brefeldin A was added 1 h after R848 and cells were incubated for other 10 h. Then cells were fixed, permeabilized and stained with allophyocyanin (APC)-labeled IL-12p40/p70 specific monoclonal antibodies (PharMingen) and (PE)-labeled anti CD83 specific monoclonal antibodies (Immunotech). Cytokines were measured in culture supernatants after 48 h of activation with the various stimuli using commercially available ELISA kits for TNF, IL-6, IL-1β, IL-10 and for human and mouse IL-12p70 (R&D Systems).
Immunoblot analysis
Equal amounts of protein lysates were loaded on SDS-PAGE and gels were transferred on PVDF membrane. Filters were blocked with 5% dry, skim milk and blotted with the specific primary antibody: rabbit antisera to polyclonal antibody to phosphorylated c-Jun (Ser-73) (New England Biolabs) monoclonal phosphorylated p38 (Sigma), polyclonal total p38 (Santa Cruz Biotechnologies) and monoclonal anti-IkBα (Imgenex). Blots were then incubated with the appropriate horse radish peroxidase (HRP)-conjugated secondary antiserum (Jackson Immunoresearch), and revealed with the SuperSignal WestPico chemiluminescence system (Pierce).
Real-time quantitative RT-PCR
Total RNA was extracted with Rneasy Mini prep Kit (Qiagen). The cDNA were synthesized using random hexamer nucleotides and a MMLV reverse transcriptase kit (Stratagene). Transcripts were quantified with a real-time quantitative PCR on an ABI PRISM 7700 Sequence Detector (Perkin-Elmer Applied Biosystems) using pre-developed Applied Biosystems probes and reagents according to manufacturer's instructions. For each sample, mRNA abundance was normalized to the amount of 18S rRNA and expressed as arbitrary units.
Microarrays and data analysis
MoDCs from 3 different donors were left untreated or treated with LPS, R848 and the combination of LPS plus R848. RNA was extracted with the Trizol method (Trizol, Invitrogen Life Technologies) and an additional RNA clean-up step using the RNAeasy purification kit according to the manufacturer's protocol (Qiagen, Hilden). Total RNA (5 µg) was labeled and hybridized using the GeneChip Expression 3’ Amplification One-Cycle Target Labeling kit on Affymetrix U133 2.0 Plus microarrays (Affymetrix Inc.). Washes and scanning were performed according to Affymetrix protocols using Fluidics Station 400 and GeneChip Scanner 3000. Raw signals and the Present/Absent calls were obtained using Affymetrix GCOS 1.2 software. Further data analysis was performed with GeneSpring 7.2 (Silicon Genetics). Signal values below 0.01 were set to 0.01. The percentile of all the measurements in each sample was calculated using all genes not marked absent and each gene measurement was divided by the 50th percentile. Each gene was divided by the mean of its normalized measurements in the three untreated samples. Genes which did not present a signal intensity above 50 and that were not marked present in at least two of three replicates of at least one condition were discarded: 14,550 genes passed this filter. Fold changes between treated and untreated cells at 2 and at 8 hour were calculated on genes showing a statistically differential expression in at least one of the conditions using a parametric test with variance assumed equal (ANOVA) and a P-value cut-off of 0.05, followed by the Benjamini and Hochberg false discovery rate multiple test correction. Ratios between the fold-changes were then calculated for the selected 8,828 genes. For each gene the best single agonist was selected as the one able to induce the closer Fold change over control compared to the one induced by the synergistic combination.
Supplementary Material
Acknowledgements
We thank G. Natoli and A. Macagno for critical reading and A. Martín-Fontecha for help with the experiments with mouse DCs. Supported in part by the Swiss National Science Foundation (Grant n. 31-63885), the NIH (grant n. U19AI057266/01) and by the European Community (MUVAPRED n. LSHP-CT-2003-503240).
References
- 1.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 9.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 10.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 11.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 13.Bin LH, Xu LG, Shu HB. TIRP, a novel Toll/interleukin-1 receptor (TIR) domain-containing adapter protein involved in TIR signaling. J Biol Chem. 2003;278:24526–24532. doi: 10.1074/jbc.M303451200. [DOI] [PubMed] [Google Scholar]
- 14.Oshiumi H, et al. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-β. J Biol Chem. 2003;278:49751–49762. doi: 10.1074/jbc.M305820200. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad-Nejad P, et al. Bacterial CpG-DNA and lipopolysaccharides activate Tolllike receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 20.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 22.Schulz O, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 24.Edwards AD, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 25.Reise Sousa C. Activation of sdendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 27.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 28.Langrish CL, et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 29.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 30.Gao JJ, et al. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J Immunol. 1999;163:4095–4099. [PubMed] [Google Scholar]
- 31.Sato S, et al. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 32.Yi AK, Yoon JG, Hong SC, Redford TW, Krieg AM. Lipopolysaccharide and CpG DNA synergize for tumor necrosis factor-α production through activation of NF-κB. Int Immunol. 2001;13:1391–1404. doi: 10.1093/intimm/13.11.1391. [DOI] [PubMed] [Google Scholar]
- 33.Jurk M, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, et al. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14:783–791. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 38.Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 39.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto M, et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκB-ζ. Nature. 2004;430:218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 43.Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol. 2003;33:2832–2841. doi: 10.1002/eji.200324073. [DOI] [PubMed] [Google Scholar]
- 44.Nakahara T, et al. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int Immunol. 2004;16:1701–1709. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.