Abstract
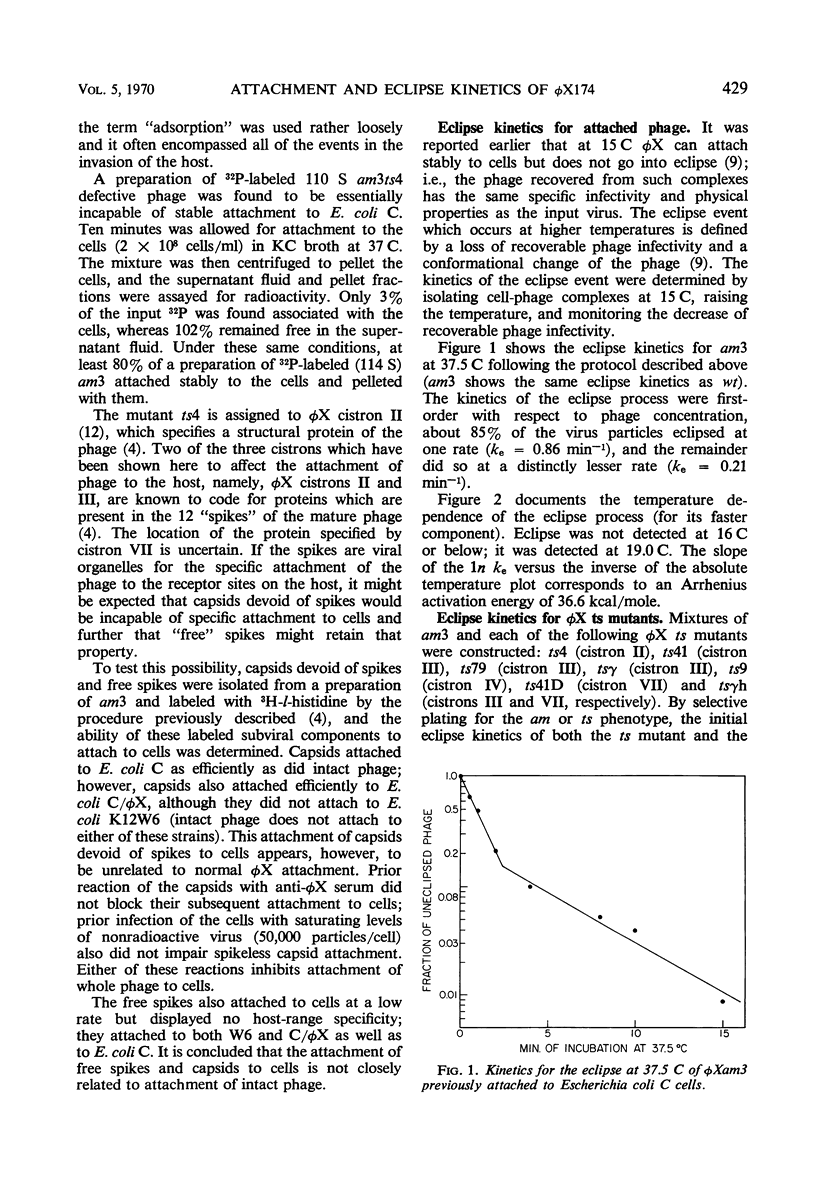
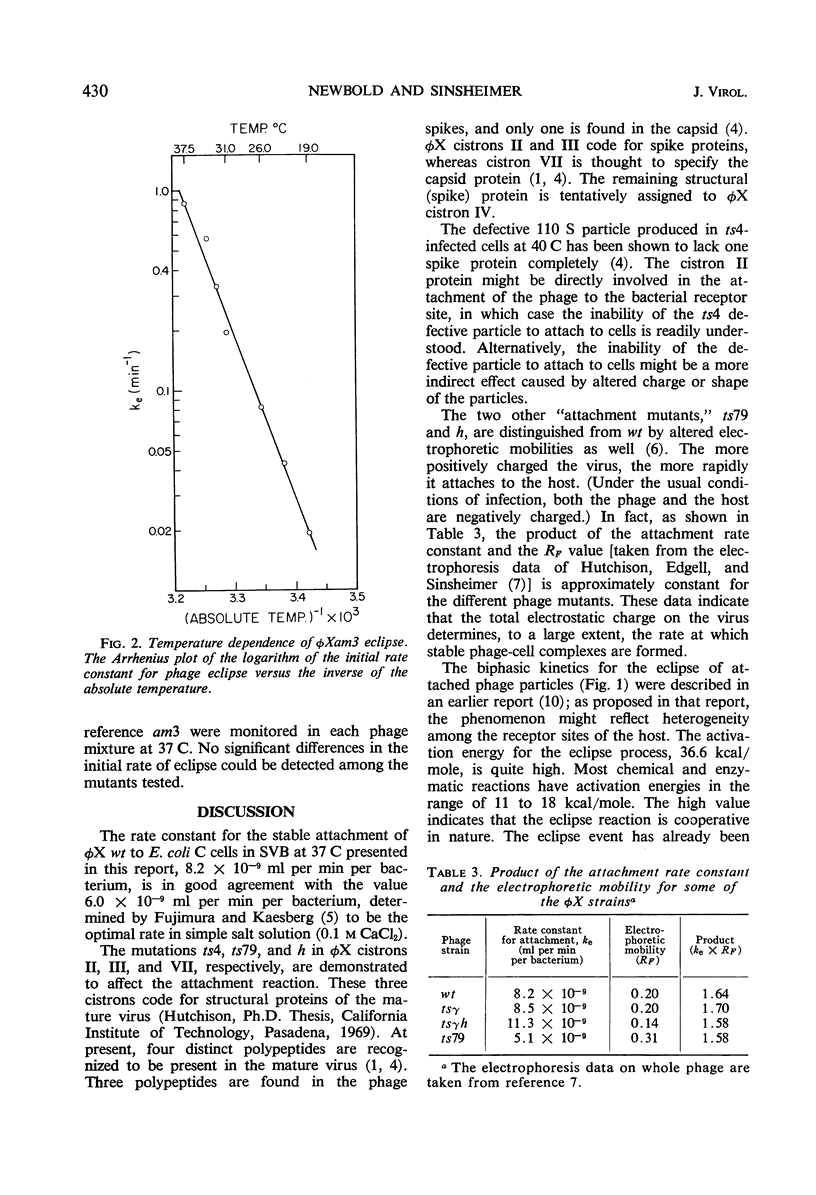
The products of φX cistrons II, III, and VII are demonstrated to affect the attachment of the phage to its host Escherichia coli C; therefore, by inference, these cistrons influence, directly or indirectly, the structure of proteins in the virus particle. Two of the mutations which alter attachment kinetics, ts79 in cistron III and h in cistron VII, also affect the electrophoretic mobility of the virus and emphasize the role of charge in the attachment interaction with the host. The kinetics for attached phage to go into “eclipse” are first-order and biphasic; about 85% of the phage eclipse at one rate (ke = 0.86 min−1) and the remainder do so at a distinctly lower rate (ke = 0.21 min−1). No φX cistrons yet identified affect the eclipse process. The lowest temperature at which eclipse is detected is 19 C. The Arrhenius activation energy for phage eclipse has the high value of 36.6 kcal/mole, indicating the cooperative nature of the event.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess A. B., Denhardt D. T. Studies on phiX174 proteins. I. Phage-specific proteins synthesized after infection of Escherichia coli. J Mol Biol. 1969 Sep 28;44(3):377–386. doi: 10.1016/0022-2836(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 3. Phage maturation and lysis after synchronized infection. J Mol Biol. 1965 Jul;12(3):641–646. doi: 10.1016/s0022-2836(65)80318-7. [DOI] [PubMed] [Google Scholar]
- Dowell C. E. Cold-sensitive mutants of bacteriophage phi-X-174. I. A mutant blocked in the eclipse function at low temperature. Proc Natl Acad Sci U S A. 1967 Sep;58(3):958–961. doi: 10.1073/pnas.58.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- FUJIMURA R., KAESBERG P. The adsorption of bacteriophage phi-X174 to its host. Biophys J. 1962 Nov;2:433–449. doi: 10.1016/s0006-3495(62)86866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Edgell M. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XII. Phenotypic mixing between electrophoretic mutants of phi-X174. J Mol Biol. 1967 Feb 14;23(3):553–575. doi: 10.1016/s0022-2836(67)80125-6. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- RUECKERT R. R., ZILLIG W. Biosynthesis of virus protein in Escherichia coli C in vivo following infection with bacteriophage phi-X-174. J Mol Biol. 1962 Jul;5:1–9. doi: 10.1016/s0022-2836(62)80056-4. [DOI] [PubMed] [Google Scholar]



