Abstract
Whereas human pro-social behavior is often driven by empathic concern for another, it is unclear whether non-primate mammals experience a similar motivational state. To test for empathically motivated pro-social behavior in rodents, a free rat was placed in an arena with a cagemate trapped in a restrainer. Within days, the free rat acted deliberately and quickly to open the restrainer and free the cagemate. Rats did not open an empty or object-containing restrainer. Rats freed cagemates even when rewarding social contact was prevented. When liberating a cagemate was pitted against chocolate contained within a second restrainer, rats opened both restrainers and in most trials, shared the chocolate. Thus, rats behave pro-socially in response to a conspecific’s distress, providing strong evidence for biological roots of empathy.
Pro-social behavior refers to actions that are intended to benefit another. One common motivator of pro-social behavior in humans is empathic concern, an other-oriented emotional response elicited by and congruent with the perceived welfare of an individual in need. The separation of one’s own affective state from another’s is crucial for the pro-social manifestation of empathic concern (1, 2). For example, an individual who simply shares another’s state of distress via emotional contagion may become stuck within a state of immobility that prevents him or her from moving to action. In contrast, appreciation of another’s distress without experiencing overwhelming emotional distress is conducive to pro-social behavior (3). To determine whether rodents are capable of helping behavior motivated by empathic concern, we tested whether the presence of a trapped cagemate induces a pro-social motivational state in rats, leading them to open the restrainer door and liberate a trapped cagemate.
During each test session, the free rat was allowed to roam freely in an arena with a centrally located restrainer. Inside the restrainer was a cagemate (trapped cagemate condition, fig. S1A), a plush toy rat (object condition), or nothing (empty condition). To test whether the actions of the free rat were due to the trapped condition of the cagemate rather than the mere presence or inaccessibility of the cagemate, an additional group of rats (2+empty condition) was tested. Two free cagemates were placed in the arena and separated by a perforated screen; the restrainer was on one side of the screen with the “free” rat (fig. S1B).
To open the restrainer door, rats had to apply enough force to tip the door over to the side (fig. S1C). Door-opening by the free rat allowed the trapped cagemate to leave the restrainer. In preliminary experiments, some rats developed learned helplessness if they failed to open the door within the first few test days. To avoid this, the experimenter opened the door halfway in trials when the rat failed to open the door by a set time point. After this experimental manipulation, either rat fully opened the door and the trapped rat escaped from the restrainer; rats then remained in the arena for half the initial period. Door-opening was only counted as such if the free rat opened the door prior to the half-way opening. Sessions were repeated for 12 days, once daily (4).
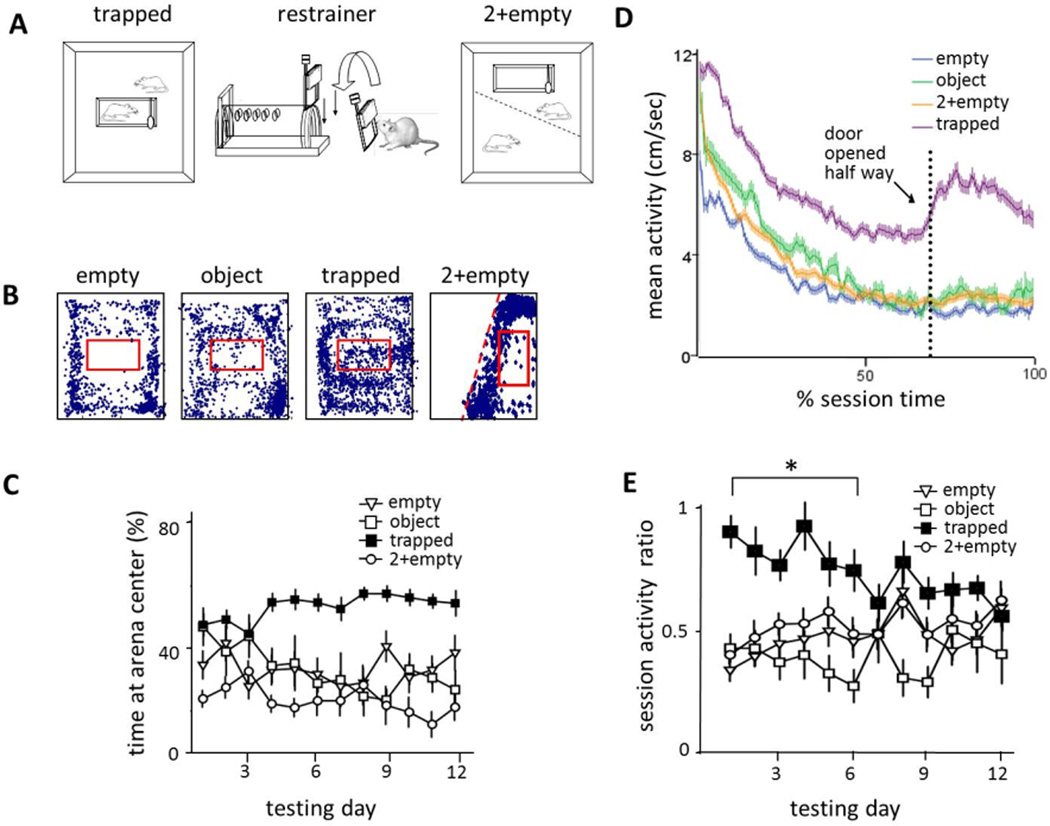
Rats in the trapped cagemate condition showed restrainer-centered activity (Fig. 1A; Movie S1) and learned to open the door and liberate the trapped cagemate within a few days. Rats circled the restrainer, digging at and biting it, and initiated contact with the trapped rat through holes in the restrainer. They spent more time near the restrainer in the arena center (Fig. 1B), and showed greater movement speed (hereafter termed activity, Fig. 1C) than rats in the control conditions. Prior to learning to open the restrainer door and liberate the trapped rat (mean: day 6.9 ± 2.9), free rats in the trapped cagemate condition stayed significantly more active in the second half relative to the first half of sessions than did rats in the control conditions (Fig. 1D). These results suggest that rats are motivated to move and act specifically when in the presence of a trapped cagemate.
Fig. 1.
Rats showed activity focused on the restrainer (red box in A) only if it contained a trapped cagemate. Rats were placed in an arena with a trapped cagemate (n=30, 6 females), an empty restrainer (n = 20, 6 females) a restrainer containing an object (n=8 males) or with an empty restrainer and a free cagemate located across a perforated screen (n=12 males). (A) The locations, plotted at 0.5 Hz, of a representative free rat from each of the 4 groups on the first day of testing are illustrated. (B) The mean (±SEM) amount of time that a rat was >5 cm away from the arena wall is shown for each of the 12 days of testing. Rats in the trapped cagemate condition spent more time in the arena center than did rats in the control conditions (P<0.001, mixed model analysis, post-hoc PLSD). (C) The mean (±SEM) activity across the testing session (averaged across all days) for each group of rats is shown. Rats in the trapped cagemate condition moved significantly faster than rats in all other conditions (P<0.001, mixed model analysis, post-hoc PLSD). (D) The mean ratio (±SEM) of the average activity during the second half of sessions relative to the average activity during the first half is shown for each day of testing. On days 1–6, rats in the trapped cagemate condition stayed significantly more active in the second half relative to the first half of sessions than did rats in the control conditions (P<0.001 mixed model analysis, PLSD).
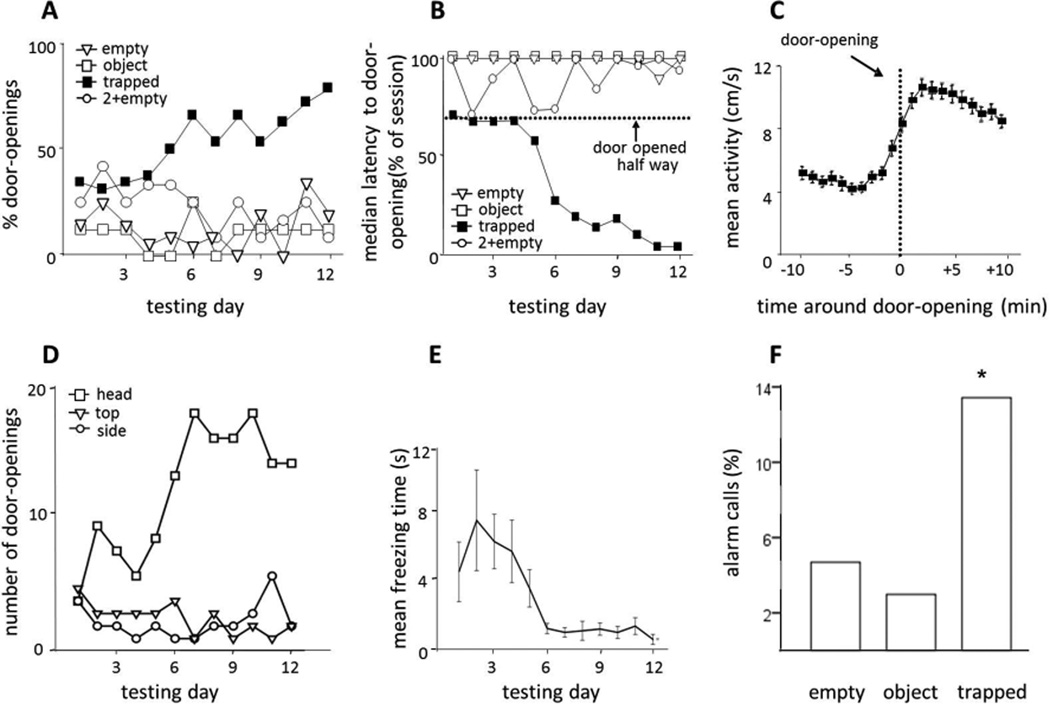
The proportion of rats in the trapped cagemate condition that opened the door increased across the 12 days of the experiment (Fig. 2A). No such increase was observed for rats in any of the control conditions. The time to door-opening decreased for rats in the trapped condition but not for those in control conditions (Fig. 2B; Movie S2). In the final days of testing, most rats (23/30) in the trapped condition opened the door within several minutes of the start of the session, and were classified as “openers” (see Supplementary Methods for definition), whereas the rats in control conditions either never opened the door or did so only after it was opened halfway. Only 5 of 40 in the control conditions (1/8, object; 2/20, empty; 2/12, 2+empty) became openers (P<0.001, chi-square). Rats who failed to consistently open the door were termed “non-openers”. When activity was aligned to the opening of the door, there was a sharp increase in activity just before and extending for more than 10 minutes afterward (Fig. 2C). These data suggest that the liberation of a trapped cagemate is a salient event for the free rat.
Fig. 2.
Rats placed with a trapped cagemate learned to open the restrainer door in a goal-directed fashion. (A) The proportion of rats in the trapped cagemate condition that opened the door increased across the days of testing. (B) The median time to door-opening is shown across all days of testing. Only rats in the trapped cagemate condition opened the door at decreasing latencies. The dashed line indicates the time point when the door was opened half way by the experimenter. (C) Rats in the trapped cagemate condition showed a sharp increase in activity at the moment that the restrainer door was opened (time 0). Activity from all trials, regardless of whether the door was opened before or after the experimenter opened the door halfway, was averaged. (D) The count of opening type (with head, from side, from top) is shown across days of testing. As free rats learned to open the door, they developed a consistent style, suggesting goal-directed behavior. (E) Initially, rats showed freezing behavior immediately after the door fell over, but as they learned to open the door, they were no longer startled by the door-opening and no longer froze. (F) More alarm calls were recorded in the trapped cagemate condition (n=67 sample files) than in the empty (n=64) and object (n= 67) conditions (p<0.05 ANOVA, PLSD<0.05).
On the first days of testing, rats in the trapped condition opened the door in any of three ways: from the side, from the top, or straight on by pushing it up with their head. On later days, (days 6–12) they consistently opened the door with their heads (Fig. 2D). Furthermore, rats initially froze after the door fell over, suggesting that door-opening (which made a sound) was an unexpected event. But in later days of testing (days 6–12), they did not freeze (Fig. 2E), demonstrating that door-opening was the expected result of a deliberate action. Hence, door-opening by the free rat in the trapped condition showed the characteristics of goal-directed behavior.
Ultrasonic (22–23 kHz) vocalizations collected from multiple testing arenas were analyzed to determine whether rats emitted alarm calls during testing. Rats in the trapped cagemate condition made significantly more alarm calls (15%) than rats in the empty and object conditions (3–5%, Fig. 2F) in randomly sampled files from all 12 days of testing. The greater frequency of alarm calls in the trapped cagemate condition than in control conditions suggests that the former paradigm was stressful. Further analysis showed that days 1–3 contained 20–27% alarm calls for male rats in the trapped cagemate condition. In 90% of files containing spontaneous alarm calls on day 1, the trapped rat was identified as the source of the calls, and in the remaining samples, we were not able to unambiguously identify the caller. Since alarm calls were relatively infrequent, it is unlikely that the free rat was acting to terminate alarm calls.
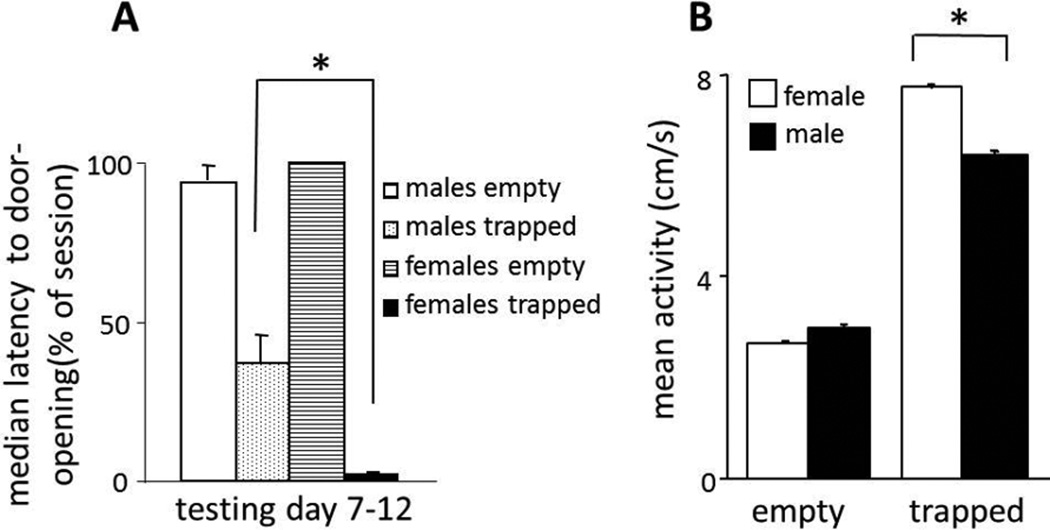
Females were tested in the trapped cagemate (n=6) and empty (n=6) conditions. Consistent with previous findings that human and non-human females are more empathic than males (5–7), all female rats in the trapped condition became door-openers whereas a third of the male rats were non-openers. Further, female rats in the trapped condition opened the restrainer door at a shorter latency than did males (Fig. 3A). Females in the empty condition never opened the restrainer door. Female rats were also more active than males in the trapped cagemate condition but there was no sex difference in the empty condition (Fig. 3B).
Fig. 3.
(A) Females in the trapped cagemate condition (n=6) opened the door at consistently shorter latencies than did males (n=24) on days 7–12 (P<0.01, Mixed model analysis). When median door-opening latencies on days 7–12 were averaged, there was no difference between males (n=14) and females (n=6) in the empty condition. (B) The mean (±SEM) activity was greater for females than males in the trapped cagemate condition (P<0.001, ANOVA) but there was no gender-related difference in the empty condition.
To examine whether individual differences in boldness influence door-opening behavior, we tested the latency for approach to the ledge of an opened cage (see Supplementary Methods). Interestingly, animals who eventually proved to be openers showed significantly lower latencies for approach than non-openers, suggesting that openers were bolder (fig. S2). This demonstrates that individual trait differences factor into the individual expression of pro-social behavior.
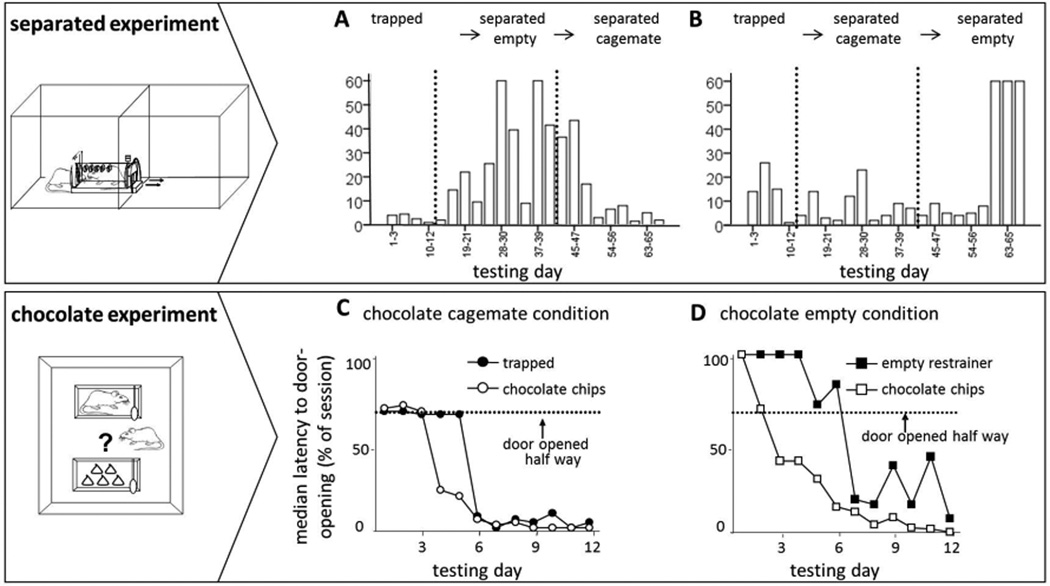
To determine whether the anticipation of social interaction is necessary to motivate door-opening, we tested an additional cohort of rats in a modified setup in which the trapped rat could only exit into a separate arena (separated condition, fig. S1D). Rats (12 pairs) were first exposed to the trapped cagemate condition (12 days); three rats did not open the door on any of the last 3 days in the trapped cagemate condition (days 10–12) and were not tested further. In a second stage, rats were placed in the separated setup with a restrainer that was either empty (separated empty) or contained a cagemate (separated cagemate) for 29 days of testing (Fig. 4A). In a third stage (27 days), conditions were reversed so that rats in the separated cagemate condition during stage 2 were then tested in the separated empty condition and vice versa. Thus, all 9 free rats were tested in counter-balanced order with both an empty and full restrainer. Rats placed in the separated cagemate condition either continued to or returned to opening the door at short latency as they had in the trapped cagemate condition (Fig. 4A). In contrast, when rats were placed in the separated empty condition, they eventually stopped opening the door of the empty restrainer. Thus, individual rats opened the door of a restrainer containing a cagemate but not that of an empty restrainer, indicating that expectation of immediate social contact is not necessary for pro-social behavior in rats.
Fig. 4.
(A) In the separated experiment, rats (n=9) opened the door for a trapped cagemate even when no contact was possible between the two animals after door-opening. The latencies to door-opening are plotted across all the days of the study. Rats extinguished door-opening when the restrainer was empty, but opened the door when the restrainer contained a cagemate. This pattern was observed regardless of whether rats were tested first with an empty restrainer (n=4; top) or with a trapped cagemate (n=5; bottom; P<0.001, mixed model analysis, PLSD). (B) In the chocolate experiment, rats (n=9) opened the door for a trapped cagemate as well as for a restrainer containing 5 chocolate chips. The median time to door-opening is shown across all days of testing. On days 6–12, rats opened both restrainers at similarly short latencies. (C) Rats in the chocolate empty condition (n=6) opened the empty restrainer at significantly longer latencies than the chocolate restrainer (P<0.01). The dashed lines in B–C indicate the time point when the door was opened half way by the experimenter.
In order to examine the relative value of liberating a trapped cagemate, we tested a cohort of rats in a cagemate vs. chocolate paradigm. The free rat was placed in an arena with two restrainers, one containing the trapped cagemate and the other containing five chocolate chips (chocolate cagemate condition, fig. S1E). In the control condition, one restrainer was empty while the other contained chocolate (chocolate empty condition). For rats in the chocolate cagemate condition, there was no difference in the door-opening latencies for the two restrainers during days 6–12 (Fig. 4B). In contrast, rats in the chocolate empty condition opened the chocolate-containing restrainer significantly more quickly than they opened the empty one (Fig. 4C). These results show that the value of freeing a trapped cagemate is on a par with accessing chocolate chips. It is interesting to note that the average day on which rats learned to open the chocolate restrainer was 5.8 ± 2.1, similar to that for rats in the trapped cagemate condition (6.9 ± 2.9). Thus, even when motivated by access to highly palatable food, door-opening was neither easy nor instinctual.
Free rats in the chocolate cagemate condition could potentially eat all 5 chocolate chips themselves. This was expected since prior to testing, these rats ate on average >7 chocolate chips in one meal. Remarkably, we found that free rats shared the chocolate chips with their freed cagemate in half of all trials (52%) and 61% of all trials on days 6–12. Rats in the chocolate empty condition ate on average 4.8 ± 0.7 chips out of the five available. In contrast, free rats in the trapped condition ate fewer chips, 3.5 ± 1.5 on average (P<0.01), with trapped rats eating the remainder (1.5 ± 1.4).
Our study shows that rodents behave pro-socially when they perceive a conspecific experiencing non-painful psychological restraint stress (8, 9), acting to end that distress through deliberate action. In contrast to previous work (10–13), the present study shows pro-social behavior which is accomplished by the deliberate action of a rat. Pro-social behavior occurred in the absence of training or social reward, and even when in competition with highly palatable food. While empathy appears to be the motivation for the pro-social behavior observed in the present study, it is clearly impossible to unambiguously and definitively prove the interior motivation of a non-verbal animal (14). For example, it is possible that rats acted in order to stop the alarm calls of the trapped rats (15). Since the overall occurrence of alarm calls in randomly selected samples was low (~15%), this explanation is unlikely. Alternatively, rats could have been attracted to the trapped cagemate by curiosity. This is also unlikely, as door-opening behavior in the separated cagemate condition persisted for over a month, a time period over which curiosity habituates (16). Finally, door-opening could be coincidental, brought about by the high activity levels of rats in the trapped cagemate condition. This is unlikely since once rats learned to open the door, they did so almost immediately after being placed in the arena, by using a consistent style, and were unsurprised by the door-opening. We also know that door-opening is not an easy task, since rats needed several days in order to master opening the restrainer even when chocolate chips were the motivating reward. Thus, the most parsimonious explanation for the observed helping behavior is that rats free their cagemate in order to end distress, either their own or that of the trapped rat, that is associated with the circumstances of the trapped cagemate.
Previous research has provided clear evidence that rodents experience emotional contagion (5, 10, 11, 17–19), a response resulting in a similar emotion being aroused in the observer as a direct result of perceiving the expressed emotion of another (20, 21). Emotional contagion and the accompanying affective arousal is necessary for empathically motivated helping behavior, but the motivation to help and protect another is separate from the ability to experience the same type of emotion that another is experiencing. The manifestation of pro-social behavior in humans requires down-regulation to prevent an individual from becoming overwhelmed by the negative emotion aroused by another’s distress (22). In the current study, the free rat did not simply mimic another rat’s distress, i.e., express emotional contagion, but also acted with intention to liberate a trapped rat, suggestive that rats can down-regulate emotional distress to act upon empathic concern and thus improve the well-being of a conspecific.
Although the existence of empathy in non-humans continues to gather support in the scientific community, the idea remains highly controversial (14, 23–27). In mammals, empathic concern and helping behavior have evolved in the context of parental care (21, 28–30); females must understand the emotions and needs of their offspring, and respond appropriately to ensure their survival (31). Nurturing has evolved beyond the mother-child bond, especially in social animals where the ability to understand and respond to the affective state of another conspecific is crucial for successful navigation in the social arena. In fact, the autonomic and endocrine reactions that accompany empathy in humans derive from neural and hormonal systems, involving subcortical pathways, that are well developed in other social mammals (28). Considerable overlap exists in the phylogenetically conserved neural circuits that support social behavior and physiological homeostasis, two adaptive traits that are equally critical to survival. The simple model established here opens new avenues to better understand the biological underpinnings of empathy and pro-social behavior as well as the failure or dysfunction of empathy evident in individuals with anti-social personality disorders.
Supplementary Material
Acknowledgments
The assistance of Isabelle Boni, Aesis Brimmer, Fanny Delebecque, Dr. Kevin Hellman, Anthony Logli, Jorge Peralta, Dr. Brian Prendergast, Katie Ragsdale, David Rodgers, Michelle Sales, Bonnie Sheu, Jenny Wang, Aeja Weiss, Dr. David White, and Ken Yuan is gratefully acknowledged.
Footnotes
Supporting Online Material
Materials and Methods
Table 1
Figs. S1, S2, S3
Reference
Reference List
- 1.Batson DC. In: The Social Neuroscience of Empathy. Decety J, Ickes WJ, editors. Cambridge, MA: The MIT Press; 2009. pp. 3–15. [Google Scholar]
- 2.Decety J, Jackson PL. Behav. Cogn Neurosci. Rev. 2004;3:71. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg N, Miller PA, Fultz J, Shell F, et al. Journal of Personality and Social Psychology. 1989;57:55. doi: 10.1037//0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Materials and methods are available as supporting material on Science Online.
- 5.Langford DJ, et al. Soc. Neurosci. 2010;5:163. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- 6.Romero T, Castellanos MA, de Waal FB. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12110. doi: 10.1073/pnas.1006991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr C, Rowe AC, Blanke O. Br. J Psychol. 2010;101:277. doi: 10.1348/000712609X457450. [DOI] [PubMed] [Google Scholar]
- 8.Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Neurosci. Biobehav. Rev. 1994;18:223. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 9.Pare WP, Glavin GB. Neurosci. Biobehav. Rev. 1986;10:339. doi: 10.1016/0149-7634(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 10.Rice GE, Gainer P. J Comp Physiol Psychol. 1962;55:123. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 11.Church RM. J Comp Physiol Psychol. 1959;52:132. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 12.Lucke JF, Batson CD. Journal of Experimental Social Psychology. 1980;16:214. [Google Scholar]
- 13.Rice GEJ. Psychological Record. 1964;14:165. [Google Scholar]
- 14.Silk JB. In: The Oxford Handbook of Evolutionary Psychology. Dunbar RIM, Barrett L, editors. Oxford: Oxford University Press; 2007. pp. 115–126. [Google Scholar]
- 15.Lavery JJ, Foley PJ. Science. 1963;140:172. doi: 10.1126/science.140.3563.172. [DOI] [PubMed] [Google Scholar]
- 16.Reger ML, Hovda DA, Giza CC. Dev. Psychobiol. 2009;51:672. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon D, et al. Nat. Neurosci. 2010;13:482. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalaquett C, Thiessen D. Physiol Behav. 1991;50:221. doi: 10.1016/0031-9384(91)90524-r. [DOI] [PubMed] [Google Scholar]
- 19.Langford DJ, et al. Science. 2006;312:1967. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 20.Hatfield E, Rapson RL, Le YC. In: The Social Neuroscience of Empathy. Decety J, Ickes W, editors. Cambridge, MA: MIT Press; 2009. pp. 19–30. [Google Scholar]
- 21.Preston SD, de Waal FBM. Behav. Brain Sci. 2002;25:1. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 22.Decety J, Svetlova M. Developmental Cognitive Neuroscience. 2011 doi: 10.1016/j.dcn.2011.05.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waal FB. Annu. Rev. Psychol. 2008;59:279. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 24.Decety J. Annals of the New York Academy of Sciences. 2011 Epub ahead of print. [Google Scholar]
- 25.Warneken F, Tomasello M. Br. J Psychol. 2009;100:455. doi: 10.1348/000712608X379061. [DOI] [PubMed] [Google Scholar]
- 26.Preston S. In: Encyclopedia of Anima Behavior. Bekoff Mark., editor. vol. 2. Westport, Connecticut, London: Greenwood Press; 2004. pp. D–P. [Google Scholar]
- 27.Wills GD, Wesley AL, Moore FR, Sisemore DA. Neurosci. Biobehav. Rev. 1983;7:315. doi: 10.1016/0149-7634(83)90035-0. [DOI] [PubMed] [Google Scholar]
- 28.Carter CS, Harris J, Porges SW. In: The Social Neuroscience of Empathy. Decety J, Ickes WJ, editors. Cambridge MA: The MIT Press; 2009. pp. 169–182. [Google Scholar]
- 29.Decety J. Annals of the New York Academy of Sciences. 2011 In press. [Google Scholar]
- 30.Batson CD. Altruism in humans. New-York: Oxford University Press; 2011. [Google Scholar]
- 31.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotion. New York: Oxford University Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.