Abstract
Our goal was to develop a 3-D multi-cellular construct using primary human corneal fibroblasts cultured on a disorganized collagen substrate in a scaffold-free environment and to use it to determine the regulation of proteoglycans over an extended period of time (11 weeks). Electron micrographs revealed multi-layered constructs with cells present in between alternating parallel and perpendicular arrays of fibrils. Type I collagen increased 2–4-fold. Stromal proteoglycans including lumican, syndecan4, decorin, biglycan, mimecan, and perlecan were expressed. The presence of glycosaminoglycan chains was demonstrated for a subset of the core proteins (lumican, biglycan, and decorin) using lyase digestion. Cuprolinic blue–stained cultures showed that sulfated proteoglycans were present throughout the construct and most prominent in its mid-region. The size of the Cuprolinic-positive filaments resembled those previously reported in a human corneal stroma. Under the current culture conditions, the cells mimic a development or nonfibrotic repair phenotype.
Keywords: 3-D culture, proteoglycans, glycosaminoglycans, human fibroblasts, microscopy
INTRODUCTION
The development of 3-D multi-cellular constructs is a necessary step forward in understanding how cells organize and develop matrix. Synthetic porous constructs have been synthesized and the migration of stromal cells into the material showed cells of prototypical fibroblast morphology. Further examination revealed the deposition of stromal matrix molecules (see review by Trinkaus-Randall, 2000). While these scaffolds did provide a template for reconstitution of the cornea, the resulting lack of organization of the cells and matrix reflected the disorderly arrangement of the synthetic fibers (Trinkaus-Randall et al., 1991). The importance of developing cultures where cells retained the original cellular morphology was shown to be critical by Dr. Elizabeth Hay, whose work is honored in this volume. Dr. Hay and her collaborators demonstrated that corneal fibroblasts cultured in hydrated collagen gels possessed an elongated morphology with filopodia, which was lost when the same cells were cultured on glass (Tomasek and Hay, 1984). These studies confirm earlier work indicating that the matrix proteins are the modulators of cell shape (Tomasek et al., 1982). The studies by Hay were and are a driving force in understanding the dynamic role of the matrix on cell behavior.
To model wound repair, investigators have used scaffolding; however, these do not promote the elegant organization of the stroma that permits transmission of incident light in the cornea (see review by Ruberti et al., 2007). Recently, we developed a scaffold-free system to examine the development of long-term 3-D multi-cellular matrices. In this culture system, primary human corneal fibroblasts become multi-layered and a complex matrix is formed with collagen fibrils laid down with remarkable alignment (Guo et al., 2007).
The extracellular matrix (ECM) of the corneal stroma is composed of collagen fibrils stacked in orderly lamellae surrounded by PGs. There are two major classes of PGs; one possessing keratan sulfate (KS) side chains and the other chondroitin sulfate/dermatan sulfate (CS/DS) side chains. In vivo, the structural and biochemical properties are altered with injury and the collagen fibrils show irregular interspacing due to changes in specific PGs. These include large CS/DS glycosaminoglycan (GAG) side chains with greater sulfation and increased iduronic acid (Hassell et al., 1983). Reports also show that with injury there is a predicted increase in the ratio of CS/DS to KS GAG chains and that this is accompanied by the appearance of heparan sulfate (HS) GAG chains (Cintron et al., 1990a; Brown et al., 1995). When a monolayer of cells was cultured in vitro and stimulated by serum or TGF-β, there were short-term changes in the synthesis of PGs that were similar to those detected in wounded corneas (Brown et al., 1999). However, it was not understood if the way that the cultures were treated in monolayer altered the ultimate regulation and synthesis as these experiments were limited by the monolayer and length of culture.
In this report, we developed a 3-D multi-cellular construct using primary human corneal fibroblasts cultured on a disorganized collagen substrate in a scaffold-free environment. The cells were cultured for a maximum of 11 weeks and synthesized a multi-layered complex matrix that reached a thickness of 65 μm. We demonstrated that proteoglycans were both deposited within the matrix and secreted into the extracellular milieu. Type I collagen increased within the matrix, and sulfated proteoglycans were present within the matrix and along collagen fibrils. After 4 weeks, the cells were detected principally above the disorganized collagen substrate, but after 8 weeks, cells were detected within the substrate. Cell shape within the matrix changed both over time and throughout the depth of the matrix. The results indicate that cells can form a stable 3-D culture and express a repertoire of corneal proteoglycans. Under the current culture conditions, the cells mimic a development or nonfibrotic repair phenotype. The characterization of this system provides us an excellent opportunity to model a number of conditions using primary human cells.
RESULTS
Cellular Organization of the Construct
Previously, we demonstrated that when cells were incubated in the presence of stabilized ascorbic acid, on bare transwell polycarbonate membranes for 4 weeks, the cultures became multi-layered and the long axis of the fibroblasts showed orientation changes in the strata. In our current study, disorganized reconstituted type I collagen was deposited on polycarbonate membranes that were covalently modified to promote collagen adhesion. Five independent cultures (constructs [C 1–5]) representing 5 donor eyes were examined after 4, 8, and 11 weeks. All experiments were performed in triplicate at all time points.
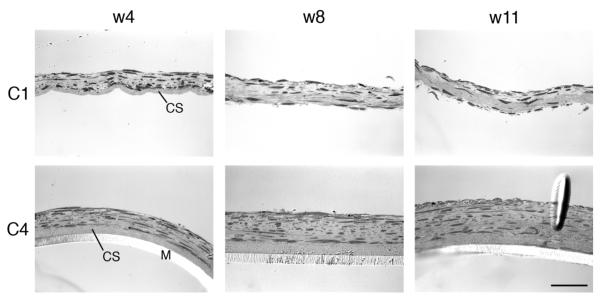
Two independent representative cultures are shown over time (Fig. 1). Constructs were examined in one of two ways, either after removal of the transwell membrane (as shown by construct 1 [C1]) or without removal of the membrane (as shown by construct 4 [C4]). After 4 weeks, the constructs were multi-layered and cells were present along the disorganized collagen substrate (Fig. 1). By 8 weeks, there was variability among the cultures. In some cultures, the disorganized collagen substrate remained intact (C1) while in others, cells had penetrated the collagen substrate (C4). One consistent observation was that extensive matrix was detected between the more apical cells and the layer of cells adjacent to the collagen substrate (Fig. 1). At 11 weeks, there was penetration into the disorganized collagen substrate, which was accompanied by deposition of matrix (Fig. 1).
Fig. 1.
Light micrographs of 2 representative constructs (C1 and C4) over time. Cross-sections are shown after 4, 8, and 11 wks of culture. C1 is shown without the transwell membrane, which was removed before processing for evaluation. C4 has the transwell membrane (M) in place. CS, collagen substrate deposited on the membrane prior to seeding the cells. Scale bar = 50 μm.
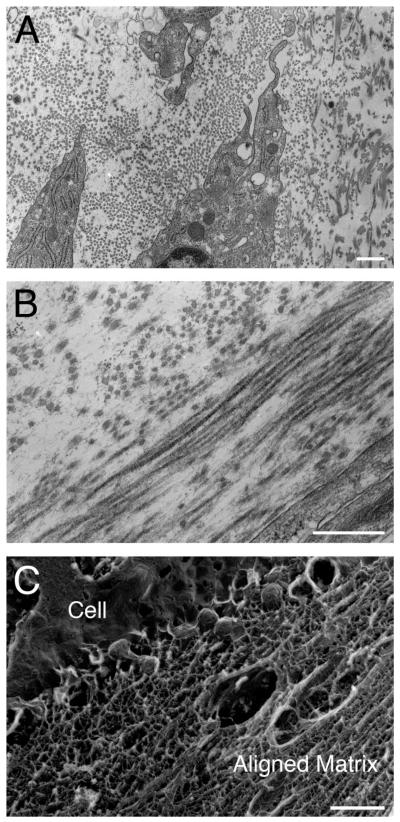
Analysis of electron micrographs revealed that the disorganized collagen substrate did not modify the organization of matrix laid down by the stromal fibroblasts. Collagen fibrils were detected throughout the matrix (Fig. 2A and B). Arrays of parallel and perpendicular matrix components were frequently observed (Fig. 2B). Quick Freeze Deep Etch (QFDE) microscopy revealed an extensive and dense matrix surrounding the cells and fibrils (Fig. 2C). In addition, the cells showed extensive rough endoplasmic reticulum (RER) at all time points (Fig. 2A).
Fig. 2.
Transmission electron micrographs of construct (C4) after 8 weeks. A: Representative image of collagen fibrils and matrix surrounding cells. Note the presence of RER in cells. B: Representative image of lamellar-like architecture and alternating arrays in the construct. C: Representative electron micrograph of cell and the extracellular matrix prepared by Quick Freeze Deep Etch (QFDE). Note that an aligned matrix is present. Scale bar = 500 nm.
To quantitate the amount of Type I collagen laid down in the constructs, a spectrofluorimetric assay for Type I collagen was performed on the 3-D cultures. Measurements were made on disorganized collagen deposited on membranes that were not seeded with cells and these were averaged and then subtracted from the experimental values. All 5 cultures showed an increase from 4 to 8 weeks, and in some cultures a 3-fold increase was detected (Table 1). When the same assays were performed on cells cultured on polycarbonate membranes only, Type I collagen was below the detection limits of the assay at all time points. These data indicate that the disorganized collagen that was laid down as a substrate provided a positive template for the formation of the 3-D construct and enhanced the deposition of collagen into the multi-cellular culture.
TABLE 1.
Type I Collagen Detected in the Extracellular Matrixa
| Sample construct | Week 4 (μg/mg) | Week 8 (μg/mg) |
|---|---|---|
| Construct 1 | 20.6 | 35.4 |
| Construct 2 | 11.6 | 20.2 |
| Construct 3 | 25.3 | 62.2 |
| Construct 4 | 9.6 | 29.5 |
| Construct 5 | 53.4 | 67.8 |
Quantitation of Type I Collagen in constructs 1–5 at week 4 and week 8.
Cytoarchitecture of the Cell Constructs
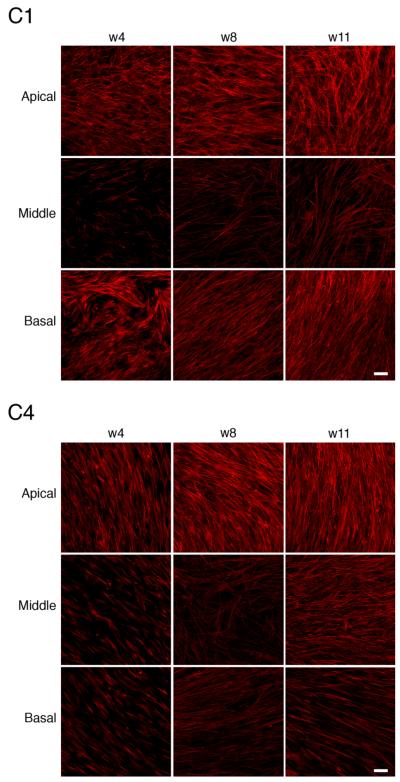
The cellular architecture was monitored over time by staining the cellular constructs with F-actin and ToPro 3, a nuclear marker (data not shown). Changes throughout the depth of the matrix were examined by imaging in z with optical sections of 0.5 μm using confocal laser scanning microscopy. Three images of two representative constructs (C1 and C4) are presented at 4, 8, and 11 weeks in Figure 3. They ranged in thickness at 4 weeks from 25 to 36 μm and at 11 weeks from 30 to 60 μm. At all time points, a dense layer of aligned cells was present at the apical and basal aspect of the construct. Cells in the mid-portion were sparser than in the apical and basal regions (Fig. 3). The directionality of the fibroblasts alternated throughout the depth of the construct, which was seen also in our previous studies on polycarbonate membranes (Guo et al., 2007). Thicker cultures (C4) demonstrated more change in cell directionality than thinner cultures (C1). The change of directionality is observed in the movies of C1 (1.5 revolutions) and C4 (0.75 revolutions) at 8 weeks (white line). The distance covering the apical to mid section for C1 was 11.5 μm and from the mid to basal section C1 was 7 μm, while C4 consisted of 25 and 10 μm, respectively, in each region. Supplemental Movies through the z-stacks for C1 and C4 can be viewed at www.interscience.wiley.com/jpages/1058-8388/suppmat.
Fig. 3.
Confocal micrographs of 2 representative constructs (C1 and C4) at weeks 4, 8, and 11. The alignment of cells throughout the depth of the constructs is detected using rhodamine phalloidin, which labels F-actin filaments. Three different regions of each construct (apical, middle, and basal) are shown. The distance between optical sections is 0.5 μm. C1. week 4: The distance from apical to mid region, 7.5 μm; distance from mid to basal, 10.5 μm; week 8: distance from apical to mid, 11.5 μm; distance from mid to basal, 7 μm; week 11: distance from apical to mid, 9 μm; distance from mid to basal, 13 μm. C4. week 4: The distance from apical to mid, 5.5 μm; distance from mid to basal, 10.5 μm; week 8: distance from apical to mid, 25 μm; distance from mid to basal, 10.5 μm; week 11: distance from apical to mid, 13 μm; distance from mid to basal, 35 μm. Scale bar = 50 μm.
Expression and Deposition of Glycosaminoglycans
The presence of proteoglycans was examined in both the media and in the construct. As the cells were cultured on transwells, the media in both the well below and in the transwell were collected weekly and analyzed to determine if there was polarity of secretion. Sulfated glycosaminoglycans (GAGs) secreted into the media showed an initial increase at 2 weeks, which was followed by a decrease over the next couple of weeks and then a gradual 3- to 4-fold increase for the duration of the time course. Three representative time courses are shown in Figure 4. While there was variability in the sulfated GAGs secreted into the media inside (upper well;U) or outside (lower well;L) the transwell, the trend for all of the cultures was remarkably similar. In contrast, when a collagen substrate was not laid down, the sulfated GAGs did not change over time (data not shown) indicating that changes in sulfation occurred with a more complex matrix.
Fig. 4.
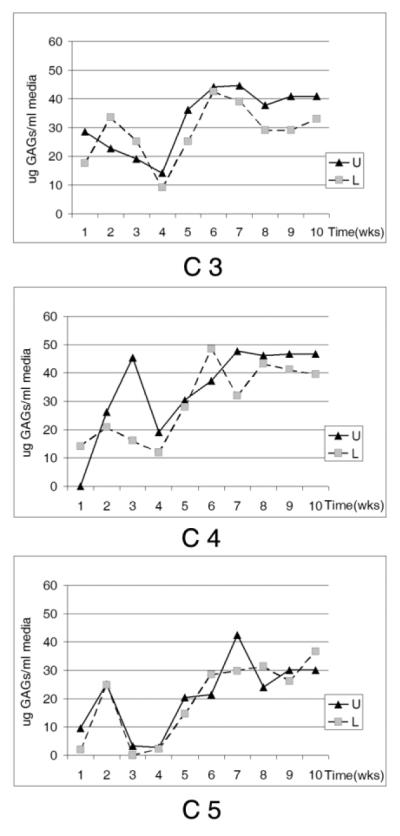
Sulfated GAGs in media from 3 representative constructs. Media collected weekly from transwells (U) and from underlying culture wells (L), filtered, fractionated, desalted, and analyzed by dimethylmethylene blue (DMB).
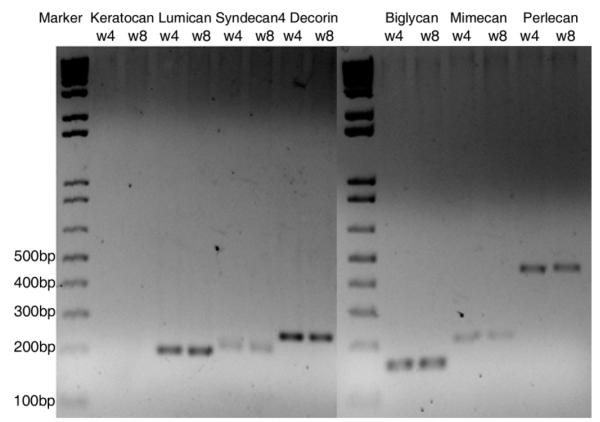
To examine if there was a difference in expression of proteoglycans (PGs) at 4 and 8 weeks that correlated with the secreted GAG value, RT-PCR and Western blot analysis was performed. Chondroitin sulfate, KS, and HS PGs were detected using RT-PCR of samples pooled from all 5 constructs. Decorin, biglycan, lumican, mimecan, syndecan4, and perlecan were detected at both time points. However, keratocan was not detected, which supported the cellular data in Figures 1 and 2 indicating that the cells were fibroblastic (Fig. 5). Unfortunately, samples were not available for analysis at week 11. A subset of the PGs (biglycan, decorin, and lumican) was examined from pooled samples using Western blot analysis. Electrophoresis of the secreted PGs without prior enzyme treatment resulted in poorly resolved smears. Digestion with chondroitinase ABC or keratanase was used to identify protein cores (Reinboth et al., 2006) (Fig. 6). Interestingly, there are high molecular weight bands that react with biglycan and decorin. In the decorin blot, the larger mass protein is a minor component. However, in the biglycan blot the protein is prominent and while it is unclear what these bands are, a number of possibilities exist. One is that dimerization has been shown to occur in solution and the PGs shown here were secreted into the medium (Scott et al., 2006), while another is that the antibody recognizes the enzyme that was left in the lysate and in the absence of primary antibody there were no bands detected (data not shown).
Fig. 5.
Proteoglycan expression in constructs at 4 and 8 weeks. Total RNA from fibroblasts was reverse transcribed and the cDNA was PCR-amplified. Aliquots of the PCR reaction were analyzed by agarose gel electrophoresis. Transcripts of the predicted sizes were obtained.
Fig. 6.
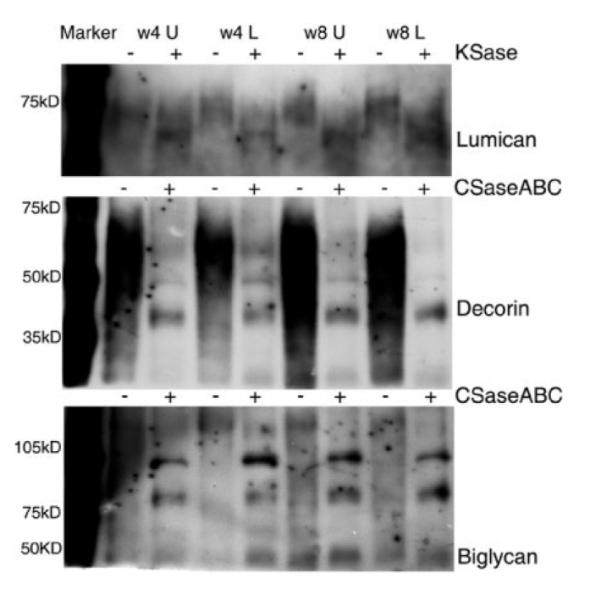
Analysis of CSPGs and KSPGs. Medium from 5 constructs were pooled at 4 and 8 weeks, filtered, fractionated, and desalted. Pools were taken from transwell (U) and culture wells (L). Equivalent protein concentrations were loaded on SDS-PAGE and immunoblots probed with antibodies to decorin, lumican, and biglycan.
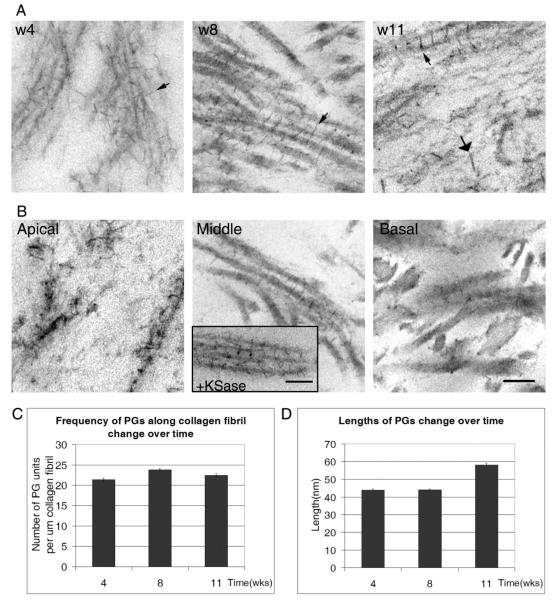
To resolve the PGs that were present in the 3-D culture, the constructs were stained with Cuprolinic blue, which binds sulfated moieties. Cells were incubated in the Cuprolinic blue solution in the presence or absence of polysaccharide lyases and examined. The PGs appeared as electron-dense filaments or rods and were present either along collagen fibrils or in the extracellular matrix. The density and lengths of the filaments have been described in the literature and are proposed to indicate phases of development or injury (Cintron et al., 1990a,b). The change in the population of sulfated PGs was evaluated over time. The presence of the sulfated PGs is shown at 4, 8, and 11 weeks at the mid-level of the construct (Fig. 7A). At 4 weeks, the arrangement of the filaments was not orderly; however, by 8 weeks the PGs were predominantly present in an axial arrangement along fibrils (small arrows). This correlated with the appearance of organized collagen fibrils at 8 weeks (Figs. 2, 7A). We observed a small increase in PGs along collagen fibrils between weeks 4 and 8, which correlated with an increase in collagen during this time period (Fig. 7C, and Table 1). In addition, by 11 weeks extensive matrix was present and was accompanied by an increase in the length and thickness of the proteoglycan filaments (large arrow) (Fig. 7A,D).
Fig. 7.
Transmission electron micrographs of Cuprolinic blue dye stained proteoglycans in constructs (A, B). A: Four, 8 and 11 weeks. Small arrows show axial alignment of filaments (w4 and 8) and large arrows indicate large filaments (w11). B: Electron micrographs at 8 weeks of construct 4 throughout the depth of the construct. Inset: Electron micrograph showing the presence of filaments after digestion with keratanase and the appearance of short filaments. C: Calculation of PGs along the collagen fibril. D: Change in length of PGs over time. Scale bar = 100 nm.
In addition, the constructs were examined throughout the depth of the culture at the apical, mid, and basal level (Fig. 7B). The basal level was defined as the region adjacent to or within the collagen substrate. There were changes in the lengths of the filaments throughout the construct. While sulfated PGs were prominent in the middle level, they were sparse at the basal level. In the apical region, PGs appeared to have an irregular arrangement, which may be associated with the higher cell density (Figs. 1, 7). When cultures were incubated with keratanase prior to staining with Cuprolinic blue, there was a loss of longer filaments (see inset, Fig. 7B).
DISCUSSION
Previously, we demonstrated that untransformed human corneal fibroblasts had the ability to deposit a complex matrix comprised in a scaffold-free system. The cells cultured in medium using a stabilized Vitamin C derivative produced a 3-D multi-cellular construct, which contained organized arrays of collagen (Guo et al., 2007). The ability to develop a 3-D natural construct without external scaffolding allows the cells and the matrix that they synthesize and deposit to further modify the culture. Our current studies examine the formation of a 3D cell culture when polycarbonate membranes were coated with disorganized collagen. This model will allow us to use human stromal fibroblasts to study development, injury, and/or pathologies of the matrix. For the first 4 weeks of culture, cells appear spindle-shaped and tend to be aligned to the newly synthesized collagen. Interestingly, over the 3-month culture period there was a change in cell morphology in the mid-portion of the construct from a classical fibroblastic-shape to a more dendritic morphology that did not stain extensively with phalloidin. At the same time, there was an increase in the number of cell layers and an increase in the deposition of collagen and PGs. We believe that this deposition is most extensive in the middle regions. The presence of the fibroblast and or keratocyte morphology in the matrix assembled by the cells mimics the normal corneal tissue and earlier work where hydrated collagen gels were assembled and cells cultured (Tomasek and Hay, 1984). We interpret these results as a transition from a synthetic to a more quiescent or homeostatic state that is potentially similar to that of a native stroma (Brown et al., 1995). However, keratocan, a marker of keratocytes, was not detected with RT-PCR, while HSPGs (perlecan and syndecan), markers of a development and non-fibrotic repair phenotype, were detected (Corpuz et al., 1996; Yang et al., 2006; Giros et al., 2007; Migueloto and Zorn, 2007; Trinkaus-Randall, 2000).
The composition of PGs and collagens with a small diameter were hypothesized to be critical for maintaining the proper structure for the cornea to function as a transparent tissue. The healthy corneal stroma is known to contain two major classes of proteoglycans, one possessing KS side chains and the other possessing CS side chains. During development and injury or disease of the cornea, it is well known that there are changes in biochemical properties of the matrix proteins (Funderburgh and Chandler, 1989; Midura and Hascall, 1989; Cintron et al., 1990a,b; Ruggiero et al., 1996; Trinkaus-Randall, 2000). Over the 3-month culture period, the multicellular constructs contained a complement of PGs and collagen. The expression of PGs was associated with a change in the sulfation of the GAGs. An initial decrease in the secretion of sulfated GAGs was followed by a 3-fold increase over 11 weeks of culture. Similar changes in the synthesis of GAGs and collagen were detected when corneal epithelial cells were cultured on a lens capsule (Meier and Hay, 1974). The increase in secreted GAGs was accompanied by an increase in the number of GAGs along a collagen fibril and in the length of the Cuprolinic blue dense filaments, all of these indicative of a non-fibrotic or development phenotype. The PGs were detected in cross-section, perpendicular to the length of fibrils, and as elongated filaments along collagen (as described by Young et al., 2005) (Fig. 7A,B). In addition, the GAG chains detected at 4 and 8 weeks (Cuprolinic blue filaments) had a similar size compared to those reported for the human corneal stroma (Muller et al., 2004). While we detect an increase in average size at 11 weeks of culture, we hypothesize that the increase in the number of GAGs represents those detected in the stromal matrix. Filaments such as these have been described in rabbit cornea following a corneal wound (Cintron et al., 1990a). These morphological changes were accompanied by biochemical changes in length, isoforms, and sulfation of GAGs, which gradually returned over time (Cintron et al., 1978, 1990a; Hassell et al., 1983).
The synthesis and expression of small leucine-rich PGs such as decorin and biglycan are demonstrated in this culture and are proposed to play important roles in fibrillogenesis, tissue repair, and regulation of transforming growth factor-β (Iozzo, 1998). Interestingly, these may or may not require the sulfated form of the GAG. Danielson et al. (1997) showed that collagen fibrils displayed lateral fusion in decorin null mice. In addition, in vitro experiments have demonstrated that decorin can alter fibrillar ultrastructure (Birk and Lande, 1981; Neame et al., 2000; Brown et al., 2002). In addition to the presence of decorin and biglycan, we demonstrated that lumican is present as a PG, which is a critical marker of the stromal matrix (Song et al., 2003; Kao et al., 2006). The KSPGs are the most abundant carbohydrate in the cornea and they have been shown to play a role in maintaining transparency (Funderburgh et al., 1991). The respective cores lumican, keratocan, and mimecan all link to KS via N-linked oligosaccharides (Funderburgh et al., 1993). Interestingly, the expression of lumican has been shown to modulate keratocan, which is not expressed in our culture system (Carlson et al., 2005). Furthermore, modifications in the structure of KS have been shown to be critical in the morphogenesis of the stromal matrix (Hayashida et al., 2006). Together, these suggest that modifications made to the culture incubations could alter the regulation of the cells. Much of our work and our observations are a direct tribute to the work of Dr. Elizabeth Hay who as recently as 2005 wrote a review on the role of the mesenchymal cell in development and how altering regulation of specific proteins modulates communication between cells (Hay, 2005).
In summary, we have shown that human fibroblast cells can be cultured as long-term 3-D cultures without an external supporting scaffold and that they promote the deposition and sulfation of PGs. The PGs are present throughout the cell-synthesized ECM. Our culture developed an apical basal polarity, which reflects that present in a corneal stroma. It is an excellent model for understanding the mechanisms of development or repair. The culture system that we have developed will be manipulated with the ultimate goal that we can model development, wound repair, and a number of stromal diseases by modulating serum levels, altering the composition of specific growth factors, and finally by genomic manipulation of human cells.
EXPERIMENTAL PROCEDURES
All procedures adhered to the Declaration of Helsinki. Human corneas were obtained from the National Disease Research Interchange (NDRI, Philadephia, PA).
Cell Culture
Primary cultures of human corneal fibroblasts from 5 donors were established prior to seeding the construct (Guo et al., 2007). Briefly, corneal epithelium and endothelium were removed and the stroma was minced into pieces. The explants were cultured for 1 to 2 weeks in Eagle’s minimum essential medium (EMEM) (ATCC, Manassas, VA) containing 10% fetal bovine serum (FBS) and antibiotic/antimycotic (Sigma, St. Louis, MO). Cells were passaged once prior to their use in the development of the construct.
Culture of Construct
Disorganized pepsin extracted bovine dermal collagen (3 mg/ml; Purecol, Fremont, CA) was laid down on polycarbonate transwells. To enhance adhesion, the membrane was treated to covalently couple the collagen to the membrane (Carbodiimide Kit; Polysciences, Warrington, PA). Primary human corneal fibroblasts were plated onto the disorganized collagen-coated transwells at a seeding density of 0.5 × 106 cells/ml. The cells were cultured in EMEM defined previously plus 1 mM 2-O-a-D-glucopyanosyl-L-ascorbic acid (Wako Chemicals USA, Inc., Richmond, VA). The medium was changed 3 times a week and collected for GAG analysis. The cultures were examined by light microscopy, as described (Guo et al., 2007), at 4, 8, and 11 weeks.
Microscopy
Light microscopy
The constructs were either removed from the membrane with Dispase II or kept on the membrane. They were then fixed and processed for TEM as described in Guo et al. (2007) and Trinkaus-Randall and Gipson (1984). Thick sections were cut and photographed with a Nikon Eclipse E800 (Nikon, Melville, NY) equipped with a SPOT Camera (Micro Video Instruments, Avon, MA)
Transmission Electron Microscopy and Cuprolinic Blue Staining
The tissue was processed as described above. Sections were cut and images were taken on a Philips 300 TEM (Eindhoven, The Netherlands). In addition, an electron-dense reagent, Cuprolinic blue, which defines the size and directional orientation of GAG chains, was used. Cuprolinic blue has been shown to identify sulfated GAGs and to demonstrate the association of sulfated GAGs with matrix molecules, such as collagen (Scott and Haigh, 1988). The tissue was incubated in the presence or absence of polysaccharide lyases (Brown et al., 1999), stained with 1% Cuprolinic blue (in buffer containing 0.3 M MgCl2) for 24 ht (Gong et al., 1992), dehydrated, and processed for electron microscopy.
Quick-Freeze Deep-Etch Electron Microscopy
Small sections were cut from the fibroblast constructs. These specimens, while still attached to the membrane, were rinsed with PBS to remove any excess medium, then mounted tissueside-up onto specimen carriers using a 2% Laponite solution (Rockwood Additives, Cheshire, UK) as an adhesive and cushioning material. After mounting the specimens, excess PBS was removed with filter paper, and the exposed surface of the tissue was rapidly slam frozen using a portable cryogen (Delaware Diamond Knives, Wilmington, DE). The frozen constructs were transferred into a modified Cressington CFE-40 freeze fracture/freeze etch system (Cressington Scientific Instrument, Watford, UK) for replication. During replication, the specimens were superficially fractured and etched at −100°C for 25 min. Rotary shadowed replicas of the etched surfaces of the constructs were created by evaporation of platinum/carbon (for contrast) at a 20° angle onto the rotating construct, followed by evaporation of pure carbon (for replica strength) at a 90° angle. Tissue was then digested overnight with household bleach. The cleaned replicas were picked up on copper 600-mesh grids. Grids were viewed and photographed with a JEOL JEM-1000 (JEOL Ltd., Tokyo, Japan).
Confocal Microscopy
A portion of the construct was prepared on the polycarbonate membrane as a flat mount for analysis by confocal microscopy. It was fixed with 4% paraformaldehyde, washed with phosphate buffered saline (PBS), blocked with 1% bovine serum albumin (BSA) in PBS, and stained overnight with rhodamine phalloidin (1: 80; Molecular Probes, Eugene, OR), a marker of F-actin filaments, and To-Pro-3 (1:1000), a marker of cell nuclei. Continuous Z-sections were taken of the flat mounts from the apical to the basal surface with a Leica TCS-SP2 confocal microscope (Leica Microsystems, Heidelberg, Germany). Optical sections with a 0.5-μ step size were stacked for the basal, middle, and apical regions of each construct. Thickness from basal to mid- and from mid-region to apical surface was determined for each construct.
Glycosaminoglycan Analysis
The media were collected weekly from the different cultures, filtered, and then fractionated using Vivapure Maxispin columns (Anion exchange; Sartorius Stedim Biotech S.A., Aubagne, France). The samples were concentrated and desalted using Amicon Ultracell YM-3 (Millipore, Billerica, MA). The GAGs were quantitated using the dimethylmethylene blue assay (DMB) (Brown et al., 1992). Briefly, samples were mixed with the DMB reagent and absorbance (525 nm) was read immediately. Concentrations were determined from standard curves of CS (Sigma, St. Louis, MO).
Proteoglycan Core Protein Analysis
Total protein was determined using the BCA assay according to the manufacturer’s instructions (Pierce, Rockford, IL). Proteoglycan core proteins were identified using selective polysaccharidases in conjunction with SDS-PAGE and immunoblotting. The lysates were digested with chondroitinase ABC for 3 hr at 37°C to remove the chondroitin chains or keratanase to remove the keratan sulfate chains. Aliquots were analyzed using SDS-PAGE and Western blot analysis (Brown et al., 2002). Equivalent amounts of protein from each lysate were subjected to SDS-PAGE and transferred to Polyscreen PVDF membrane (PerkinElmer, Boston, MA) by the semi-dry method. Positive controls were run (data not shown). Non-specific binding was blocked with BSA in a Tris buffer (10 mM Tris, 100 mM NaCl, 0.1%Tween-20). Membranes were probed with primary antibodies of appropriate concentrations in BSA, washed and incubated with horseradish peroxidase-conjugated (HRP) secondary antibody in BSA (Boucher et al., 2007). The primary monoclonal antibodies used were directed against decorin, biglycan, and lumican (R&D system, Minneapolis, MN). The secondary antibody was HRP-goat anti-mouse (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Visualization was performed by enhanced chemiluminesence (PerkinElmer, Boston, MA) and quantified with the Kodak Imaging system.
Determination of Collagen
Type I collagen was determined using the Human Type I Collagen Detection kit (Chondrex, Inc., Redmond, WA). The extraction and ELISA were performed according to the manufacturer’s directions. The assay was read at 490 nm on a Molecular Devices plate reader. The antibody detects the native conformation of Type I collagen and does not recognize denatured Human Type I collagen.
RT-PCR
Reverse transcriptase polymerase chain reaction (RT-PCR) was used to characterize the presence of proteoglycans. Total cellular RNA was isolated from the constructs using TRIZOL (Invitrogen, Carlsbad, CA). The RNA was reverse transcribed and the cDNA was PCR-amplified. Positive controls were run for verification but are not present in Figure 5. The primers were designed based on the sequences for decorin, biglycan, keratocan, perlecan, mimecan, lumican, and syndecan4 (see Table 2).
TABLE 2.
Primer Sequencesa
| Target sequence | Primer (forward) | Primer (reverse) |
|---|---|---|
| Keratocan | tattcctggaaggcaaggtg | attccatgggacactcgaag |
| Lumican | tgatctgcagtggctcattc | aaaagagcccagctttgtga |
| Syndecan4 | tctctcgcacatttccacag | actggggaaggggtttaatg |
| Decorin | atgtgtctacgtgcgctctg | ctgaaaatggcaggcaaaat |
| Biglycan | ggactctgtcacacccacct | agctcggagatgtcgttgtt |
| Mimecan | agcaggccaaaaccaatatg | gatgtgtatgggtgggaagg |
| Perlecan | tgagggcatacgatggcttgt | ggagatgaccctgagcagcatct |
Primers for the target sequences above were used for RT-PCR.
Statistical Analysis
The length of the Cuprolinic blue–stained PG filaments and the collagen intervals between those PG units were measured using Image J (NIH; Bethesda, MD: http://rsb.info.nih.gov/ij/). The populations were randomly assigned and measured. Measurements of at least 50 filaments were taken. More than 30 collagen fibrils containing at least 3 PG filament units were measured and the number of units/μm collagen fibril was calculated. Results were averaged and expressed as ± SEM and Student’s t-test was performed.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Matthew Nugent and Haiyan Gong for critical discussion of the manuscript and Rozanne Richman and Pat Pearson for their excellent technical expertise. We thank Ilene Boucher for excellent technical assistance with the movies.
Grant sponsor: NIH; Grant numbers: EY015500, EY06000, EY05665; Grant sponsor: MA Lion’s Eye Research Fund, Inc.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Birk DE, Lande MA. Corneal and scleral collagen fiber formation in vitro. BiochimBiophys Acta. 1981;670:362–369. doi: 10.1016/0005-2795(81)90108-2. [DOI] [PubMed] [Google Scholar]
- Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT, Applebaum E, Banwatt R, Trinkaus-Randall V. Synthesis of stromal glycosaminoglycans in response to injury. J Cell Biochem. 1995;59:57–68. doi: 10.1002/jcb.240590108. [DOI] [PubMed] [Google Scholar]
- Brown CT, Nugent MA, Lau FW, Trinkaus-Randall V. Characterization of proteoglycans synthesized by cultured corneal fibroblasts in response to transforming growth factor beta and fetal calf serum. J Biol Chem. 1999;274:7111–7119. doi: 10.1074/jbc.274.11.7111. [DOI] [PubMed] [Google Scholar]
- Brown CT, Lin P, Walsh MT, Gantz D, Nugent MA, Trinkaus-Randall V. Extraction and purification of decorin from corneal stroma retain structure and biological activity. Protein Expr Purif. 2002;25:389–399. doi: 10.1016/s1046-5928(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, Birk DE, Funderburgh JL, Jester JV, Kao WW. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron C, Hassinger LC, Kublin CL, Cannon DJ. Biochemical and ultrastructural changes in collagen during corneal wound healing. J Ultrastruct Res. 1978;65:13–22. doi: 10.1016/s0022-5320(78)90017-5. [DOI] [PubMed] [Google Scholar]
- Cintron C, Covington HI, Kublin CL. Morphologic analyses of proteoglycans in rabbit corneal scars. Invest Ophthalmol Vis Sci. 1990a;31:1789–1798. [PubMed] [Google Scholar]
- Cintron C, Gregory JD, Damle SP, Kublin CL. Biochemical analyses of proteoglycans in rabbit corneal scars. Invest Ophthalmol Vis Sci. 1990b;31:1975–1981. [PubMed] [Google Scholar]
- Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. Bovine sulfate keratan sulfate proteoglycans 37A. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Chandler JW. Proteoglycans of rabbit corneas with nonperforating wounds. Invest Ophthalmol Vis Sci. 1989;30:435–442. [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Physical and biological protperties of keratan sulfate proteoglycan. Biochem Soc Trans. 1991;19:871–816. doi: 10.1042/bst0190871. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Brown SJ, Vergnes JP, Hassell JR, Mann MM, Conrad GQ. Sequence and structural implications of a bovine keratan sulfate proteoglycan core protein. Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J Biol Chem. 1993;268:11874–11880. [PubMed] [Google Scholar]
- Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol. 2007;5:7–29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Freddo TF, Johnson M. Age-related changes of sulfated proteoglycans in the normal human trabecular meshwork. Exp Eye Res. 1992;55:691–709. doi: 10.1016/0014-4835(92)90174-q. [DOI] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:4056–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362–369. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- Hay Elizabeth D. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanims that creat it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci USA. 2006;103:13333–13338. doi: 10.1073/pnas.0605441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. Focus on molecules: lumican. Exp Eye Res. 2006;82:3–4. doi: 10.1016/j.exer.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Hay Elizabeth D. Stimulation of extracellular matrix synthesis in the developing cornea by glycosaminoglycans. Proc Nat Acad Sci. 1974;71:2310–2313. doi: 10.1073/pnas.71.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura RJ, Hascall VC. Analysis of the proteoglycans synthesized by corneal explants from embryonic chicken. II. Structural characterization of the keratan sulfate and dermatan sulfate proteoglycans from corneal stroma. J Biol Chem. 1989;264:1423–1430. [PubMed] [Google Scholar]
- Miqueloto CA, Zorn TM. Characterization and distribution of hyaluronan and the proteoglycans decorin, biglycan and perlecan in the developing embryonic mouse gonad. J Anat. 2007;211:16–25. doi: 10.1111/j.1469-7580.2007.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LJ, Pels E, Schurmans LR, Vrensen GF. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res. 2004;78:493–501. doi: 10.1016/s0014-4835(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinboth Betty, Thomas John, Hanssen Eric, Gibson Mark A. Big-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation and participates in ternary complexing with these macromolecules. J Biol Chem. 2006;281:7816–7824. doi: 10.1074/jbc.M511316200. [DOI] [PubMed] [Google Scholar]
- Ruberti J, Zieske JD, Trinkaus-Randall V. Corneal tissue replacement. In: Lanza, Langer, Vacanti, editors. Principles of tissue engineering. vol. 68. Elsevier; 2007. pp. 1021–1043. [Google Scholar]
- Ruggiero F, Burillon C, Garrone R. Human corneal fibrillogenesis. Collagen V structural analysis and fibrillar assembly by stromal fibroblasts in culture. Invest Ophthalmol Vis Sci. 1996;37:1749–1760. [PubMed] [Google Scholar]
- Scott JE, Haigh M. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitindermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in ‘critical-electrolyte-concentration’ techniques. Biochem J. 1988;253:607–610. doi: 10.1042/bj2530607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PG, Dodd CM, Bergmann EM, Sheehan JK, Bishop PN. Crystal structure of the Biglycan dimer and evidence that dimerization is essential for folding and stability of class I small leucine-rich repeat proteoglycans. J Biol Chem. 2006;281:13324–13332. doi: 10.1074/jbc.M513470200. [DOI] [PubMed] [Google Scholar]
- Song J, Lee YG, Houston J, Petroll WM, Chakravarti S, Cavanagh HD, Jester JV. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest Ophthalmol Vis Sci. 2003;44:548–557. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J Cell Biol. 1984;99:536–549. doi: 10.1083/jcb.99.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, a-actinin, and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Trinkaus-Randall V. Cornea: biological responses. In: Lanza R, Langer R, Chick E, editors. Principles of tissue engineering. 2nd ed Academic Press; 2000. pp. 471–491. [Google Scholar]
- Trinkaus-Randall V, Gipson IK. Role of calcium and calmodulin in hemidesmosome formation in vitro. J Cell Biol. 1984;98:1565–1571. doi: 10.1083/jcb.98.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus-Randall V, Banwatt R, Cappecchi J, Leibowitz HM, Franzblau C. In vivo fibroplasia of a porous polymer in the cornea. IOVS. 1991;32:3245–3251. [PubMed] [Google Scholar]
- Yang W, Gomes RR, Brown AJ, Burdett AR, Alicknavitch M, Farach-Carson MC, Carson DD. Chondrogenic differentiation on perlecan domain I, collagen II, and bone morphogenetic protein-2-based matrices. Tissue Eng. 2006;12:2009–2024. doi: 10.1089/ten.2006.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RD, Tudor D, Hayes AJ, Kerr B, Hayashida Y, Nishida K, Meek KM, Caterson B, Quantock AJ. A typical composition and ultrastructure of proteoglycans in the mouse corneal stroma. Invest Ophthalmol Vis Sci. 2005;46:1973–1978. doi: 10.1167/iovs.04-1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.