Abstract
Secondary pulmonary infections by encapsulated bacteria including Streptococcus pneumoniae and Staphylococcus aureus following influenza represent a common and challenging clinical problem. The reasons for this polymicrobial synergy are still not completely understood, hampering development of effective prophylactic and therapeutic interventions. While it has been commonly thought that viral-induced epithelial cell damage allows bacterial invasiveness, recent studies by several groups have now implicated dysfunctional innate immune defenses following influenza as the primary culprit for enhanced susceptibility to secondary bacterial infections. Understanding the immunological imbalances that are responsible for virus-bacteria synergy will ultimately allow the design of effective, broad-spectrum therapeutic approaches for prevention of enhanced susceptibility to these pathogens.
Respiratory viruses such as influenza virus are known to cause severe disease and to be associated with pneumonia, particularly in the very young and aged populations, and in individuals with serious medical comorbidities. In addition, respiratory virus infection can often lead to increased susceptibility to secondary bacterial infections. The mechanisms responsible for this viral-bacterial synergy have remained elusive and historically have been attributed to virus-induced lung tissue damage (1,2). However, by exploiting recently developed animal models, a dysfunctional host antibacterial immune response during influenza infection has been implicated as the major contributor to secondary bacterial susceptibility (3). This paper now reviews recent scientific progress that has shed new insight into this major clinical problem.
The Clinical Scenario and Relevant Animal Models
It is well-known that bacterial pneumonia often occurs following influenza infection. These secondary infections predominantly involve a select group of bacteria, including Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pyogenes. Such co-infections may be particularly problematic during influenza pandemics. Indeed, reviews of published autopsy case reports revealed that over 90% of deaths during the 1918 influenza pandemic likely resulted from secondary pneumococcal pneumonia (4,5). One could argue that antibiotics were not available in 1918 and thus, secondary bacterial infections would likely not represent a serious problem today. Nevertheless, most deaths in the 1957–58 “Asian influenza” pandemic were still due to secondary bacterial pneumonia, even with the availability of antibiotics (6). In one study, 75% of confirmed fatal cases of influenza in the 1957–58 pandemic had bacteriological and histological evidence of bacterial pneumonia, mainly due to S. aureus or S. pneumoniae (7). The remaining fatal cases appeared to be caused primarily by influenza viral pneumonia. Furthermore, in the more recent 2009 H1N1 (swine flu) pandemic, over 50% of the people who died showed histologic and microbiologic evidence of bacterial pneumonia (8). Strikingly, in one report, 43% of the children who died from the H1N1 virus in the USA from April to August, 2009, had laboratory-confirmed bacterial co-infections, including all 6 children that had culture or pathology results reported and no recognized, high-risk medical conditions (9). In another report, it was found that among 317 pediatric deaths associated with the H1N1 virus from April, 2009 to January, 2010, 28% had evidence of bacterial co-infection, predominantly S. aureus and S. pneumoniae (10). It should be recognized that, given the difficulty and uncertainty of detecting and cultivating bacteria from the lungs of deceased patients, the numbers of co-infected patients in all of these studies could be significantly higher.
Co-infections are also a continuing problem with seasonal influenza. Approximately 90,000 people die from bacterial infections in the USA each year and over the past 20 years, methicillin-resistant S. aureus (MRSA) has emerged as a growing problem for both hospital- and community-acquired pneumonia. Indeed, more people die from MRSA than from HIV (11,12). In addition, new variants of MRSA continue to emerge as pulmonary pathogens and have been associated with both community outbreaks and post-influenza pneumonia (13,14). It has been estimated that bacterial co-infections are found in 4–30% of adults and in 22–33% of children that are hospitalized with community-acquired viral pneumonia (15). Again, most of these infections are due to S. aureus or S. pneumoniae.
The mouse infection model is well-accepted for studying influenza infection. In both humans and mice, influenza virus titers in the lung reach a peak at 3 to 5 days after infection and the virus begins to be cleared thereafter, with resolution of infection nearly completed by days 10–12. Murine models of virus-bacterial co-infection have also been established by several groups (16–20) and these models appear to accurately mimic clinical observations regarding the high susceptibility to secondary bacterial infection following influenza, with greatly enhanced disease severity and fatality rates. The viral strain most commonly used for murine co-infection studies is the mouse-adapted H1N1 A/PR/8/34 but nonadapted H1N1 CAL/04/09 has also been employed (21). The greatest susceptibility to secondary bacterial infection in both humans and mice is seen around day 7, at the time of influenza virus clearance, and lasts approximately one week (Fig. 1). Nonetheless, there are differences in the detailed experimental conditions utilized in different mouse studies, and these differences are mainly related to whether the individual focus is on understanding influenza-induced susceptibility to secondary bacterial infection or the resulting poor disease outcome. For example, many studies (22–24) have used very high bacterial challenge doses, particularly when studying influenza and S. aureus co-infection, which leads to extensive neutrophil recruitment and exacerbation of inflammation, a clinical feature that ultimately can result in bacterial pneumonia and a poor outcome. Similarly, some studies (25,26) have focused on the late stages of bacterial infection (24 hr or later after secondary infection), again when there is an influx of neutrophils into the lung and intense inflammatory responses due to bacterial outgrowth. Thus, investigators using high doses of challenge bacteria and/or investigating the latter stages of infection typically end up studying neutrophil function, either their antibacterial activities or accompanying inflammatory lung damage. On the other hand, our experiments have indicated that a normal mouse can effectively clear up to approximately 105 pneumococci very early (within 4–12 hrs); higher challenge doses require recruitment of neutrophils for survival (3). We have used this system to examine phagocytic function very early after bacterial infection, thus avoiding the confounding issue of whether the observed pathology is due to failure to control the initial bacterial infection versus the overwhelming inflammatory response following the infection. We suggest that using the smallest viral and bacterial doses necessary to observe pathogen synergy, a situation which most closely mimics the natural clinical scenario, is optimal for studying the mechanism of influenza-induced susceptibility to secondary bacterial infection.
Figure 1.
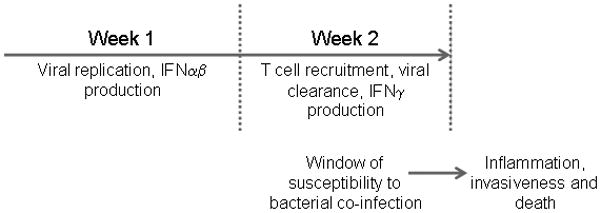
Kinetics of influenza virus infection and susceptibility to bacterial co-infection.
Virus-Mediated Lung Damage
The mechanisms responsible for synergy between influenza virus and bacterial infections have remained puzzling since 1918. It is clear that increased susceptibility to various encapsulated bacteria occurs following influenza infection (27), suggesting a general defect. Influenza virus replicates preferably in epithelial cells, which leads to direct damage to the airway epithelium. Historically, the generally accepted mechanism responsible for microbial synergy is that this virus-induced damage to the epithelial barrier provides increased attachment sites for bacteria, resulting in invasive disease (1,2). Influenza-induced lung tissue damage in both humans and mice is greatest on day 6 after infection (28), which generally correlates with the time of greatest susceptibility to bacteria. However, viral strains that cause minimal epithelial cell damage still enhance subsequent bacterial infection in mice (29,30). Influenza neuraminidase and up-regulation of platelet-activating factor receptor expression during murine viral infection may increase bacterial adherence (31,32), although use of mice deficient in platelet-activating factor receptor or treatment with a competitive receptor antagonist had no influence on survival rates after bacterial infection (19,33). Furthermore, genetic deletions that modify viral neuraminidase expression do not affect susceptibility of mice to secondary bacterial pneumonia (34). Finally, there was no correlation between human mortality and virus attack rates in 1918 (35), suggesting factors other than simply viral-induced lung damage.
Influenza-Induced Suppression of Antibacterial Innate Immunity
A concept that has recently gained traction in the field is that innate bacterial clearance in the lung is somehow impaired by influenza infection. Alveolar macrophages are the major cell population in the normal airway and these cells form the first line of defense against respiratory infection. A deficiency in alveolar macrophage-mediated phagocytosis following influenza has been reported (36–38). However, in most of the reported studies, inhibition of phagocytosis was only partial. For example, in the study by Warshauer et al. (38), 90% of Staphylococcus epidermidis were killed by alveolar macrophages obtained from uninfected mice versus only 68–73% killing by macrophages from influenza infected animals. Jakab and colleagues (39,40) reported defective phagolysosome formation by alveolar macrophages from virus-infected mice but no defect in phagocytosis, while Nugent and Pesanti (41) found no defect in either uptake or killing. The discrepancies in results from these various laboratories could be due to several factors - differences in the days elapsed since initial virus infection and/or secondary bacterial challenge, differences in doses of virus and bacteria used, and variations in the combinations of virus and bacterial strains studied.
More recently, it has been found that alveolar macrophage-mediated clearance of S. pneumoniae that occurs within 4–6 hrs following intranasal bacterial challenge, is in fact, significantly inhibited by prior influenza virus infection, with maximal inhibition occurring on days 7–8 following viral infection (3). Interestingly, this is when effector T cells have migrated into the lung airways to initiate recovery from viral infection (42) and is the time of peak IFN-γ expression in the pulmonary tract. Indeed, while bacterial clearance is suppressed in wild-type mice after influenza infection, this suppression is nearly absent in virus-infected IFN-γ−/− mice and in wild-type mice treated with neutralizing anti-IFN-γ mAb after influenza infection (3). Furthermore, treatment of non-virus infected mice with exogenous IFN-γ can mimic viral infection and result in inhibition of alveolar macrophage-mediated bacterial phagocytosis (3). A critical role for IFN-γ in inhibiting phagocytosis of S. aureus by alveolar macrophages has similarly been reported (43). Further experiments showed that the relatively high levels of IFN-γ in the lung following influenza caused inhibition of MARCO expression (3), the scavenger receptor necessary for recognition of non-opsonized pneumococci by alveolar macrophages (44). Thus, although low doses of IFN-γ are traditionally known to enhance intracellular killing of bacteria, high levels of IFN-γ in the lung downregulate expression of the scavenger receptors required for bacterial recognition by lung macrophages, such as MARCO (3) and the mannose receptor (45), and thus, inhibits binding and uptake by these cells. It has been suggested that impaired NK cell activity during secondary S. aureus infection leads to inhibition of alveolar macrophage phagocytosis and enhanced susceptibility to invasive bacterial disease, possibly due to decreased TNF-α expression (46), but this potential mechanism has yet to be fully examined.
It should be noted that, in normal mice infected only with pneumococci, increased expression of IFN-γ can enhance TNF-α expression and thereby lead to increased neutrophil recruitment (47). However, in animals previously infected with influenza virus, TNF-α production is decreased even in the presence of IFN-γ (3,48). This is probably related to the finding that influenza infection leads to de-sensitization of TLR4-mediated signaling (48). However, while pneumolysin made by pneumococci is a ligand for TLR4, there is no evidence that immunity to S. aureus depends upon TLR4. Furthermore, this TLR signaling defect is relatively long-lasting and still observed several months after viral infection. Thus, a potential effect on TLR signaling does not track with clinical susceptibility to secondary bacterial infection, which normally occurs within a one week window following influenza virus infection. Nevertheless, this effect may be related to defects in restoration of lung homeostasis (see below).
In agreement with earlier human studies (49–51), McNamee and Harmsen (23) reported significant neutrophil dysfunction in the lungs of influenza virus and pneumococcus doubly- infected mice. However, neutrophil impairment was observed on both day 3 and day 6 after influenza infection while enhanced susceptibility to S. pneumoniae infection was seen only on day 6. Expression of inhibitory IL-10 that is induced by 2,3-dioxygenase in influenza virus- infected hosts has been reported to be partially responsible for the increased susceptibility to secondary bacterial infection, likely due to an effect on neutrophil function (22,25). However, only a minimal decrease in susceptibility to secondary bacterial infection is observed in IL-10−/− mice (3,25). Furthermore, IL-10−/− mice clear influenza infection more quickly than wild-type animals due to earlier induction of adaptive immunity (52,53). This in turn, alters the window of enhanced susceptibility to bacterial infection, an effect that may account for the earlier findings.
Shahangian et al. (54) found that mice deficient for the IFNαβ-R were partially resistant to secondary infection with S. pneumoniae following influenza, and this effect correlated with production of neutrophil chemoattractants. A similar function for IFNα and IFNβ was reported in a mouse model of upper respiratory tract pneumococcal colonization (55). An important role for the Th17 pathway in this effect was shown by the finding that IL-17, IL-22, and IL-23 were decreased following co-infection with influenza virus and S. aureus, and that this decrease was dependent upon type 1 IFN (56). Furthermore, intentional overexpression of IL-23 during influenza led to markedly improved bacterial clearance. Similarly, Ivanov et al. (57) recently reported that IL-22-deficient mice were significantly more susceptible to pneumococcal infection following influenza. It has been found in other infection models that IL-17-producing γδ T cells can be particularly suppressed by type 1 IFN (58) and indeed, this has been reported to occur during secondary pneumococcal infections following influenza (59). In summary, it appears that in addition to decreased alveolar macrophage function following lung viral infection, it is likely that induced type 1 IFN production can also inhibit IL-17 mediated neutrophil recruitment, possibly by targeting γδ T cells (Fig. 2).
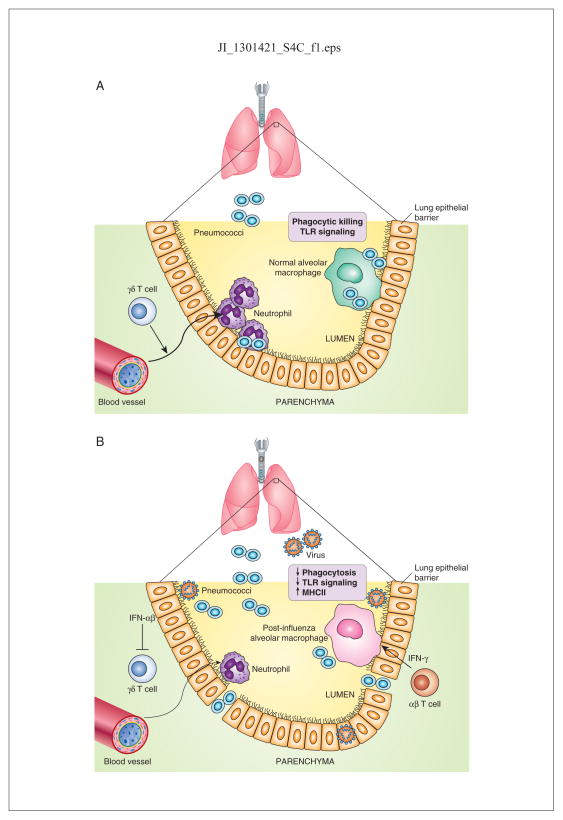
Figure 2.
A model for the influence of influenza infection on innate antibacterial immunity. (A), In the normal, uninfected lung, resident alveolar macrophages provide the first line of defense against encapsulated bacteria such as pneumococci. If macrophage defenses are overwhelmed, neutrophils are recruited to the airways through the action of IL-17 and related cytokines, likely produced by γδ T cells. (B) During influenza infection, CD4 and CD8 T cells are recruited into the lung to help resolve viral infection. These T cells secrete copious amounts of IFN-γ that binds to alveolar macrophages and modifies their properties, including inhibition of scavenger receptor expression, such as MARCO, and upregulation of MHC class II. In addition, IFN-αβ produced by virally-infected epithelial cells inhibits IL-17 production and thus, neutrophil recruitment is diminished. Not shown is the infiltration of other inflammatory myeloid cells into the lung following influenza. The result is that while adaptive immunity designed to establish anti-viral immune memory is enhanced, innate antibacterial defenses are suppressed. By approximately Day 14 following influenza infection, levels of type I and type II IFN are decreased and innate defenses against bacterial invasion are restored.
Defects in Restoration of Lung Homeostasis
As summarized above, multiple studies have demonstrated that impaired antibacterial immunity predominantly contributes to lethal influenza and bacterial co-infection, and inhibited innate antibacterial immunity is associated with dysregulated pulmonary cytokine responses following influenza infection. On the other hand, these immune regulators, such as type I IFN (60), IL-10 (52), and IL-17 (61,62), often have opposite influences on protective antiviral immune responses. Failure to maintain either appropriate antiviral or antibacterial immune responses can have detrimental effects on the outcome of co-infection. This may help explain the greatly enhanced disease severity and fatality rates associated with influenza and bacterial co-infection.
In addition to enhanced bacterial outgrowth, virus-induced lung damage and loss of associated repair responses may also contribute to a lethal outcome following secondary bacterial infection (63). Moreover, it is known that at the recovery stage of influenza infection, multiple anti-inflammatory immune responses are up-regulated to restore airway epithelial integrity and lung homeostasis, including CD200-CD200R interactions (64,65), innate lymphoid cell function (66), T cell-mediated IL-10 production (67), and PGE2 expression (68). Although there is still a lack of direct evidence that these immune regulators inhibit macrophage or neutrophil antibacterial activities following influenza infection (69,70), it was found that influenza infection leads to de-sensitization of alveolar macrophage TLR signaling and susceptibility to secondary bacterial infection even long after viral clearance (48).
Recent co-infection studies have mostly focused on the mechanism responsible for influenza-induced susceptibility to secondary bacterial infection. However, it should be noted that broad pulmonary inflammatory infiltration is a key clinical feature of bacterial pneumonia. Overwhelming bacterial infection may explain widespread lung pathology at later phases of infection. However, excessive inflammation was found to be independent of pulmonary bacterial burden (20). Additionally, immunopathogenesis of influenza and pneumococcal co-infection can be directly mediated by viral virulent factors such as PB1-F2 (71). Nonetheless, excessive inflammatory responses after establishment of secondary bacterial infection comprises another difficulty for the clinical management of disease (72,73), and is likely the reason for enhanced disease severity and mortality despite appropriate antibiotic treatment.
Conclusions
Based on the various findings discussed above, it appears that an elicited adaptive immune response against viral infection (an intracellular pathogen) impairs innate immune defenses against bacterial infection (an extracellular pathogen). This would explain why secondary bacterial infections in the clinic occur at a time when the virus begins to be cleared from the lung and the patient enters the recovery stage. Although some investigators find a large decrease in total numbers of alveolar macrophages in influenza-infected lungs (74), other studies have not observed a significant reduction in numbers but rather, a modified phenotype (3,60,75). This is accompanied by a change in function of the phagocytic lung cell population from cells that mediate basal levels of innate protection through phagocytosis and production of pro-inflammatory cytokines, to cells better attuned to antigen presentation and induction of adaptive immune responses. In fact, while alveolar macrophage expression of the scavenger receptor, MARCO, is down-regulated by virus-induced IFN-γ, MHC class II expression is increased (3). Considering the fact that murine alveolar macrophages normally inhibit adaptive immune responses (76,77), their modification on day 7 of influenza infection, together with type 1 IFN-mediated inhibition of neutrophil recruitment (Fig. 2), may be a mechanism that evolved to allow enhanced induction of specific anti-influenza T cell memory in the respiratory tract, albeit at the temporary expense of innate protection against bacterial pathogens. This new paradigm should ultimately allow development of novel immune intervention strategies for the broad-spectrum prevention and management of secondary bacterial infections following influenza.
Acknowledgments
Supported by NIH grant RO1 AI075312.
References
- 1.MacCallum WG. Pathological anatomy of pneumonia associated with influenza. Johns Hopkins Hosp Rep. 1921;20:149. [Google Scholar]
- 2.Opie EL, Blake FG, Rivers TM. The pathology and bacteriology of pneumonia following influenza. In: Opie EL, Blake FG, Small JC, Rivers TM, editors. Epidemic Respiratory Disease. The Pneumonias and Other Infections of the Respiratory Tract Accompanying Influenza and Measles. C.V. Mosby; St. Louis: 1921. pp. 107–281. [Google Scholar]
- 3.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26:D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hers JF, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 8.Gill JR, Zong-Mei S, Ely SF, Guinee J, Beasley MB, Suh J, Deshpande C, Mollura DJ, Morens DM, Bray M, Travis WD, Taubenberger JK. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection - United States, April–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:941–947. [PubMed] [Google Scholar]
- 10.Cox CM, Blanton L, Dhara R, Brammer L, Finelli L. 2009 Pandemic Influenza A (H1N1) Deaths among Children - United States, 2009–2010. Clin Infect Dis. 2011;52:S69–S74. doi: 10.1093/cid/ciq011. [DOI] [PubMed] [Google Scholar]
- 11.Taubes G. The bacteria fight back. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 13.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 14.DeRyke CA, Lodise TP, Jr, Rybak MJ, McKinnon PS. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest. 2005;128:1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 15.Pavia AT. What is the role of respiratory viruses in community-acquired pneumonia? Infect Dis Clin North Am. 2013;27:157–175. doi: 10.1016/j.idc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of vaccine-induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J Virol. 2010;84:4105–4108. doi: 10.1128/JVI.02621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LN, Dias P, Han D, Yoon S, Shea A, Zakharov V, Parham D, Sarawar SR. A mouse model of lethal synergism between influenza virus and Haemophilus influenzae. Amer J Pathol. 2010;176:800–811. doi: 10.2353/ajpath.2010.090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–515. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol. 2011;186:987–993. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 22.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, vander PT, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006;193:214–222. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- 23.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler J, Breitbach K, Renner C, Heitsch AK, Bast A, van RN, Vogelgesang S, Steinmetz I. NADPH-oxidase but not inducible nitric oxide synthase contributes to resistance in a murine Staphylococcus aureus Newman pneumonia model. Microbes Infect. 2011;13:914–922. doi: 10.1016/j.micinf.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 25.van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, van der PT. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 26.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J Virol Methods. 2001;94:173–186. doi: 10.1016/s0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 27.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6:303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nugent KM, Pesanti EL. Tracheal function during influenza infections. Infect Immun. 1983;42:1102–1108. doi: 10.1128/iai.42.3.1102-1108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alymova IV, Portner A, Takimoto T, Boyd KL, Babu YS, McCullers JA. The novel parainfluenza virus hemagglutinin-neuraminidase inhibitor BCX 2798 prevents lethal synergism between a paramyxovirus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2005;49:398–405. doi: 10.1128/AAC.49.1.398-405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134:1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 31.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 32.van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Florquin S, Shimizu T, Ishii S, Jansen HM, Lutter R, van der PT. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L194–L199. doi: 10.1152/ajplung.00050.2005. [DOI] [PubMed] [Google Scholar]
- 33.McCullers JA, Iverson AR, Murray PJ. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand J Immunol. 2008;40:11–17. doi: 10.1080/00365540701477568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chockalingam AK, Hickman D, Pena L, Ye J, Ferrero A, Echenique JR, Chen H, Sutton T, Perez DR. Deletions in the neuraminidase stalk region of H2N2 and H9N2 avian influenza vrus subtypes do not affect postinfluenza secondary bacterial pneumonia. J Virol. 2012;86:3564–3573. doi: 10.1128/JVI.05809-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanks GD, Brundage JF. Pathogenic responses among young adults during the 1918 influenza pandemic. Emerg Infect Dis. 2012;18:201–207. doi: 10.3201/eid1802.102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakab GJ. Mechanisms of bacterial superinfections in viral pneumonias. Schweiz Med Wochenschr. 1985;115:75–86. [PubMed] [Google Scholar]
- 37.Nickerson CL, Jakab GJ. Pulmonary antibacterial defenses during mild and severe influenza virus infection. Infect Immun. 1990;58:2809–2814. doi: 10.1128/iai.58.9.2809-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warshauer D, Goldstein E, Akers T, Lippert W, Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977;115:269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- 39.Jakab GJ, Warr GA, Sannes PL. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980;27:960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakab GJ, Green GM. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976;57:1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nugent KM, Pesanti EL. Effect of influenza infection on the phagocytic and bactericidal activities of pulmonary macrophages. Infect Immun. 1979;26:651–657. doi: 10.1128/iai.26.2.651-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 43.Hang DTT, Choi EJ, Song JY, Kim S, Kwak J, Shin YK. Differential effect of prior influenza infection on alveolar macrophage phagocytosis of Staphylococcus aureus and Escherichia coli: involvement of interferon-γ production. Microbiol Immunol. 2011;55:751–759. doi: 10.1111/j.1348-0421.2011.00383.x. [DOI] [PubMed] [Google Scholar]
- 44.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris N, Super M, Rits M, Chang G, Ezekowitz RA. Characterization of the murine macrophage mannose receptor: demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood. 1992;80:2363–2373. [PubMed] [Google Scholar]
- 46.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol. 2010;184:2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 47.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes IFN-γ dependent neutrophil recruitment in the lung and improves protection against respiratory S. pneumoniae infection. Infect Immun. 2007;75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin RR, Couch RB, Greenberg SB, Cate TR, Warr GA. Effects of infection with influenza virus on the function of polymorphonuclear leukocytes. J Infect Dis. 1981;144:279–280. doi: 10.1093/infdis/144.3.279. [DOI] [PubMed] [Google Scholar]
- 50.Craft AW, Reid MM, Low WT. Effect of virus infections on polymorph function in children. Br Med J. 1976;1:1570. doi: 10.1136/bmj.1.6025.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramson JS, Giebink GS, Mills EL, Quie PG. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981;143:836–845. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- 52.Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84:5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;0:0. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol. 2013;87:6911–6924. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, Ho L, Sauer JD, Iwakura Y, Metzger DW, Monack DM. Type I IFN signaling constrains IL-17A/F secretion by γδ T cells during bacterial infections. J Immunol. 2010;184:3755–3767. doi: 10.4049/jimmunol.0902065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of γδ T Cells. J Virol. 2012;86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6Chi monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Zou Z, Yan Y, Wang W, Wang C, Chen Z, Xing L, Tang C, Ju X, Guo F, Deng J, Zhao Y, Yang P, Tang J, Wang H, Zhao Z, Yin Z, Cao B, Wang X, Jiang C. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kash JC, Walters KA, Davis AS, Sandouk A, Schwartzman LM, Jagger BW, Chertow DS, Li Q, Kuestner RE, Ozinsky A, Taubenberger JK. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. mBio. 2011;2:1–9. doi: 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 65.Rygiel TP, Rijkers ES, de RT, Stolte EH, d van V, Rimmelzwaan GF, Boon L, van Loon AM, Coenjaerts FE, Hoek RM, Tesselaar K, Meyaard L. Lack of CD200 enhances pathological T cell responses during influenza infection. J Immunol. 2009;183:1990–1996. doi: 10.4049/jimmunol.0900252. [DOI] [PubMed] [Google Scholar]
- 66.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizumura K, Hashimoto S, Maruoka S, Gon Y, Kitamura N, Matsumoto K, Hayashi S, Shimizu K, Horie T. Role of mitogen-activated protein kinases in influenza virus induction of prostaglandin E2 from arachidonic acid in bronchial epithelial cells. Clin Exp Allergy. 2003;33:1244–1251. doi: 10.1046/j.1365-2222.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- 69.Hussell T, Cavanagh MM. The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochem Soc Trans. 2009;37:811–813. doi: 10.1042/BST0370811. [DOI] [PubMed] [Google Scholar]
- 70.Snelgrove RJ, Godlee A, Hussell T. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 2011;32:328–334. doi: 10.1016/j.it.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 71.McAuley JL, Hornung F, Boyd KL, Smith AM, McKeon R, Bennink J, Yewdell JW, McCullers JA. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karlstrom A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis. 2009;199:311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCullers JA, English BK. Improving therapeutic strategies for secondary bacterial pneumonia following influenza. Future Microbiol. 2008;3:397–404. doi: 10.2217/17460913.3.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial super-infections. J Immunol. 2013 doi: 10.4049/jimmunol.1300014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 76.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]