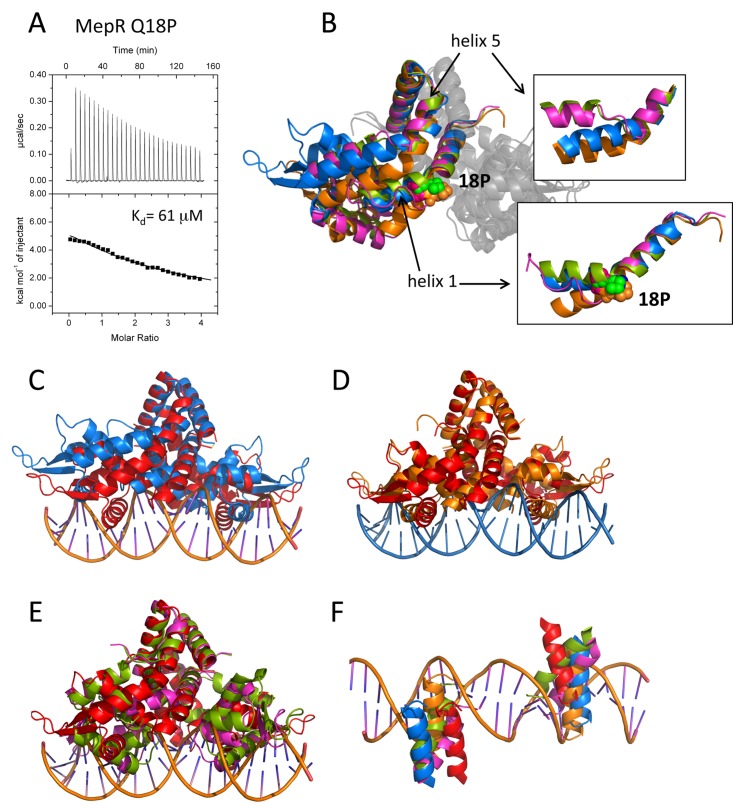
FIG 4 .
The effect of the Q18P substitution on the structure and function of MepR. (A) Titrations of mepR operator DNA with MepR(Q18P). The solid lines represent theoretical fits to the experimental data (closed squares); fitting parameters are provided in Table 1. (B) The overlay of the four MepR(Q18P) dimers located in the asymmetric unit after alignment of their dimerization domains. Only one monomer of each dimer is shown in color. P18 is shown as appropriately colored spheres. The insets show the effects of Q18P mutation on helix 1 and helix 5, which are indicated by black arrows. (C to E) The alignment of the WT MepR-DNA model (WT MepR is shown in red) with each of the four MepR(Q18P) conformational variants (shown in blue, green, orange, and magenta). The structures were aligned as in panel B. (F) The locations of the recognition helices of the four MepR(Q18P) conformational variants and the WT MepR protein as they would be found in consecutive major grooves of B-DNA. The color code is the same as in panels C to E. A more detailed description of the structural distortions of MepR helices 1 and 5 effected by the Q18P mutation is provided in Text S2 and Fig. S4 in the supplemental material.