Abstract
The tree-like architecture of the mammary gland is generated by branching morphogenesis, which is regulated by many signals from the microenvironment. Here we examined how signaling downstream of phosphoinositide 3-kinase (PI3K) regulates different steps of mammary branching using three-dimensional culture models of the mammary epithelial duct. We found that PI3K was required for both branch initiation and elongation. Activated Akt was enhanced at branch initiation sites where its negative regulator, PTEN, was blocked by signaling via Sprouty2 (SPRY2); inhibiting Akt prevented branch initiation. The pattern of SPRY2 expression, and thus of Akt activation and branch initiation, was controlled by mechanical signaling from endogenous cytoskeletal contractility. In contrast, activated GTP-bound Rac1 localized to the leading edge of nascent branches and was required for branch elongation. These data suggest that the PI3K network integrates mechanical and biochemical signaling to control branching morphogenesis of mammary epithelial cells.
Keywords: Morphodynamics, Patterning, Mechanical stress
Introduction
Ramified epithelia such as the mammary gland develop via branching morphogenesis, which generates functionally efficient, complex, but well-ordered tissue architectures (Hogan, 1999). Epithelial branching morphogenesis is a reiterative process that can be divided into several unique steps, including primary bud formation, branch initiation, and branch elongation (Affolter et al., 2003; Zhu and Nelson, 2012). During branching of the murine mammary gland, terminal end buds (TEBs) form at the tips of the ducts and invade into the surrounding stroma in response to stimulation from ovarian hormones. New primary branches then form through bifurcation of the TEBs and secondary side branches sprout laterally from the primary ducts. This process is reiterated until the entire mammary fatpad is filled with the epithelial tree (Sternlicht, 2006).
Branching of an epithelium typically arises through changes in cell shape, size, division, motility, invasiveness, or combinations thereof (Affolter et al., 2003; Zhang and Vande Woude, 2003; Zhu and Nelson, 2012). These cellular level processes are regulated by different signals including hormones, growth factors, receptor tyrosine kinases (RTKs), extracellular matrix (ECM) molecules, and endogenous mechanical stress (Gjorevski and Nelson, 2011;Metzger and Krasnow, 1999). The phosphoinositide 3-kinase (PI3K)/Akt signaling network can be activated by several RTKs to regulate a wide array of cellular functions (Martelli et al., 2006). Upon activation by growth factor stimulation, PI3K produces the lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P2, PIP3), which in turn binds to the serine/threonine protein kinase Akt (Matsui et al., 2003), recruiting it to the membrane for subsequent activation by phosphorylation. Akt regulates a variety of transcription factors as well as several cytoplasmic proteins (Manning and Cantley, 2007). For example, Akt promotes cell invasion by increasing the production of matrix metalloproteinase-9 (MMP9) (Kim et al., 2001) and modulates cell migration by phosphorylating p21-activated kinase (PAK) (Zhou et al., 2003).
In normal tissue, PI3K signaling can be attenuated by PTEN, a ubiquitously expressed tumor suppressor protein that dephosphorylates PIP3 into PIP2 (Polak and Buitenhuis, 2012). PTEN is subject to regulation at the transcriptional level, as well as at the post-translational level by phosphorylation (Tamguney and Stokoe, 2007). The phosphorylated carboxy terminal tail of PTEN interacts with its catalytic domain, serving as a pseudosubstrate and therefore causing auto-inhibition (Odriozola et al., 2007;Tamguney and Stokoe, 2007). One of the proteins that regulates PTEN post-translationally is Sprouty2 (SPRY2), leads to dephosphorylation of PTEN at several sites, increasing its activity and thereby decreasing PI3K signaling (Edwin et al., 2006).
This regulation of the PI3K pathway is potentially relevant for branching morphogenesis. SPRY2 encodes an RTK inhibitor (Hacohen et al., 1998; Kramer et al., 1999), and was first identified as a novel antagonist of fibroblast growth factor (FGF) signaling during development of the Drosophila trachea. In SPRY mutants, overactivation of the FGF pathway induces ectopic branching from the stalks of the primary trachea (Hacohen et al., 1998). SPRY2 similarly acts as a negative regulator of branching morphogenesis in several other organs. Targeted overexpression of SPRY2 in mouse peripheral lung epithelium using the surfactant protein-C (SP-C) promoter reduces branching and inhibits epithelial proliferation (Mailleux et al., 2001). Conversely, transiently reducing the levels of SPRY2 in cultured mouse lung explants results in a dramatic increase in branching as well as in the expression of lung epithelial marker genes (Tefft et al., 1999). Consistently, tissue-specific overexpression of SPRY2 in the ureteric bud causes severe defects in kidney development including a reduction in organ size owing to inhibition of ureteric branching (Chi et al., 2004). SPRY2 is thus considered as a negative regulator of branching morphogenesis (Mailleux et al., 2001; Tefft et al., 1999) and is well poised to modulate PI3K signaling via PTEN.
Whereas the PI3K pathway has also been implicated in branching of the mammary gland (Li et al., 2002; Renner et al., 2008;Utermark et al., 2012), it remains unclear what downstream signals are involved in the regulation of the different steps of the branching process. Conditional deletion of PTEN in the mouse mammary gland via the use of MMTV-Cre leads to accelerated ductal extension and enhanced lateral branching during puberty (Li et al., 2002). Similarly, MMTV-mediated transgenic expression of an activated myristoylated p110α protein, the catalytic subunit of PI3K, leads to increased ductal branching (Renner et al., 2008). In addition to Akt, PI3K activates Rac1 (Kolsch et al., 2008), which promotes the formation of lamellipodia (Ridley et al., 1992) and induces the expression of various MMPs (Mack et al., 2011), which are themselves required for mammary branching morphogenesis (Fata et al., 2007; Simian et al., 2001; Wiseman et al., 2003). PI3K is thus considered to be a positive regulator of branching by mammary epithelial cells (Zhu and Nelson, 2012).
Here we used organotypic culture models to characterize how signaling downstream of PI3K regulates the branch initiation and extension steps of mammary epithelial branching morphogenesis. We found that Akt is required for branch initiation downstream of growth factor signaling, and its activity is controlled by the levels of phosphorylated PTEN. In contrast, activated Rac1 found at the tips of the branches is required for branch elongation. Phosphorylated PTEN and Akt are regulated by endogenous mechanical stress, which signals through SPRY2. Therefore, the PI3K pathway couples mechanical signaling and biochemical signaling in its regulation of branching morphogenesis by mammary epithelial cells.
Materials and methods
Cell culture and reagents
Functionally normal EpH4 mouse mammary epithelial cells were cultured in DMEM/F12 growth medium (Hyclone) supplemented with 2% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals), 50 μg/ml gentamicin (Sigma), and 5 μg/ml insulin (Sigma). NMuMG mouse mammary epithelial cells (ATCC) were cultured in DMEM supplemented with 10% FBS, 50 μg/ml gentamicin, and 10 μg/ml insulin. EpH4 and NMuMG cells have been widely used in studies of mammary epithelial branching morphogenesis. Cells were grown in a 37 °C incubator with 5% CO2 and treated with the following reagents diluted to the concentrations indicated: LY294002 (50 μM; Cell Signaling); wortmannin (1 μM; Cell Signaling); Akt inhibitor IV (5-(2-benzothiazolyl)-3-ethyl-2-[2-(methylphenylamino)ethenyl]-1-phenyl-1 H-benzimidazolium iodide, 20 μM; EMD Millipore); Y27632 (10 μM; Tocris); blebbistatin (25 μM; Sigma); calyculin A (0.5 nM; Calbiochem); NSC23766 (100 μM; Tocris).
shRNA and expression constructs
shRNAs targeting the Mus musculus sequence of SPRY2 (NM_011897) were purchased from Open Biosystems (Table S1). Control scrambled shRNA (Sarbassov et al., 2005), constitutively active PI3K plasmid pCAG-Myr-p110-IH (Takahashi et al., 2003), and control plasmid pCAGEN (Matsuda and Cepko, 2004) were obtained from AddGene.
Transfections
One day before transfection, cells were seeded in 10-cm dishes at 60–70% confluence. The following day, transfections were performed using 10 μg of each relevant plasmid and SuperFect Transfection Reagent (Qiagen).
Preparation and branching of cell clusters
Clusters of mouse mammary epithelial cells were prepared by overnight shaking (180 rpm at 37 °C for 15 h) in the presence of 0.083% (w/v) of pluronic F108 (BASF) as previously described (Lee et al., 2011). Cell clusters ~100 μm in diameter were collected by centrifugation (200 rpm for 1 min) and embedded within 4 mg/ml of non-pepsinized native type I collagen (Koken), which was gelled as described previously (Pavlovich et al., 2011). A cell-free layer of collagen was placed underneath the layer containing clusters. Growth medium including no growth factor, EGF (4.2 nM, Invitrogen), or HGF (4.2 nM, Sigma) was added to the samples to induce branching.
Engineered mammary epithelial tubules
Elastomeric stamps of poly(dimethylsiloxane) (PDMS; Sylgard 184, Ellsworth Adhesives) containing a relief of the desired tissue geometry were fabricated using a combination of photolithography and soft lithography (Gomez and Nelson, 2011; Nelson et al., 2008). Briefly, stamps were rendered non-adhesive by coating with 1% (w/v) bovine serum albumin in phosphate-buffered saline (PBS). Stamps were placed atop a drop of collagen, which was then gelled as described above. After removing the stamps, a suspension of mammary epithelial cells was allowed to settle within the molded collagen cavities. Any extra cells were washed away with growth medium and a gelled collagen lid was placed on top of the sample. The epithelial cells formed cylindrical tissues of the shape and size of the collagen cavities, and remained quiescent until induced to branch by addition of medium containing EGF or HGF.
Imaging and immunofluorescence analysis
The branching pattern of microfabricated tubules was measured as described previously (Nelson et al., 2006). Briefly, samples were fixed in 4% paraformaldehyde for 15 min, stained for nuclei using Hoechst 33342 (Invitrogen), and visualized using a Hamamatsu Orca CCD camera attached to a Nikon Eclipse Ti microscope. The binarized images of 50 tubules were stacked with ImageJ software to obtain a pixel frequency map, which was subsequently color-coded in Adobe Photoshop.
For immunofluorescence analysis, samples were fixed, permeabilized (0.5% Igepal CA-630 for 10 min, then 0.1% Triton X-100 in PBS for 15 min), and blocked with 10% goat serum (Atlanta Biologicals) overnight. Samples were then incubated with primary antibodies against pAkt (Cell Signaling), pPTEN (Sigma), or cleaved caspase-3 (Cell Signaling), followed by incubation with Alexa Fluor-conjugated secondary antibodies (Invitrogen). Signal intensity as a function of spatial location was measured by taking a line scan through the midline of each tubule, and the end/middle ratio was calculated by dividing the signal intensity at the two ends by the signal intensity in the middle.
To visualize active GTP-bound Rac1, samples were fixed and permeabilized as described above. After blocking with 10% goat serum, the tissues were incubated with GST-PAK-PBD (Cytoskeleton) (final concentration 2.5 μg/100 μl of blocking solution) overnight, followed by rabbit anti-GST antibody (Sigma) and secondary goat anti-rabbit antibody.
Immunoblotting
Whole cell lysates were prepared from mammary epithelial cells transfected with pCAG-Myr-p110-IH or pCAGEN control (AddGene) using RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 50 mM Tris, pH 8.0, and protease inhibitor cocktail). Immunoblot analysis was performed using antibodies against p110α (Cell Signaling), pAkt (Cell Signaling), Akt (Cell Signaling), pPTEN (Sigma), PTEN (Sigma), SPRY2 (Abcam) or β-actin (Cell Signaling).
Statistical analysis
All experiments were conducted independently at least three times. The Student’s t-test was used to compare branch lengths and the percentage of branching clusters between conditions. P values < 0.05 were considered to be statistically significant.
Results
The PI3K pathway is required for branching morphogenesis of mammary epithelial cells
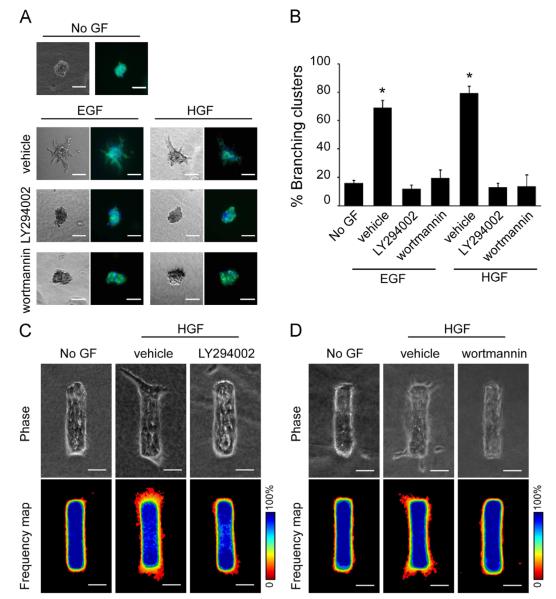
We used two complementary three-dimensional (3D) culture models to recapitulate the mammary epithelium and investigate the role of the PI3K pathway in the multi-step process of branching morphogenesis. In the first model, clusters of EpH4 mouse mammary epithelial cells were embedded within 3D ECM gels of type I collagen (Chen et al., 2009; Hirai et al., 1998; Lee et al., 2011;Pavlovich et al., 2011; Simian et al., 2001). Robust branching morphogenesis occurred after the addition of epidermal growth factor (EGF) or hepatocyte growth factor (HGF) (Fig. 1A and B). These branches were multicellular in morphology and extended up to 100 μm into the surrounding collagen gel, as reported by several previous studies using this system (Chen et al., 2009; Hirai et al., 2007; Lee et al., 2011; Mori et al., 2013; Simian et al., 2001). Strikingly, we observed that branching was completely blocked in the presence of 50 μM LY294002 or 1 μM wortmannin (Fig. 1A and B), both of which are potent inhibitors of PI3K (Walker et al., 2000). We observed similar results in clusters of NMuMG mouse mammary epithelial cells (Fig. S1), a widely used model for the mammary epithelium (Owens et al., 1974).
Fig. 1.
Branching morphogenesis induced by EGF or HGF is blocked by PI3K inhibitors. (A) Clusters of mammary epithelial cells were embedded in collagen gel and treated with no growth factor (No GF), EGF or HGF with or without PI3K inhibitors and monitored for branching after 24 h. Shown are phase contrast and fluorescence images depicting nuclei (blue) and actin (green). Scale bars, 100 μm. (B) Branching in the cluster assay was quantified by counting the percentage of clusters that branched in each well. *Indicates p<0.05 compared with No GF control. (C, D) Mammary epithelial tubules were treated with No GF or HGF with or without LY294002 (C) or wortmannin (D) for 24 h. Shown are phase contrast images and frequency maps of 50 tubules stained for nuclei. Scale bars, 50 μm. Color bars indicate frequency.
In the second 3D culture system, mammary epithelial tubules of specific and reproducible pre-defined geometries were engineered within collagen gels using replica micromolding (Gjorevski and Nelson, 2010, 2012; Lee et al., 2011; Nelson et al., 2006, 2008;Pavlovich et al., 2011). When treated with HGF, branches formed specifically at the ends of cylindrical tubules (Fig. 1C and D). Consistent with the results in 3D clusters, branching morphogenesis of the engineered tubules was blocked in the presence of LY294002 or wortmannin (Fig. 1C and D). Together, these results suggest that the PI3K pathway is necessary for mammary epithelial cells to branch in response to either EGF or HGF.
Inhibiting Akt blocks branching morphogenesis of mammary epithelial cells
The PI3K pathway regulates divergent physiological processes that include cell proliferation, growth, differentiation, apoptosis, metabolism, and motility (Martelli et al., 2006). Many of these functions relate to the ability of PI3K to lead to activation of the serine/threonine kinase, Akt (Engelman, 2009). Akt thus acts as a critical signaling node downstream of PI3K.
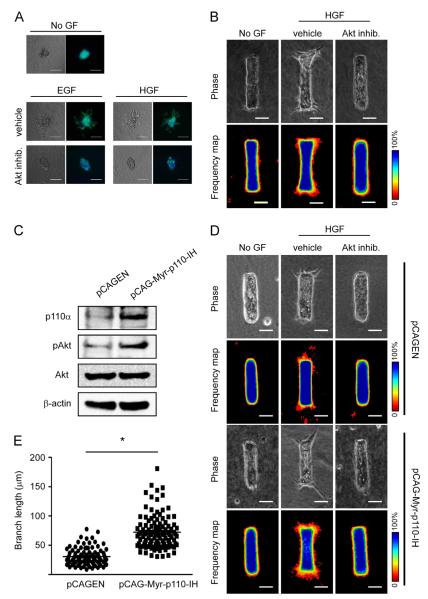
To explore the role of downstream effectors of PI3K in the regulation of branching of mammary epithelial cells, we first disrupted the activity of Akt using a benzimidazole-derivative pharmacological inhibitor. Branching morphogenesis was evaluated using both the 3D cluster and engineered tubule assays, as described above. In both assays, branching was blocked in the presence of the Akt inhibitor (Fig. 2A and B; Fig. S1). To confirm that PI3K regulates branching of mammary epithelial cells via Akt, we expressed ectopically an activated form of the p110α subunit of PI3K while simultaneously disrupting Akt activity with the pharmacological inhibitor. Expression of activated p110α was confirmed by immunoblotting analysis (Fig. 2C). Surprisingly, expression of activated p110α was not sufficient, by itself, to induce branching in the absence of treatment with growth factors (Fig. 2D). However, when induced to branch, tubules expressing activated p110α extended branches that were significantly longer than those of controls (Fig. 2E). The initiation of these branches was completely blocked when tubules expressing activated p110α were treated with the Akt inhibitor (Fig. 2D). These data suggest that activation of Akt is required for PI3K-mediated branching morphogenesis of mammary epithelial cells.
Fig. 2.
Inhibiting Akt blocks branching morphogenesis of mammary epithelial cells. (A) Clusters of mammary epithelial cells were embedded in collagen gel and treated with no growth factor (No GF), EGF or HGF with or without Akt inhibitor (20 μM) and monitored for branching after 24 h. Shown are phase contrast and fluorescence images depicting nuclei (blue) and actin (green). Scale bars, 100 μm. (B) Mammary epithelial tubules were treated with No GF or HGF with or without Akt inhibitor for 24 h. Shown are phase contrast images and frequency maps of 50 tubules stained for nuclei. Scale bars, 50 μm. Color bars indicate frequency. (C) Mammary epithelial cells were transfected with pCAGEN control vector or pCAG-Myr-p110-IH plasmid. Total protein was assessed by immunoblotting to determine the expression levels of p110α, pAkt, total Akt, and β-actin. (D) Transfected mammary epithelial cells were used to form tubules, which were treated with No GF or HGF with or without Akt inhibitor for 24 h. Shown are phase contrast images and frequency maps of 50 tubules stained for nuclei. Scale bars, 50 μm. Color bars indicate frequency. E. Quantification of branch lengths from engineered tubules transfected with pCAGEN or pCAG-Myr-p110-IH plasmid. *p<0.05.
Akt is activated by HGF and phosphorylated Akt (pAkt) is sustained at future branch sites
One advantage of the engineered tubule assay is that the initial geometry of the tubules is tightly controlled and the positions at which they branch are highly predictable and reproducible. This predictability permits examination of the signals that influence branching within the tissues at time points before branches have initiated. In the cylindrical tubules, branches initiate specifically at the two ends. That the Akt inhibitor completely blocked branching led us to examine the spatial distribution of activated Akt, which is characterized by phosphorylation at its carboxy terminal tail (Yang et al., 2002). Immunofluorescence analysis revealed that the phosphorylation of Akt increased rapidly and homogeneously throughout the mammary epithelial tubules within 30 min after treatment with HGF (Fig. 3A). At 2 h after induction with HGF, the pAkt signal in the body of the tubules had decreased, whereas the signal at the ends of the tubules was sustained (Fig. 3A and B). By contrast, total Akt was evenly distributed throughout the tubules (Fig. 3C). At this time point, we also observed that cells at the ends of the tubules had formed lamellipodia that projected out into the surrounding collagen (Fig. 3A, arrow). Thereafter, the pAkt signal gradually decreased and had reduced to background levels by 4 h after addition of growth factor (Fig. 3A).
Fig. 3.
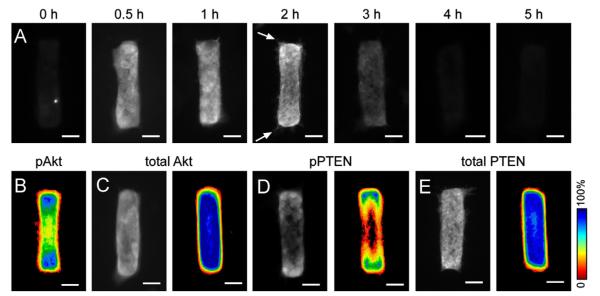
pAkt and pPTEN are sustained at future branch sites. (A) Tubules were treated with HGF for different time periods and stained for pAkt. Scale bars, 50 μm. (B) Frequency map of 50 tubules stained for pAkt after 2 h of treatment with HGF. (C-E) Immunofluorescence images and frequency maps of 50 tubules stained for total Akt (C), phosphorylated PTEN (D) or total PTEN (E) after 2 h of treatment with HGF. Scale bars, 50 μm. Color bars indicate frequency.
PTEN is the primary inhibitory regulator of Akt phosphorylation and the PI3K pathway. Phosphorylation of PTEN decreases its membrane localization and activity (Tamguney and Stokoe, 2007). To investigate how the spatial distribution of pAkt is regulated, we examined the levels of phosphorylated PTEN (pPTEN) in the tubules. We found that pPTEN was also enhanced in the cells located at the ends of the tubules (Fig. 3D), which was similar to the spatial distribution of pAkt. In contrast, total PTEN showed an even distribution within the tubules (Fig. 3E). These data suggest that a local reduction in PTEN activity at the ends of the tubules leads to enhanced phosphorylation and activation of Akt, thus permitting branch initiation.
High mechanical stress at the ends of the tubules sustains activation of the PI3K pathway
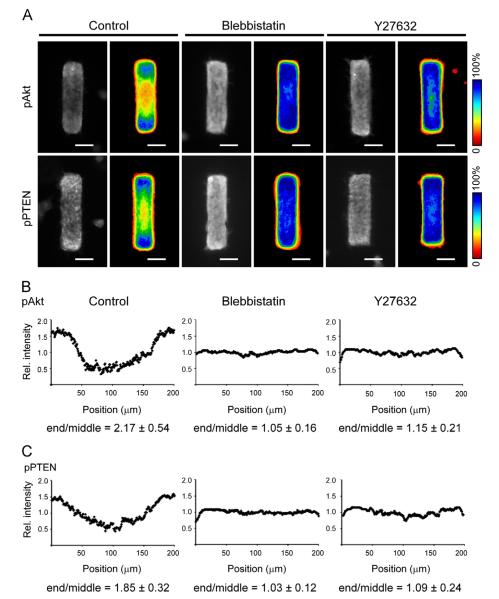
The mechanical properties of the microenvironment have been shown to alter activation of the PI3K pathway (Hoshino et al., 2007; Rice et al., 2010). Shear stress activates Akt and Akt-related signaling proteins in vascular smooth muscle cells and this activation is closely correlated with shear-induced vascular smooth muscle cell reorientation (Rice et al., 2010). When bovine aortic endothelial cells are exposed to cyclic strain, the levels of pPTEN and pAkt increase in a time-dependent manner (Hoshino et al., 2007). We found previously that mechanical stress is distributed non-uniformly across engineered epithelial tissues, with higher stress concentrated at the ends of the tubules (Gjorevski and Nelson, 2010, 2012). These observations led us to hypothesize that the high mechanical stress at the ends of the tubules was responsible for the locally sustained activation of the PI3K pathway. To explore this possibility, we modulated cytoskeletal contractility to perturb the endogenous mechanical stress profile within the tissue. Contractility of the actin cytoskeleton is enhanced by signaling through the Rho effector, Rho kinase (ROCK) and phosphorylation of myosin light chain. Treatment with either Y27632, which inhibits the activity of ROCK (Davies et al., 2000), or with blebbistatin, a small molecule inhibitor of non-muscle myosin II (Kovacs et al., 2004), reduce actomyosin-mediated contractility and thus mechanical stress at the ends of the engineered tubules (Gjorevski and Nelson, 2010). In vehicle-treated control samples, we observed elevated levels of pAkt and pPTEN at the ends of the tubules (Fig. 4A–C). However, treatment with either blebbistatin or Y27632 led to a homogenous distribution of both pAkt (Fig. 4A and B) and pPTEN (Fig. 4A and C) with the tubules. Conversely, increasing contractility with the myosin light chain phosphatase inhibitor calyculin A (Ishihara et al., 1989) led to an increase in the relative levels of pAkt at the ends of the tubules (Fig. S2). These data suggest that the spatial distributions of pAkt and pPTEN are regulated in part by endogenous mechanical stress within the epithelial tissues.
Fig. 4.
Spatial distribution of pAkt and pPTEN is affected by altering cellular contractility. (A) Mammary epithelial tubules were treated with HGF with or without blebbistatin or Y27632 for 2 h. Shown are immunofluorescence images and frequency maps of 50 tubules stained for pAkt and pPTEN. Scale bars, 50 μm. Color bars indicate frequency. (B, C) Average line scan of intensity of pAkt (B) or pPTEN (C) along the tubules. P<0.0001 for blebbistatin or Y27632 as compared to control.
The PI3K pathway is negatively regulated by SPRY2 at branch sites during branching morphogenesis of mammary epithelial cells
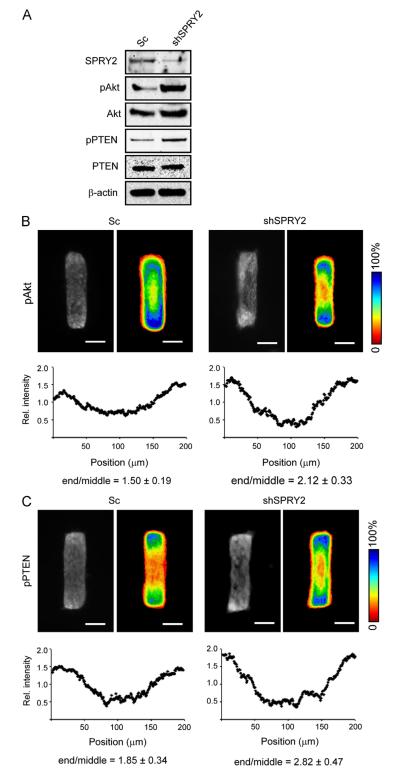
Previous studies have suggested that SPRY2 negatively regulates branching morphogenesis of the Drosophila trachea and several mammalian organs (Hacohen et al., 1998; Mailleux et al., 2001; Tefft et al., 1999). We were able to confirm a similar inhibitory role for SPRY2 in our 3D culture model. Small hairpin RNA (shRNA)-mediated knockdown of SPRY2 (shSPRY2) in mouse mammary epithelial cells led to ectopic and unpatterned branching along the entire length of the tubules (Fig. S3). SPRY2 has been reported to lead to dephosphorylation of the inhibitory phosphates on the carboxy terminus of PTEN, thereby increasing PTEN levels and activity (Edwin et al., 2006). Indeed, we found that shRNA-mediated knockdown of SPRY2 increased the levels of pPTEN and pAkt (Fig. 5A). We therefore sought to determine whether SPRY2 affects the spatial distribution of pPTEN and pAkt within the 3D engineered tissues. Tubules comprised of mammary epithelial cells expressing shSPRY2, but not scrambled shRNA, showed higher relative levels of pAkt and pPTEN when treated with HGF (Fig. 5B and C). We obtained similar results using a second set of SPRY2-specific shRNAs (Fig. S4). These results suggest that SPRY2 blocks mammary epithelial branching morphogenesis in part by activating PTEN and thus inhibiting PI3K signaling along the trunk of the tissue.
Fig. 5.
Loss of SPRY2 leads to elevated levels of pPTEN and pAkt. (A) Mammary epithelial cells were transfected with scrambled shRNA (Sc) or shRNA targeting SPRY2 (shSPRY2). Levels of total and phosphorylated Akt and PTEN were analyzed by immunoblot. (B, C) Mammary epithelial cells were transfected with Sc or shSPRY2 and used to generate mammary epithelial tubules. Tubules were treated with HGF for 2 h and stained for pAkt (B) or pPTEN (C). Scale bars, 50 μm. Color bars indicate frequency. P<0.0001 for shSPRY2 as compared to Sc control.
Inhibiting Rac1 blocks branch extension
Our data revealed that Akt was activated soon after growth factor stimulation to promote branch initiation, and that the pAkt signal reduced to background levels after about 4 h, suggesting that signaling through effectors other than Akt were responsible for branch elongation. In addition to Akt, Rac1 has been implicated as a key effector downstream of PI3K (Kolsch et al., 2008). To investigate the role of Rac1 in the regulation of mammary branching by PI3K, we treated mammary epithelial tubules with the Rac inhibitor NSC23766, which prevents Rac1 activation by Rac-specific guanine nucleotide exchange factors (GEFs) without affecting the activation of Cdc42 or RhoA (Nassar et al., 2006). Treatment with NSC23766 did not completely block branching from the tubules, but instead significantly reduced the lengths of the branches that formed (Fig. 6A). We obtained similar results using the 3D cluster assay (Fig. 6B and C; Fig. S1). These data suggest that activation of Rac1 is required for growth factor-stimulated branch extension, but not branch initiation.
Fig. 6.
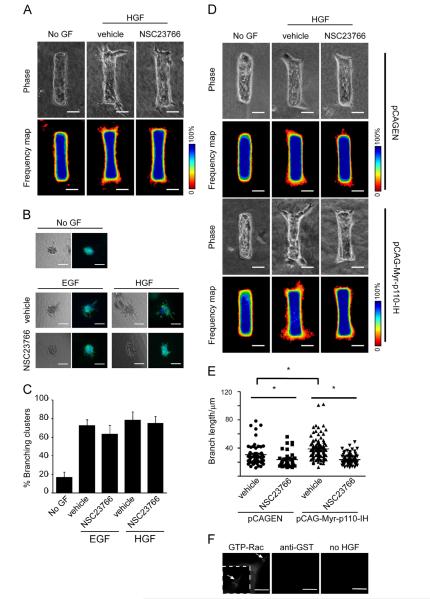
Inhibiting Rac1 activity blocks branch extension. (A) Mammary epithelial tubules were treated with no growth factor (No GF) or HGF with or without the Rac1 inhibitor NSC23766 for 24 h. Shown are phase contrast images and frequency maps of 50 tubules stained for nuclei. Scale bars, 50 μm. Color bars indicate frequency. (B) Clusters of mammary epithelial cells were embedded in collagen gel and treated with No GF, EGF or HGF with or without NSC23766 and monitored for branching after 24 h. Shown are phase contrast and fluorescence images depicting nuclei (blue) and actin (green). Scale bars, 100 μm. (C) Branching in the cluster assay was quantified by counting the percentage of clusters that branched in each well. (D) Mammary epithelial cells were transfected with pCAGEN control vector or pCAG-Myr-p110-IH plasmid. Transfected cells were used to form tubules, which were treated with No GF or HGF with or without NSC23766 for 24 h. Shown are phase contrast images and frequency maps of 50 tubules stained for nuclei. Scale bars, 50 μm. Color bars indicate frequency. (E) Quantification of branch length from engineered tubules transfected with pCAGEN or pCAG-Myr-p110-IH vector and treated with or without NSC23766. *P<0.05. (F) Staining for activated Rac1. Shown is active GTP-bound Rac1 in HGF-treated, untreated, and GST-only control samples. Scale bars, 50 μm.
Our experiments showed that inhibiting Akt prevented branching completely whereas inhibiting Rac1 permitted branch initiation but prevented branch extension. These findings, along with the evidence that the activation of Akt reduced to background levels within 4 h of growth factor stimulation, led us to hypothe size that PI3K regulates branch extension through Rac1. To test this hypothesis directly, we transfected activated p110α into mammary epithelial cells while simultaneously blocking Rac activity using NSC23766. In the presence of HGF, expression of activated p110α resulted in longer branches than control plasmid-transfected cells (Fig. 6D and E). However, this effect was blocked by treatment with NSC23766 (Fig. 6D and E). These data suggest that activated p110α induces branch extension through Rac1.
Activated Rac1 is found at the leading edge of cells in the elongating branches
As shown by many studies, PI3K signaling through Rac1 is important for cell motility and invasion (Ridley et al., 1992). Rac1 has been implicated in establishing and maintaining the leading edge of motile cells, and Rac1 is dynamically activated at specific locations in the extending leading edge in different cell types including neutrophils and T cells (Cernuda-Morollon et al., 2010;Gardiner et al., 2002). However, no studies have reported the localization of activated Rac1 within morphogenetic tissues. Activated GTP-bound Rac1 can be detected in whole cell lysates by virtue of its ability to associate with the p21-binding domain (PBD) of the Rac1 effector protein PAK. To examine the spatial localization of activated Rac1 during branch extension, we took advantage of a GST-PAK-PBD fusion protein which only recognizes GTP-bound Rac1 (Cernuda-Morollon et al., 2010; Joffre et al., 2011). This in situ analysis revealed that Rac1 was activated at the tips of the cells leading the branches (Fig. 6F), consistent with a requirement for the activation of this GTPase during branch extension. These data thus suggest that activated Rac1 is spatially positioned to mediate PI3K-induced branch extension.
Discussion
Here we investigated how the PI3K pathway regulates different steps of branching morphogenesis of mammary epithelial cells in culture. Our results suggest that PI3K regulates branch initiation and extension through distinct pathways (Fig. 7). High mechanical stress leads to sustained levels of phosphorylated activated Akt at future branch sites, and this activation is required for branch initiation. In contrast, Rac1 is activated at the leading edge of nascent branches and required for branch extension. The levels of pAkt are controlled by pPTEN, which in turn is regulated by mechanical signaling via SPRY2. Manipulating PI3K, Akt, Rac1, or SPRY2 thus alters branching morphogenesis of cultured mammary epithelial cells by tuning branch initiation or extension without inducing major alterations in cell death (Fig. S5).
Fig. 7.
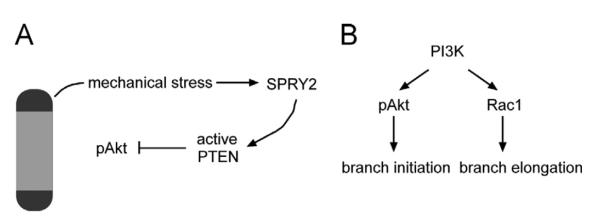
Roles of Akt, Rac1, PTEN, and SPRY2 in mammary epithelial branching morphogenesis. (A) pAkt at the ends of the tubules is required for branch initiation. In contrast, Rac1 is activated at the leading edge of nascent branches and required for branch extension. (B) High mechanical stress leads to high levels of SPRY2 at future branch sites, which leads to dephosphorylation and activation of PTEN along the trunk of the tissue. This in turn results in less pAkt in the body of the tubules, thus blocking ectopic branching from this location.
The PI3K pathway has also been implicated in the regulation of branching morphogenesis of several other epithelial tissues. In a study which used the PTENloxp/loxp; SP-C-rtTA+/−; TetO-Cre+/− mice, loss of PTEN resulted in impaired branching morphogenesis of the airways of the lung (Yanagi et al., 2007). Organ cultures of the ureteric bud taken from E10.5 mouse embryos revealed that inhibiting PI3K, but not MEK1 or p38 MAPK, completely blocks GDNF-dependent outgrowth, which suggests that induction of the ureteric bud requires PI3K (Tang et al., 2002). PI3K is also essential for branching after the ureteric bud has invaded the metanephric mesenchyme, since LY294002 inhibits branching in E11.5 metanephric kidneys (Tang et al., 2002). It is interesting to note that in our 3D culture models, the PI3K pathway is necessary but not sufficient to induce branching. In the absence of growth factors, we did not observe any branching in the tubules comprised of cells ectopically expressing activated p110α. However, in the presence of growth factors, activated p110α induced the extension of significantly longer branches. Transgenic mice overexpressing PI3K in the mammary gland also do not display excessive ductal branching until stimulated by hormones at puberty (Renner et al., 2008), even though complete knockout of p110α significantly blocks mammary branching and ductal extension (Utermark et al., 2012). It is therefore likely that, in parallel to PI3K, other growth factor-induced signaling pathways are required to induce branching morphogenesis of mammary epithelial cells.
Our data suggest an important link between PI3K signaling and SPRY2 expression in the regulation of branching morphogenesis of mammary epithelial cells. SPRY proteins are well appreciated as antagonists of FGF signaling during branching morphogenesis of the Drosophila tracheal system (Hacohen et al., 1998; Kramer et al., 1999). In that organ, SPRY is expressed in the tip cell (the actively branching cell) and antagonizes FGF-induced signaling in the adjacent stalk cells, thereby inhibiting ectopic secondary branching (Hacohen et al., 1998). Here, we found a similar pattern of expression in mammary epithelial tubules, with high levels of SPRY2 in cells located at future branch sites. We also found a similar inhibitory phenotype, although in this case, SPRY2 at branch sites activates PTEN in the trunk of the tubules (analogous to the stalk cells), thus leading to inhibition of Akt in those cells and preventing ectopic branching. How SPRY2 accomplishes this non-cell autonomous inhibition of branching in any organ remains a mystery, but our data suggest its reach is wider than the MAPK pathway previously defined. Indeed, SPRY2 was recently shown to signal through both MAPK and PI3K in the prostate epithelium (Schutzman and Martin, 2012).
Since the initial identification of SPRY in Drosophila, four mammalian SPRY proteins (SPRY1–4) have been discovered based on sequence similarity (Edwin et al., 2009; Guy et al., 2009). The expression of SPRY1, 2 and 4 is ubiquitous in both embryos and adults, whereas SPRY3 is restricted to the brain and testes (Leeksma et al., 2002; Minowada et al., 1999; Panagiotaki et al., 2010). Though evidence suggests that RTK signaling regulates the expression levels of SPRY2 (Cabrita and Christofori, 2008), it is not clear whether other types of regulation are involved. Our data suggest a novel and prominent role for endogenous mechanical stress. Indeed, we found that SPRY2 expression increases in the presence of calyculin A, which enhances cytoskeletal contractility, and decreases in the presence of blebbistatin, which reduces contractility (Fig. S6). Although mechanical stress is well appreciated to regulate signal transduction and gene expression in several tissues, its effects on SPRY proteins are only beginning to be uncovered. SPRY1 is upregulated following mechanical loading in the heart (Huebert et al., 2004), and mechanical strain results in increased expression of Spred2, a SPRY-related protein, in osteoblasts (Ott et al., 2009). Our data suggest that mechanical stress regulates the expression of SPRY2 in mammary epithelial cells, thus controlling PTEN activation, Akt phosphorylation, and branch initiation. Global changes in tissue contractility lead to concentrated spatial patterns of mechanical stress (Gjorevski and Nelson, 2010), which explains why mechanical stress and the PI3K pathway are linked specifically and preferentially at branch sites. It will be interesting to determine precisely how mechanical stress alters the expression of SPRY2, both in the context of normal morphogenesis as well as in disease.
In summary, the work presented here demonstrates that the PI3K pathway integrates biochemical signaling from growth factors with mechanical signaling from endogenous cytoskeletal contractility to regulate the branching morphogenesis of mammary epithelial cells in culture. Given the subtle differences between conditions and the fact that pathways appear to intersect in a time-dependent manner during branching morphogenesis (Lee et al., 2011), we expect that the integration between these signaling pathways is complex. The ability to parse spatial and temporal changes in signaling is a key advantage of 3D epithelial culture models such as those used here; these simplified systems recapitulate many aspects of mammary epithelial morphogenesis (Lo et al., 2012; Nelson and Bissell, 2005), but lack the heterogeneous mesenchymal environment that also contributes to development of native tissues. Future studies exploring how PI3K and mechanical signaling are specifically regulated and how they work together to define the pattern of branching in vivo will provide insight into their respective roles during physiological and pathological development.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (GM083997 and HL110335), Susan G. Komen for the Cure (FAS0703855), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, and the Camille & Henry Dreyfus Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Abbreviations
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GEF
guanine nucleotide exchange factor
- HGF
hepatocyte growth factor
- MMP
matrix metalloproteinase
- PAK
p21-activated kinase
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- ROCK
Rho kinase
- RTK
receptor tyrosine kinase
- SPRY2
sprouty2
- TEB
terminal end bud
- 3D
three-dimensional
Footnotes
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2013.04.029.
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollon E, Millan J, Shipman M, Marelli-Berg FM, Ridley AJ. Rac activation by the T-cell receptor inhibits T cell migration. PLoS One. 2010;5:e12393. doi: 10.1371/journal.pone.0012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Nelson CM, Khauv D, Bennett S, Radisky ES, Hirai Y, Bissell MJ, Radisky DC. Homology with vesicle fusion mediator syntaxin-1a predicts determinants of epimorphin/syntaxin-2 function in mammary epithelial morphogenesis. J. Biol. Chem. 2009;284:6877–6884. doi: 10.1074/jbc.M805908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin F, Anderson K, Ying C, Patel TB. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol. Pharmacol. 2009;76:679–691. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin F, Singh R, Endersby R, Baker SJ, Patel TB. The tumor suppressor PTEN is necessary for human Sprouty 2-mediated inhibition of cell proliferation. J. Biol. Chem. 2006;281:4816–4822. doi: 10.1074/jbc.M508300200. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 2007;306:193–207. doi: 10.1016/j.ydbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr. Biol. 2002;12:2029–2034. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr. Biol. (Camb) 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Mapping of mechanical strains and stresses around quiescent engineered three-dimensional epithelial tissues. Biophys. J. 2012;103:152–162. doi: 10.1016/j.bpj.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez EW, Nelson CM. Lithographically defined two- and three-dimensional tissue microarrays. Methods Mol. Biol. 2011;671:107–116. doi: 10.1007/978-1-59745-551-0_5. [DOI] [PubMed] [Google Scholar]
- Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? 203, 191–202J. Endocrinol. 2009;203:191–202. doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J. Cell. Biol. 1998;140:159–169. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Nelson CM, Yamazaki K, Takebe K, Przybylo J, Madden B, Radisky DC. Non-classical export of epimorphin and its adhesion to alphav-integrin in regulation of epithelial morphogenesis. J. Cell Sci. 2007;120:2032–2043. doi: 10.1242/jcs.006247. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Nishimura K, Sumpio BE. Phosphatase PTEN is inactivated in bovine aortic endothelial cells exposed to cyclic strain. J. Cell Biochem. 2007;100:515–526. doi: 10.1002/jcb.21085. [DOI] [PubMed] [Google Scholar]
- Huebert RC, Li Q, Adhikari N, Charles NJ, Han X, Ezzat MK, Grindle S, Park S, Ormaza S, Fermin D, Miller LW, Hall JL. Identification and regulation of Sprouty1, a negative inhibitor of the ERK cascade, in the human heart. Physiol. Genomics. 2004;18:284–289. doi: 10.1152/physiolgenomics.00098.2004. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011;13:827–837. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell. Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- Lee K, Gjorevski N, Boghaert E, Radisky DC, Nelson CM. Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 2011;30:2662–2674. doi: 10.1038/emboj.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeksma OC, Van Achterberg TA, Tsumura Y, Toshima J, Eldering E, Kroes WG, Mellink C, Spaargaren M, Mizuno K, Pannekoek H, de Vries CJ. Humans prouty 4, a new ras antagonist on 5q31, interacts with the dual specificity kinase TESK1. Eur. J. Biochem. 2002;269:2546–2556. doi: 10.1046/j.1432-1033.2002.02921.x. [DOI] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Lo AT, Mori H, Mott J, Bissell MJ. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J. Mammary Gland Biol. Neoplasia. 2012;17:103–110. doi: 10.1007/s10911-012-9251-7. [DOI] [PubMed] [Google Scholar]
- Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle. 2011;10:1571–1581. doi: 10.4161/cc.10.10.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD, Nelson CM, Chen CS, Zhang H, Bascom JL, Seiki M, Bissell MJ. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin beta1. Development. 2013;140:343–352. doi: 10.1242/dev.084236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Cancelas J, Zheng J, Williams DA, Zheng Y. Structure-function based design of small molecule inhibitors targeting Rho family GTPases. Curr. Top. Med. Chem. 2006;6:1109–1116. doi: 10.2174/156802606777812095. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Inman JL, Bissell MJ. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat. Protocol. 2008;3:674–678. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J. Biol. Chem. 2007;282:23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- Ott CE, Bauer S, Manke T, Ahrens S, Rodelsperger C, Grunhagen J, Kornak U, Duda G, Mundlos S, Robinson PN. Promiscuous and depolarization-induced immediate-early response genes are induced by mechanical strain of osteoblasts. J. Bone Miner. Res. 2009;24:1247–1262. doi: 10.1359/jbmr.090206. [DOI] [PubMed] [Google Scholar]
- Owens RB, Smith HS, Hackett AJ. Epithelial cell cultures from normal glandular tissue of mice. J. Natl. Cancer Inst. 1974;53:261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Panagiotaki N, Dajas-Bailador F, Amaya E, Papalopulu N, Dorey K. Characterization of a new regulator of BDNF signaling, Sprouty3, involved in axonal morphogenesis in vivo. Development. 2010;137:4005–4015. doi: 10.1242/dev.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich AL, Boghaert E, Nelson CM. Mammary branch initiation and extension are inhibited by separate pathways downstream of TGFbeta in culture. Exp. Cell Res. 2011;317:1872–1884. doi: 10.1016/j.yexcr.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak R, Buitenhuis M. The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood. 2012;119:911–923. doi: 10.1182/blood-2011-07-366203. [DOI] [PubMed] [Google Scholar]
- Renner O, Blanco-Aparicio C, Grassow M, Canamero M, Leal JF, Carnero A. Activation of phosphatidylinositol 3-kinase by membrane localization of p110alpha predisposes mammary glands to neoplastic transformation. Cancer Res. 2008;68:9643–9653. doi: 10.1158/0008-5472.CAN-08-1539. [DOI] [PubMed] [Google Scholar]
- Rice KM, Kakarla SK, Mupparaju SP, Paturi S, Katta A, Wu M, Harris RT, Blough ER. Shear stress activates Akt during vascular smooth muscle cell reorientation. Biotechnol. Appl. Biochem. 2010;55:85–90. doi: 10.1042/BA20090258. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schutzman JL, Martin GR. Sprouty genes function in suppression of prostate tumorigenesis. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1217204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- Tamguney T, Stokoe D. New insights into PTEN. J. Cell Sci. 2007;120:4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev. Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr., Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr. Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, Iglehart JD, Roberts TM, Muller WJ, Zhao JJ. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012;26:1573–1586. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J. Clin. Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J. Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell Biol. 2003;23:8058–8069. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Nelson CM. PI3K signaling in the regulation of branching morphogenesis. BioSystems. 2012;109:403–411. doi: 10.1016/j.biosystems.2012.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.