Abstract
Glioblastoma multiforme (GBM) are aggressive and uniformly fatal primary brain tumors characterized by their diffuse invasion of the normal appearing parenchyma peripheral to the clinical imaging abnormality. Hypoxia, a hallmark of aggressive tumor behavior often noted in GBMs, has been associated with resistance to therapy, poorer survival, and more malignant tumor phenotypes. Based on the existence of a set of novel imaging techniques and modeling tools, our objective was to assess a hypothesized quantitative link between tumor growth kinetics (assessed via mathematical model and routine MR imaging) and the hypoxic burden of the tumor (assessed via PET imaging). Our bio-mathematical model for glioma kinetics describes the spatial and temporal evolution of a glioma in terms of concentration of malignant tumor cells. This model has already been proved usefulas a novel tool to dynamically quantify the net rates of proliferation(ρ) and invasion (D) of the glioma cells in individual patients. Estimates of these kinetic rates can be calculated from routinely available pretreatment MR imaging invivo. Eleven adults withGBM were imaged pre-operatively with FMISO-PET and serial gadolinium-enhanced T1 (T1Gd) and T2-weighted MRIs to allow estimation of patient-specific net rates of proliferation (ρ) and invasion (D). Hypoxic volumes (HV) were quantified from each 18F-fluoromisonidazole (FMISO) PET scan following standard techniques. To control for tumor size variability, two measures of hypoxic burden were considered: relative hypoxia (RH), defined as the ratio of the HV to the T2-defined tumor volume, and the mean intensity on FMISO-PET scaled to the blood activity of the tracer (mean T/B). Pearson correlations between RH and the net rate of cell proliferation ρ reached significance (p < 0.04). Moreover, highly significant positive correlations were found between biological aggressiveness ratio ρ/D and both RH (p < 0.00003) and the mean T/B (p < 0.0007). Overall, biological aggressiveness assessed by serial MRI is linked with hypoxic burden assessed on FMISO-PET using a novel bio-mathematical model for glioma growth and invasion. This study suggests that patient-specific modeling of growth kinetics can provide novel and valuable insight into quantitative connections between disparate information provided by multimodality imaging.
Keywords: glioblastoma, MRI, FMISO-PET, bio-mathematical model, hypoxia
INTRODUCTION
Gliomas are uniformly fatal lesions of the brain signified by their invasive potential and their increased capacity for proliferation (1). This is especially true of glioblastoma multiforme (WHO grade IV, GBM), a highly anaplastic, rapidly proliferating, primary brain neoplasm characterized by diffuse invasion of the normal-appearing parenchyma peripheral to the abnormality seen on clinical imaging. As outlined by Stupp et al (2, 3), the current standard-of-care for newly diagnosed GBMs involves resection followed by adjuvant radiation and chemotherapy. However, GBMs typically recur within months, with a poor prognosis of 6 to 14 months (4).
Magnetic resonance imaging (MRI) and positron emission tomography (PET) can offer non-invasive means to assess individual tumor biology in vivo, thereby assisting diagnosis as well as patient-specific treatment planning. MRI provides anatomical tumor information by allowing visualization of the lesion’s structural extent. Gadolinium-enhancement on T1-weighted MRI (T1Gd MRI), allows the bulk tumor mass to be imaged, with hyperintense-appearing neoangiogenesis enclosing a hypointense region of central necrosis or dead tissue. T2-weighted MRI (T2 MRI) detects the surrounding edema associated with invading glioma cells.
Recent developments in radiopharmaceutical research has produced PET tracers that can target hypoxia, amongst other characteristic errors of disease (5–8). Imaging with radiolabeled nitroimidazoles offers a noninvasive means of assessing hypoxia. One of the earliest and most commonly used agents for hypoxia detection was the PET tracer 18F-fluoromisonidazole (FMISO), a nitroimidazole derivative (9, 10). Nitroreductases within the cell metabolize nitroimidazoles, which can act as electron acceptors when oxygen levels are low. Reduced nitroimidazoles covalently bond to intracellular macromolecules and cannot exit viable cells, such that FMISO uptake is proportional to the amount of hypoxia (11). This does not occur in necrotic tissues, due to a lack of enzyme activity required for the metabolic processing of bioreductive probes.
GBMs are characterized by hypoxia, which results from the rapid depletion of nutrients that occurs with aberrant tumor cell proliferation (12). Hypoxia has been shown to be associated with the propagation and progression of malignant tumors (13), as well as a predictor of resistance to standard radiotherapy and some varieties of concurrent chemotherapy (14). Accordingly, tumors with significant levels of hypoxia generally demonstrate a lower probability of remaining asymptomatic, as well as shorter overall survival (11, 15). By limiting tumor response to and control by therapy, hypoxia is an important adverse prognostic factor that is indicative of higher rates of recurrence and fatality. Additionally, the related necrosis is characteristic of GBM diagnoses (16). It has been suggested that the hypoxia-stimulated expression of genes for oncoproteins, glucose transporters, and glycolytic enzymes confers a growth advantage for the tumor and allows hypoxic cells to utilize the energy-saving mechanism of glycolysis (17), which may promote a more aggressive tumor phenotype.
Quick Guide: Main Model Equations.
| Equation 1 |
This is a reaction-diffusion partial differential equation used to describe the density of glioma cancer cells (c) in terms of two net rates: motility (D) and proliferation (ρ). The equation relates the temporal rate of change of glioma cell density at the spatial location x with the diffuse motility of the cancer cells near that location and the net cell proliferation of those cells locally. The net motility rate D varies depending on the location in the brain to allow for an increased velocity of migration through white matter than though grey. The (maximum) net proliferation rate ρ includes both birth and death rates and assumes logistic growth with a tissue carrying capacity k. There is a spatial heterogeneity in the net proliferation term resulting from the effect ofk. At the center of a densely packed tumor, the netproliferation term becomes 0 with a gradient to a local maximum rate of ρ for those diffusely invading cells at the periphery. Though local proliferation rates at the microscopic level will vary depending on genetic and molecular mechanisms, by consideringnet proliferation ρ the model attempts to capture the downstream effects of these mechanisms on the tumor cell population as a whole.
Major Assumptions of the Model
This model assumes glioma cell invasion throughout the brain is a diffusion process and that the coefficient of diffusion (D) can vary in space depending on the grey and white composition of the brain at that location. The model also assumes logistic growth of the tumor cell population, so that the net proliferation rate (ρ) is lower in regions of high cell density (wherec≈k) than in regions of low cell density (where c ≪ k).
Given the high degree of invasiveness and the inability of current medical imaging technology to capture the full extent of glioma invasion, bio-mathematical modeling has been used to shed light on the growth patterns of gliomas in vivo. The mathematical model developed by Swanson et al (18–20) describes growth and invasion in terms of two patient-specific parameters: the net cell dispersal rate (D) and proliferation rate (ρ). See the Quick Guide for further details. The model considers the expansion of MRI-detectable edge of the tumor as resembling a traveling wave that asymptotically approaches a constant velocity (21–23). In untreated gliomas, linear radial growth has been observed (24, 25). Further, Pallud et al (26) established that the model-predicted constant velocity (contributed to by both D and ρ) of low-grade glioma growth is a prognostic factor. Similarly, kinetic analyses of tumor growth have shown that untreated gliomas grow continuously at predictable rates before their inevitable progression to more malignant phenotypes. Growth velocity has also been seen to predict the conversion to contrast-enhancement on MRI (27). The model parameters for biological aggressiveness (D and ρ) have prognostic significance even when controlling for standard clinico-pathologic parameters1. A simulation example based on patient-specific values for D and ρ is provided in Figure 1, along with a comparison of representative multimodality patient images from which D ρ and can be obtained.
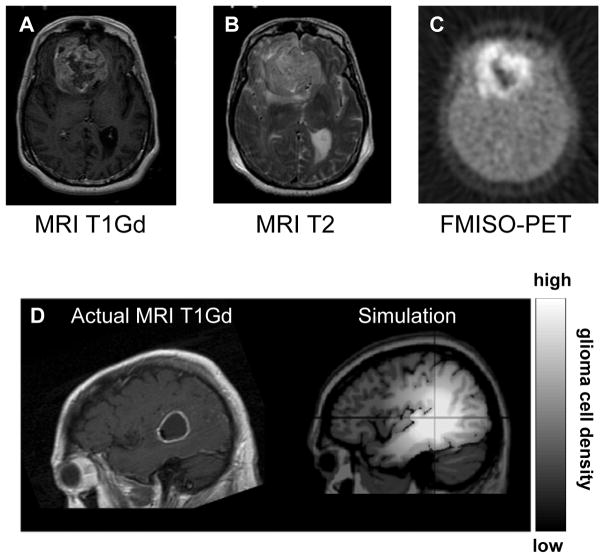
Figure 1.
A 55-yr-old woman with a temporal glioblastoma multiforme imaged pre-operatively on T1Gd MRI (A), T2 MRI (B), and FMISO-PET (C). MRI showed a contrast-enhancing tumor with a large necrotic center. A simulation of tumor expansion shown in (D) was generated by applying D and ρ measured from the serial MRI of a ring-shaped temporal glioblastoma, revealing a diffuse extent of disease peripheral to the imaging abnormality.
Given the successful application of this model to characterizing glioma kinetics in terms of net rates of proliferation and invasion, it is appropriate to explore how hypoxic burden, a hallmark of aggressive tumor behavior, relates to the model parameters that can be used as quantitative measures of biological aggressiveness. Given the model-defined biological aggressiveness metrics for each patient, this study investigates the link between anatomic glioma growth kinetics assessed on MRI and hypoxic burden as seen on FMISO-PET.
MATERIALS AND METHODS
Patients
Eleven adult patients with newly diagnosed GBM were imaged pre-operatively with FMISO-PET and T1Gd, T2 MRI. There were 7 males and 4 females, ages ranging from 37 to 73 (median = 55, mean = 57.6). Karnofsky performance scores at diagnosis ranged from 60 to 90 (median = 70, mean = 74). These patients were recruited from the University of Washington Medical Center. Each patient signed informed consent for inclusion in our study, with prior approval by the Institutional Review Board and Radiation Safety committees.
FMISO PET Imaging Protocol
18F-fluoromisonidazole (FMISO) was prepared as outlined by Lim and Berridge (28) and detailed methods are described elsewhere (29). Briefly, all PET scans were performed on an Advance Tomograph (G.E. Medical Systems) operating in a three-dimensional, high-resolution mode with 35 imaging planes covering a 15 cm axial field of view. For each patient, venous access lines were placed in each arm, one for FMISO injection and the other for blood sampling. Injections of 3.7 MBq/kg (0.1 mCi/kg) of FMISO were then administered, for a maximum of 260 MBq or 7 mCi. A single field-of-view emission scan from 120 to 140 minutes post-injection and an attenuation scan (25 minutes) of the brain with tumor were obtained. The acquired imaging data were reconstructed to determine the tumor hypoxic volume (HV) as described in later sections. During emission tomography, four venous blood samples were obtained at intervals of 5 minutes. Whole blood samples of 1 mL each were counted in a Cobra multichannel gamma well counter (Packard Corp.) that is calibrated each week in units of cpm/MBq. Blood activity of the 4 samples was averaged and then expressed as MBq/mL decay corrected to time of injection.
MRI Protocol
MRIs were acquired using a 1.5 Tesla GE system (Horizon LX Echospeed with 9.1 software). The preoperative Stealth navigation studies included axial T1 with contrast (3D gradient echo, TE/TR minimal, 1.3 mm slice thickness with no skip, FOV 26), axial T2 FSE (TE 97.3, TR 4000, 1.7 mm slice thickness with no skip, FOV 26). Follow-up scans including standard gadolinium-enhanced T1-weighted (TE minimal, TR 350), T2-weighted (TE 102, TR 4300) are obtained in 2D mode with a spin echo sequence and slice thickness of 5 mm with no inter-slice spacing.
Image Processing and Data Acquisition
The number of days between the FMISO-PET and MR images ranged from 0 to 16 with an average of 7.2 days. Spatial registration of the T2 MRI and the FMISO images to the T1Gd MRI was performed using Statistical Parametric Mapping (SPM) software (30) in order to compare regions of PET activity relative to MRI defined abnormalities. Accuracy of image co-registration was confirmed by visual inspection in addition to the optimization features provided by SPM.
Data acquisition from FMISO-PET and MRIs was performed with a semi-automated image-processing program developed in MATLAB (31), which consisted of three parts: MRI (T1Gd and T2) thresholding, FMISO-PET thresholding, and computational determination of different tumor regions of a glioma. Specific details of the techniques used are reported elsewhere (29).
The coregistered FMISO-PET images were scaled to the average venous blood concentration of FMISO activity to produce tumor/blood (T/B) values. This allowed for a three-dimensional pixel-by-pixel calculation of T/B activity ratios.
The number of pixels in the brain with a T/B ratio ≥ 1.2, indicating hypoxia, was determined and converted to milliliters to give the hypoxic volume (HV). T/B ratios previously measured with the tomograph in normal brain and muscle showed that greater than 90% of the values fell below 1.1 (32), and our cutoff ratio of 1.2 is in accordance with previous studies(9). Due to the nature of PET imaging, small non-tumorous regions of FMISO activity are typically scattered in isolated voxels throughout the brain. Designating HV as FMISO T/B ≥ 1.2 largely excludes this noise, and is also consistent with results from histological and immunohistochemical definitions of the hypoxic region. To normalize hypoxia for tumors of different sizes, we defined relative hypoxia (RH) by scaling the HV, as determined on FMISO-PET, to the T2-weighted MR-defined volume, giving the unitless hypoxic fraction RH = HV/T2 volume. Since FMISO-PET is not retained in necrotic tissue, we also considered an alternate measure of relative hypoxia, termed RH*, defined as the HV normalized to the volume of the non-necrotic T2 region (thus removing the central hypo-intensity on T1Gd).
Although the choice of a T/B cutoff of 1.2 is consistent with previous studies exploring FMISO-PET (9, 12, 29), we chose to consider a variety of possible T/B cutoffs to assure that our findings were not as a result of this relatively arbitrary cutoff choice. First, FMISO PET images for each patient were scaled using a range of T/B ratios (0.9 to 1.6). Values for HV (associated with each new cutoff choice) and the resultant RH were then obtained.
The analysis focuses on the association between the model parameters and RH, which we believe is a more accurate and biologically-based quantification of the tissue’s hypoxic burden, which controls for the overall size of the region in which hypoxia may be imaged. It would be inappropriate to compare rates (of biological aggressiveness) with volumes (e.g. HV) since the tumors are imaged at various times during their evolution. Moreover, the growth rate can be independent of size. Additionally, we considered the average T/B intensity of pixels at or above the 1.2 threshold as a measure of hypoxic density, denoted as mean T/B in our analysis. Examining the relationships between the model parameters and mean T/B allows for a complementary characterization of hypoxic burden.
Calculation of Model Parameters
Following Swanson et al (18–20), for contrast-enhancing gliomas, the bio-mathematical model allows for the calculation of the ratio of the net proliferation rate ρ to the net dispersal coefficient D (ρ/D) from a single pair of T1Gd and T2 sequences. A second sequence of MR images without intervening treatment was available for a subset of the patients included in this study (N=5), which allowed the explicit definition of the two unknown parameters D and ρ in individual patients. The velocity of radial expansion v was also determined for those cases.
Tumor Sphericity
Previous work on cell-based mathematical modeling of tumor invasion has linked more aggressive cellular phenotypes with formation of masses with finger-like protrusions (33). Further, these modeling approaches have found that hypoxia can select for these more aggressive phenotypes. We also examined the degree of sphericity in our 3D analysis of each tumor to provide an additional spatial metric of growth kinetics and aggression. Sphericity (Ψ) was calculated relating the surface area (SA, cm2) with the volume (V, cc) of each tumor on a scale from 0 to 1: . This 3D geometric index has been applied to measure a tumor’s similarity to a spherical object (34), where a sphericity value of 1 indicates that the tumor is perfectly spherical, which values less than 1 signify a more irregular and fingered shape. As the compactness measure of a three-dimensional shape, sphericity decreases with the amount of surface area. This measure was quantified for both T1Gd and T2 images. See Table 1.
Table 1.
Patient age, sex, performance status, HV (T/B ≥ 1.2), RH, mean T/B, velocity of growth v, dispersal D, cell proliferation, ρ and circularity on T1Gd and T2 MRI.
| No | Age | Sex | KPS | T1Gd Vol (cc) | T2 Vol (cc) | HV (cc) | RH | Mean T/B | Velocity(mm/yr) | D (mm2) | ρ (1/yr) | ρ/D(1/mm2) | Sphericity T1Gd | Sphericity T2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | 60 | 39.5 | 94.3 | 120.5 | 1.28 | 1.49 | - | - | - | 0.75 | 0.43 | 0.57 |
| 2 | 73 | M | 60 | 23.6 | 75.9 | 75.2 | 0.99 | 1.56 | - | - | - | 0.53 | 0.83 | 0.68 |
| 3 | 55 | F | 70 | 79.8 | 166.8 | 180.9 | 1.08 | 1.65 | - | - | - | 0.66 | 0.44 | 0.54 |
| 4 | 72 | M | 70 | 14.4 | 38.1 | 123.8 | 3.25 | 1.74 | - | - | - | 1.17 | 0.35 | 0.48 |
| 5 | 37 | M | 90 | 50.8 | 177.9 | 33.7 | 0.19 | 1.41 | 6.5 | 6.3 | 1.7 | 0.27 | 0.73 | 0.94 |
| 6 | 63 | M | 90 | 30.4 | 78.1 | 119.2 | 1.53 | 1.53 | 95.6 | 55.3 | 41.3 | 0.75 | 0.39 | 0.38 |
| 7 | 43 | M | 70 | 25.4 | 111.5 | 28.8 | 0.26 | 1.37 | - | - | - | 0.29 | 0.61 | 0.75 |
| 8 | 56 | M | 100 | 39 | 152.6 | 63.4 | 0.42 | 1.48 | - | - | - | 0.26 | 0.78 | 0.81 |
| 9 | 54 | F | 70 | 8.4 | 44.9 | 52.5 | 1.17 | 1.28 | 96.7 | 72.3 | 32.4 | 0.45 | 0.86 | 0.86 |
| 10 | 53 | F | 70 | 2.6 | 37.8 | 19.2 | 0.51 | 1.37 | 32.1 | 28.9 | 8.9 | 0.31 | 1.07 | 1.01 |
| 11 | 70 | M | 70 | 16.4 | 66.9 | 6.3 | 0.09 | 1.26 | 48.6 | 36.9 | 16 | 0.43 | 0.92 | 0.53 |
Statistical Analysis
Pearson correlation was used to assess association between the model parameters and hypoxic burden, with the p-value for statistical significance determined using Student’s T-distribution.
RESULTS AND DISCUSSION
All patients had glioblastoma multiforme as designated by WHO criteria (35). The patient age, sex, KPS at diagnosis, HV, RH, velocity of glioma expansion, the computed values of the model parameters D and ρ, and the ratio ρ/D are shown in Table 1. Values for v, D, and ρ are displayed for 5 patients for whom two imaging studies were available over a period of time prior to any operation or treatment
Correlates of Hypoxic Burden and Net Proliferation/Diffusion
The statistical significance of associations between hypoxic burden and the MRI-defined anatomical tumor burden quantified by v, D, and ρ are summarized in Table 2. A significant (Pearson) correlation was found between the net rate of cell proliferation ρ and RH (p < 0.04), as well as RH* (p < 0.04). Correlations with dispersal D did not reach significance. The mathematical model and our traveling wave approximation of glioma expansion suggest that both ρ and D contribute to v. The velocity, v, only approached significance when compared with RH.
Table 2.
P-values and R2 for the correlations of FMISO-determined measures of hypoxic burden (RH and mean T/B) and MR-defined growth characteristics (v, D and ρ). Asterisks indicate associations reaching statistical significance (p < 0.05).
| p-value | R2 | ||
|---|---|---|---|
| RH vs. | velocity v (mm/yr) | 0.057 | 0.75 |
| D (mm2) | 0.12 | 0.6 | |
| ρ (1/yr) | 0.034* | 0.82* | |
| ρ/D (yr/ mm2) | 0.000021* | 0.88* | |
| RH* vs. | velocity v (mm/yr) | 0.055 | 0.76 |
| D (mm2) | 0.12 | 0.6 | |
| ρ (1/yr) | 0.03* | 0.83* | |
| ρ/D (yr/ mm2) | 0.000018* | 0.88* | |
| Mean T/B vs. | velocity v (mm/yr) | 0.18 | 0.5 |
| D (mm2) | 0.31 | 0.33 | |
| ρ (1/yr) | 0.11 | 0.62 | |
| ρ/D (yr/ mm2) | 0.00069* | 0.74* | |
In the context of hypoxia, model-defined biological aggressiveness may also be quantitatively assessed by the ratio ρ/D based on the observation that a cell population with increased proliferation (ρ) relative to invasive capability (D) would be more likely to produce local hypoxia. The ratio ρ/D can be related to the gradient of the leading (invading) edge of the tumor. That is, a high ρ/D would correspond to a mitotically active (high ρ) tumor with relative low invasiveness (low D) leading to a relatively well demarcated nodule. Conversely, a low ρ/D would correspond to a diffusely invasive leading-edge of the tumor consistent with a tumor that has a relatively lower proliferative capacity compared to its diffuse invasion. We did note a consistent pattern for which a larger ρ also suggested a larger D, such that ρ/D also increased. The biological aggressiveness ratio ρ/D showed strong correlation with RH (p < 0.00003) and RH* (p < 0.00002). In addition, ρ/D was found to significantly correlate with mean T/B (p < 0.0007).
Correlates of Tumor Sphericity
In response to a number of computational studies suggesting that the tumor microenvironment (e.g. local hypoxia) can drive the formation of tumors that are less spherical (33) we explored the tumor sphericity as an alternate candidate measure of tumor aggressiveness. Correlation analysis was performed between sphericity and all glioma growth characteristics quantified through anatomical and functional imaging. Sphericity on T1Gd negatively correlated with RH (p < 0.04), mean T/B (p <0.02) and ρ/D (p < 0.009). Pearson correlation results for sphericity delineated on T2 also reached significance for mean T/B (p < 0.05) and ρ/D (p < 0.008).
Alternative T/B Ratios
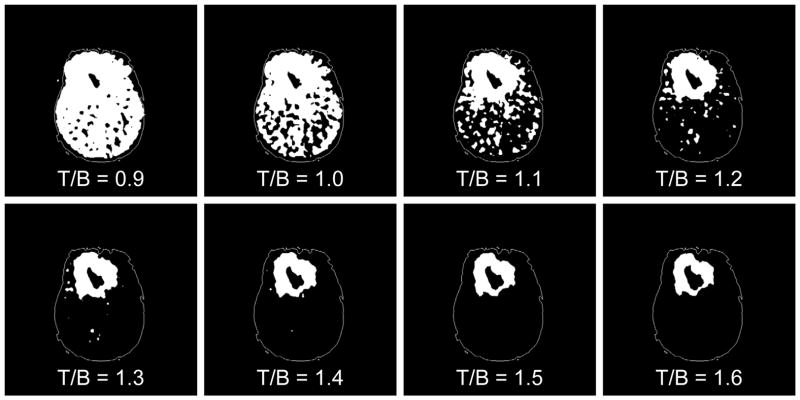
Based on the hypothesis that hypoxia would not be expected at large distances from the bulk tumor mass, for each T/B cutoff an associated HV was calculated and the portion of that HV residing beyond the tumor region was graphed (data not shown). The results showed that the HV occupying presumed non-tumorous regions precipitously declined around the 1.2 level, indicating that the FMISO activity at or above the T/B =1.2 level is largely restricted to previously defined areas of MRI abnormality. More than 99% of normal appearing brain in the hemisphere contralateral to the tumor was observed to have a T/B < 1.2. Figure 2 of a right frontal GBM displays a graphical example of an FMISO PET image subjected to thresholding at each of the T/B levels considered. These data support the reasonability of using the 1.2 T/B level as a threshold for hypoxia.
Figure 2.
An illustration of the results of applying a range of T/B ratios to the FMISO PET image of a right frontal GBM (Patient #3) to generate visualizations of the HV regions suggesting T/B = 1.2 is a reasonable threshold to define a HV separate from imaging noise.
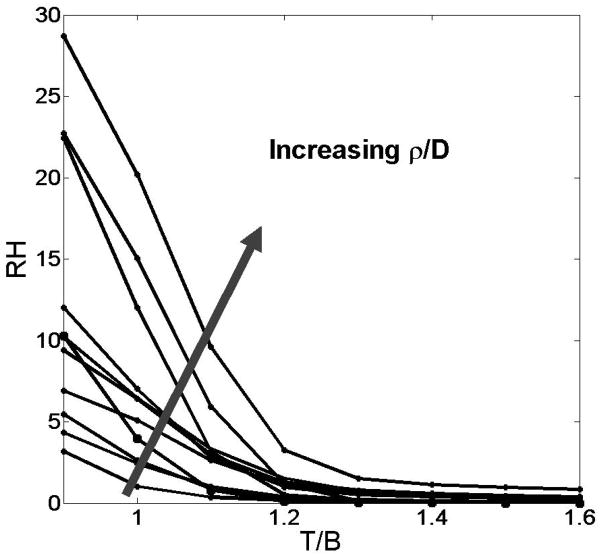
For each patient, plots were generated of RH against various threshold levels ranging from 0.9 to 1.6 in increments of 0.1, yielding the results summarized in Figure 3. RH decreased somewhat linearly as the ratio increased, as expected. Of special note is our observation that the value for the ratio ρ/D increases as a function of RH, regardless of the T/B level used. This suggests that the hypothesized “ideal” T/B = 1.2 is not a confounding factor in considering hypoxic burden as a marker of a tumor’s biological aggressiveness. The striking linearity of the scatter of the MRI-defined ρ/D relative to RH is illustrated in Figure 4. The positive relationship holds regardless of T/B threshold, which further confirms our conclusion from the results depicted in Figure 3.
Figure 3.
Plot of RH as measured at various T/B thresholds. Curves represent individual patients (N = 11), with the direction of the superimposed arrow denoting a general increase in ρ/D values.
Figure 4.
Scatter plots of RH vs. ρ/D for all patients in this study (N = 11). RH was determined over a variety of T/B levels, ranging from 1.1 to 1.6 in increments of 0.1. A strong linear relationship between the variables is shown for all thresholds; correlations were statistically significant for all T/B levels considered.
CONCLUSIONS
Hypoxia is a clinically important feature of glioblastomas, specifically as it relates to treatment resistance. The ability of FMISO-PET to visualize and quantify the hypoxic fraction of the tumor is highly relevant in clinical applications. While the gold standard for distinguishing hypoxia is often considered to be direct measurement of pO2 levels with electrodes, this technique is practically and ethically impossible for the routine monitoring of hypoxia in intracranial tumors, as the procedure must be performed intraoperatively. Previous literature has established the utility of FMISO-PET in the non-invasive assessment of hypoxia(36) and has been linked to pO2, where FMISO retention is detectable in the range of 2 –3 mm Hg and below(10, 37, 38). For cancers of the head and neck, it has been shown that hypoxia (imaged via FMISO-PET) can affect prognosis independently of other prognostic variables (39). Though there is evidence that the association between hypoxia and clinical outcome may not hold across glioma grades (40), recent multivariate analyses of GBM patient survival revealed significant correlations with the volume of FMISO-PET imageable hypoxia alone (9).
Assessing the spatio-temporal growth of GBM through a bio-mathematical model that is driven by patient-specific parameters offers a novel means of connecting overall growth kinetics (visible on MRI) to the hypoxia-guided resistance mechanism (visible on FMISO-PET). Previous analysis of this model relate the radial velocity of tumor growth to survival (41),and show that this parameter predicts conversion of non-contrast enhancing low-grade gliomas to contrast-enhancement on T1Gd MRI (27). These results imply that our bio-mathematical model accurately predicts growth of untreated tumor over time and can distinguish between the growth of variably aggressive tumors. Combining these results with conclusions from other studies that define hypoxic conditions as an important feature of aggressive tumor microenvironment (33)lead us to investigate the relation between tumor aggressiveness quantified through our model parameters and aggressive phenotype associated with hypoxia that can be imaged by FMISO-PET for human GBMs.
The positive relationship between the model parameters and hypoxic burden is consistent with our current understanding of the process by which hypoxia arises. That is highly proliferative tumors (high ρ) are thought to be more like to be hypoxic with some recent PET imaging to support this in vivo (42). Further, intuitively, those invasive gliomas for which there is a high net proliferation rate relative to the net invasion rate (ρ/D) would likely to a form a hypoxic microenviroment based on the relative time rate for depletion of resources (e.g., oxygen via increasing cell numbers via proliferation) relative to the ability of the population of glioma cells to migrate away from the potentially hypoxia-forming environment. Although, this is an intuitive concept in overall population dynamics, the positive correlation of the ratio of the net proliferation and the net invasion rate (ρ/D), with the resultant RH, RH* and mean T/B concurs with our hypothesis that hypoxia is a hallmark of aggressive tumor behavior.
This significant correlation between the model parameters for biological aggressiveness and the hypoxic burden assessed by RH, RH* and mean T/B persists across a wide range of T/B threshold values used for defining the HV. When choosing ratios below 1.2, there tends to be significant overlap of the histogram of T/Bs from contralateral normal brain and tumor regions, whereas ratios above 1.2 tend to provide for clear delineation between the normal brain and (hypoxic) tumor tissue. We explored varying the T/B cutoff across all patients to confirm that the results relating the hypoxic burden assessed at cutoff 1.2 did not significantly change the results. Since FMISO-PET has been shown to correlate with the pO2, increasing the hypoxic threshold to levels above 1.2, would correspond to selecting regions of that tumor that are increasingly hypoxic. Thus, our results suggest that at every magnitude of hypoxia (T/B >1.2), the biological aggressiveness assessed by the bio-mathematical model and MRI correlates significantly with hypoxic burden assessed by FMISO-PET.
Previous cell-based computational models of tumor growth and invasion have shown that hypoxic environments can select for cells with aggressive phenotypes, which give rise to fingered-tumor morphologies that have low sphericity in vitro (33). This suggests that when more aggressive tumor clones are selected the tumor growth pattern is more “bumpy”. To expand on these computational and experimental studies, we quantitatively investigated the association of clinically observed sphericity on MRI with hypoxia and the model parameters. Inverse correlation of tumor sphericity with RH and mean T/B imply that more fingered or abnormally shaped tumors tend to have greater hypoxic burden. Large ρ/D correlates with low sphericity, which suggests extensive infiltration and a high grade of tumor cell dissociation. The observed results are therefore the first to provide clinical-scale support for existing theoretical cell-based that suggested aggressive cell lines produce tumors that may be less spherical (33). This is a novel insight that suggests further investigation as there are many differences between the computational models that were originally suggestive of this less spherical growth pattern and the models used here to quantify aggressiveness.
This report suggests a means of quantifying the in vivo biological link between the hypoxic burden of the tumor and the overall growth characteristics of individual gliomas. The validated mathematical model we have utilized in this study is a unique tool towards this end, as it is able to quantitatively connect the overall aggressive behavior (ρ/D) of a tumor assessed on MRI to that assessed on FMISO-PET as hypoxia, a biological result of its microenviroment. These results argue that FMISO-PET measurement in untreated GBM are able to quantify an essential outcome variable and can extend our understanding of an important pathophysiological process beyond what is shown by conventional anatomic imaging.
Further investigation is necessary to clarify these links between anatomical and functional imaging, due in part to the small sample size employed in this study, as well as the brief time scale over which pre-operative imaging data is obtained. In light of the present data, it may be possible that brain tissue heterogeneity and the placement of anatomical structures in relation to individual tumors may have affected our results. In retrospect, however, the general scarcity of data available for analysis highlights the bleak clinical reality of glioblastoma. It follows that a precise, biologically-based method to better understand its growth kinetics is highly necessary.
It is important to note that cancer modeling is dictated by biology, not the mathematics. As noted previously, the bio-mathematical model used in this study attempts to capture the downstream effects of the tumor microenvironment. Variables can be considered individually or together to assess their biological significance and testability. A striking advantage of this patient-specific model is that its predictions are readily personalized through the determination of only two key parameters; this parsimonious approach is reflected throughout much of current modeling research (43). However, we realize that there are limitations to such a mathematical formulation for aggressiveness. Although the parameters consider tumor growth as an expansion of its traveling wave front, other factors can combine to determine the patient’s overall clinical outcome, notably prognostic factors include age and Karnofsky performance score (KPS), which are of a more stochastic phenomena lacking a sufficiently understood underlying mechanism that can be incorporated into the model. But developing a mathematical model is an iterative process; upon comparison to clinical results, the model can be modified and extended to more accurately emulate observed phenomena and make more realistic predictions. The future of the modeling effort will continue to develop and refine predictions regarding glioma prognosis, progression, and therapeutic efficacy.
However, these preliminary data do suggest that these bio-mathematical modeling techniques provide for a novel tool for linking data from disparate sources. In the case of this manuscript, the disparate sources are multi-modality imaging observations of individual GBM patients. At first glance, these images may appear relatively distinct and separated by technical (imaging) and biological (modeling) mechanism, however, the techniques discussed in this manuscript provide a forum for communicating across this multimodality divide.It is clear that such a multimodality analysis can provide valuable insight into quantitative understanding of the kinetics and pathophysiology of each glioma that could uniquely be used in guiding and assessing the impact of treatment in individual patients.
Footnotes
Wang CH, Rockhill JK, Mrugala M, Peacock DL, Lai A, Jusenius K, Wardlaw JM, Cloughesy T, Spence AM, Rockne R, Alvord EC, Jr., Swanson KR. Prognostic significance of growth kinetics in newly diagnosed glioblastomas revealed by combining serial imaging with a novel bio-mathematical model. Submitted to Cancer Res; 2008.
References
- 1.Swanson KR, Alvord EC, Jr, Murray JD. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Brit J Cancer. 2002;86:14–8. doi: 10.1038/sj.bjc.6600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–36. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 4.Silbergeld DL, Rostomily RC, Alvord EC., Jr The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10:179–85. doi: 10.1007/BF00146880. [DOI] [PubMed] [Google Scholar]
- 5.Mankoff DA, Peterson LM, Tewson TJ, et al. [18F]fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med. 2001;42:679–84. [PubMed] [Google Scholar]
- 6.Tewson TJ, Krohn KA. PET radiopharmaceuticals: state-of-the-art and future prospects. Semin Nucl Med. 1998;28:221–34. doi: 10.1016/s0001-2998(98)80028-7. [DOI] [PubMed] [Google Scholar]
- 7.Varagnolo L, Stokkel MP, Mazzi U, Pauwels EK. 18F-labeled radiopharmaceuticals for PET in oncology, excluding FDG. Nucl Med Biol. 2000;27:103–12. doi: 10.1016/s0969-8051(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 8.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–81. [PubMed] [Google Scholar]
- 9.Spence AM, Muzi M, Swanson KR, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14:2623–30. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasey JS, Grunbaum Z, Magee S, et al. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res. 1987;111:292–304. [PubMed] [Google Scholar]
- 11.Rajendran JG, Wilson DC, Conrad EU, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 12.Rajendran JG, Mankoff DA, O’Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–52. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 13.Tsang RW, Fyles AW, Milosevic M, et al. Interrelationship of proliferation and hypoxia in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;46:95–9. doi: 10.1016/s0360-3016(99)00408-3. [DOI] [PubMed] [Google Scholar]
- 14.Brown JM. Therapeutic targets in radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:319–26. doi: 10.1016/s0360-3016(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 15.Fyles AW, Milosevic M, Wong R, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–56. doi: 10.1016/s0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 16.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dachs GU, Tozer GM. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36:1649–60. doi: 10.1016/s0959-8049(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 18.Swanson KR, Bridge C, Murray JD, Alvord EC., Jr Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci. 2003;216:1–10. doi: 10.1016/j.jns.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Swanson KR. PhD. University of Washington; 1999. Mathematical modeling of the growth and control of tumors. [Google Scholar]
- 20.Swanson KR, Rostomily RC, Alvord EC., Jr A mathematical modelling tool for predicting survival of individual patients following resection of glioblastoma: a proof of principle. Brit J Cancer. 2008;98:113–9. doi: 10.1038/sj.bjc.6604125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JD. Mathematical biology I: an introduction. 3. New York: Springer-Verlag; 2002. [Google Scholar]
- 22.Burgess PK, Kulesa PM, Murray JD, Alvord EC., Jr The interaction of growth rates and diffusion coefficients in a three-dimensional mathematical model of gliomas. J Neuropathol Exp Neurol. 1997;56:704–13. [PubMed] [Google Scholar]
- 23.Swanson KR, Alvord EC, Jr, Murray JD. A quantitative model for differential motility of gliomas in grey and white matter. Cell Proliferat. 2000;33:317–29. doi: 10.1046/j.1365-2184.2000.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53:524–8. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 25.Swanson KR, Alvord EC., Jr A biomathematical and pathological analysis of an untreated glioblastoma. 7th European Congress of Neuropathology; 2002 Jul 13–16; Helsinki, Finland. 2002. [Google Scholar]
- 26.Pallud J, Mandonnet E, Duffau H, et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol. 2006;60:380–3. doi: 10.1002/ana.20946. [DOI] [PubMed] [Google Scholar]
- 27.Harpold HL, Alvord EC, Jr, Swanson KR. The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol. 2007;66:1–9. doi: 10.1097/nen.0b013e31802d9000. [DOI] [PubMed] [Google Scholar]
- 28.Lim JL, Berridge MS. An efficient radiosynthesis of [18F]fluoromisonidazole. Appl Radiat Isot. 1993;44:1085–91. doi: 10.1016/0969-8043(93)90110-v. [DOI] [PubMed] [Google Scholar]
- 29.Swanson KR, Chakraborty G, Wang CH, et al. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med. 2009;50:36–44. doi: 10.2967/jnumed.108.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner J, Flandin G, Henson R, et al. Wellcome Department of Imaging Neuroscience. 5. Institute of Neurology; 2005. Statistical parametric mapping (SPM). Functional Imaging Laboratory. [Google Scholar]
- 31.MATLAB. MATLAB. 2007a. Natick, MA: The MathWorks, Inc; 2007. [Google Scholar]
- 32.Rajendran JG, Krohn KA. Imaging tumor hypoxia. In: Bailey DL, Townsend DW, Valk PE, Maisey MN, editors. Positron emission tomography, principles and practice. London: Springer-Verlag; 2002. pp. 689–96. [Google Scholar]
- 33.Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–15. doi: 10.1016/j.cell.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Silva AC, Carvalho PC, Gattass M. Diagnosis of lung nodule using semivariogram and geometric measures in computerized tomography images. Comput Meth Prog Bio. 2005;79:31–8. doi: 10.1016/j.cmpb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25. doi: 10.1093/jnen/61.3.215. discussion 26–9. [DOI] [PubMed] [Google Scholar]
- 36.Cher LM, Murone C, Lawrentschuk N, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–8. [PubMed] [Google Scholar]
- 37.Rasey JS, Nelson NJ, Chin L, Evans ML, Grunbaum Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat Res. 1990;122:301–8. [PubMed] [Google Scholar]
- 38.Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–28. doi: 10.1016/s0360-3016(96)00325-2. [DOI] [PubMed] [Google Scholar]
- 39.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 40.Lally BE, Rockwell S, Fischer DB, Collingridge DR, Piepmeier JM, Knisely JP. The interactions of polarographic measurements of oxygen tension and histological grade in human glioma. Cancer J. 2006;12:461–6. doi: 10.1097/00130404-200611000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Swanson KR, Alvord EC, Jr, Murray JD. Dynamics of a model for brain tumors reveals a small window for therapeutic intervention. Discrete Cont Dyn-B. 2004;4:289–95. [Google Scholar]
- 42.Dence CS, Ponde DE, Welch MJ, Lewis JS. Autoradiographic and small-animal PET comparisons between 18F-FMISO, 18F-FDG, 18F-FLT and the hypoxic selective 64Cu-ATSM in a rodent model of cancer. Nuc Med and Biol. 2008;35:713–20. doi: 10.1016/j.nucmedbio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Churchill SW, Zajic SC. Prediction of fully developed turbulent convection with minimal explicit empiricism. AlChE J. 2002;48:927–40. [Google Scholar]