Abstract
CEP-1347 is a potent inhibitor of mixed lineage kinase (MLK), which was investigated for ameliorating HIV-associated neurocognitive disorders. CEP-1347 and atazanavir pharmacokinetics were determined when CEP-1347 50 mg twice daily was administered to HIV-infected patients (n=20) receiving combination antiretroviral therapy including atazanavir and ritonavir (ATV/RTV, 300/100 mg) once daily continuously. Co-administration of CEP-1347 and ATV/RTV resulted with significant changes in pharmacokinetics of ATV but not RTV. Specifically, an increase in ATV accumulation ratio of 15 % (p=0.007) and a prolongation of T1/2 from 12.7 to 15.9 h (p=0.002) were observed. The results suggested that co-administration of CEP-1347 with ATV/RTV in HIV-infected patients might result in limited impact on ATV but not on RTV pharmacokinetics.
Keywords: CEP-1347, Atazanavir, Ritonavir, HIV, Drug interactions, Mixed lineage kinases
Introduction
CEP-1347 is a proprietary, semi-synthetic molecule derived from the naturally occurring indolocarbazole, K252a (Kaneko et al. 1997). By competing for ATP binding, CEP-1437 acts as a potent inhibitor of the mixed lineage kinase (MLKs), which are a family of upstream serine/threonine protein kinases of the mitogen-activated protein kinase kinases (MAP3K) group. The MAP3K drug group is involved in regulating signaling of c-Jun NH2 terminal kinases (JNKs) and p38 (Gallo and Johnson 2002; Wang et al. 2004). MAP3K is also relevant to HIV-associated neurocognitive disorders (HAND), as JNKs and p38 are implicated in neuronal apoptosis and microglial activation (Bogoyevitch et al. 2004; Harper and LoGrasso 2001; Nakajima et al. 2004; Suzuki et al. 2004). Considering that: (1) neuronal apoptosis occurs in HIV encephalitis (Gelbard et al. 1995; Petito and Roberts 1995) and is likely mediated by both viral proteins and products of glial activation (Haughey et al. 2004; Kruman et al. 1998; Lannuzel et al. 1997; New et al. 1998; Perry et al. 1998; Talley et al. 1995; Xu et al. 2004; Zhang et al. 2003); and that (2) microglial activation is strongly correlated with neurocognitive severity (Glass et al. 1993); MLKs may be a potential target for therapeutic intervention for HAND.
Most of the laboratory work conducted so far with CEP-1347 has focused on prevention of neuronal apoptosis. This was performed in a variety of neuronal populations including cortical, dopaminergic, sympathetic, and sensory neurons (Bodner et al. 2002; Bozyczko-Coyne et al. 2001; Harris et al. 2002; Namgung and Xia 2000; Pirvola et al. 2000; Saporito et al. 1999, 1998, 2000; Trotter et al. 2002; Troy et al. 2001; Ylikoski et al. 2002). The demonstrated efficacy of CEP-1347 in preclinical models of Parkinson's disease (PD) has led to a large phase II clinical trial, which showed good safety and tolerability but no efficacy in persons with early-stage PD (Parkinson Study Group PRECEPT Investigators 2007). CEP-1347 has also been shown to rescue rat hippocampal neurons and rat dorsal root ganglion neurons exposed to the HIV neurotoxin gp120 (Bodner et al. 2002, 2004). Furthermore, CEP-1347 reduces the activation of microglia and astrocytes by HIV-1 Tat (Lund et al. 2005), thus dampening neuroinflammation. We have recently shown targeting MLKs with CEP-1347 results in beneficial effects in in vitro models of HIV neurotoxicity (Sui et al. 2006) and have extended those findings in an animal model of HIV encephalitis (Eggert et al. 2010).
Atazanavir (ATV) is a commonly prescribed HIV-1 protease inhibitor (PI) that requires once daily administration and has a relatively favorable side effect profile compared to other PIs. The pharmacokinetic characteristics of ATV include absorption that is food- and gastric acid-dependent, and moderate plasma protein binding (86 %) to both alpha-acid glycoprotein and albumin. ATV undergoes extensive hepatic metabolism via CYP3A isoenzymes and is also an inhibitor of CYP3A4. The interaction between ATV and CEP-1347 is unknown. CEP-1347 is extensively metabolized, primarily via oxidation or hydrolysis at the molecule's sulfur centers. Excretion in rats and monkeys is predominantly via the biliary tract to the bowel, resulting in the recovery of at least 95 % of single oral doses in feces. CEP-1347 has previously been shown to be an inhibitor of CYP3A4/5 activity in vitro, as well as of CYP1A2, CYP2C8, CYP2C9, and CYP2D6 activities, with Ki values that range from 0.8 to 5.4 μM.
The objective of the present study was to extend our earlier works in order to investigate the pharmacokinetic interactions between CEP-1347 and a commonly used combination anti-retroviral therapy, atazanavir/ritonavir (ATV/RTV), in HIV-1-infected patients in anticipation of a HAND clinical trial.
Results
Demographics and safety
A total of 20 HIV-infected patients (16 males and 4 females) completed the study. All patients were on stable antiretroviral regimen including ATV/RTV and TDF/FTC for at least 4 weeks prior to enrollment and no antiretroviral regimen change for the duration of the study. Baseline patient demographics are summarized in Table 1. The mean age of subjects who completed the study was 44 years. All were within 30 % of their ideal body weight (mean, 82 kg). Of the 20 patients completing the study, ethnicity was as follows: 11 Caucasians and 9 African Americans. All patients tolerated study medications well, with all reported adverse events consistent with what has previously been reported for ATV/RTV and tenofovir treatment. None of the clinical laboratory abnormalities measured in any of the patients were considered to be clinically significant by the study physician. At day 21, study completion, plasma viral load had decreased from 3.80±3.70 to 2.41±2.03 log copies/mL.
Table 1. Baseline patient demographics and clinical characteristics.
| Patients (n=20) | |
|---|---|
| Age | 44 (9) |
| Gender | Male, 80 % |
| Race | Caucasian, 55 % African American, 45 % |
| Duration of HIV (years) | 14 (7) |
| Plasma HIV-1 RNA (log copies/mL) | 3.8 (4.4) |
| CD4 Cell Count (mm3) | 399 (204) |
| Weight (kg) | 82 (17) |
| Antiretroviral therapy | ATV/RTV+TDF/FTC |
Note: Values are the arithmetic mean (standard deviation) for patients who participated in the present study
Effects of CEP-1347 on ATV/RTV pharmacokinetics
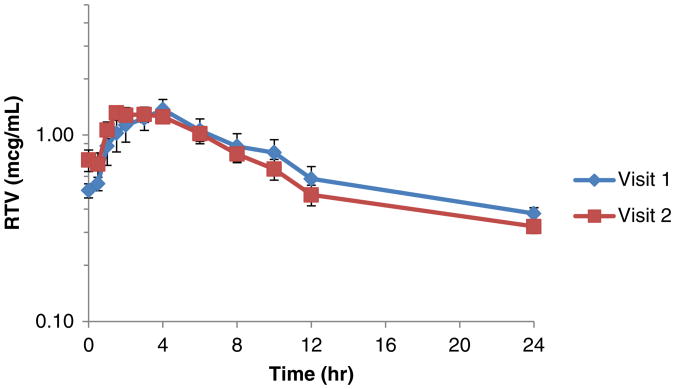
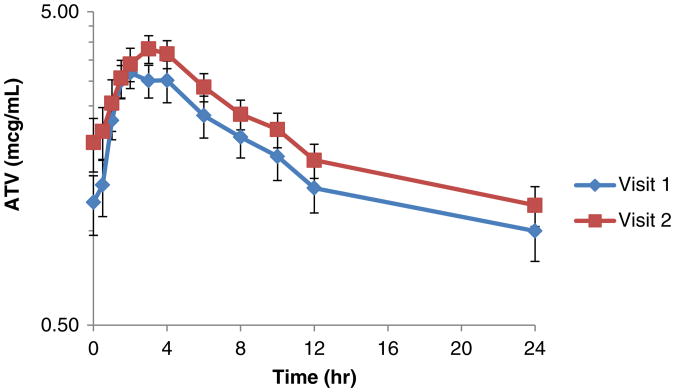
The major PK parameters of RTV remained similar with concurrent use of CEP-1347 (Table 2). In contrast, significant differences were observed in two PK parameters for ATV during CEP-1347 co-administration (Table 3), with an increase in ATV accumulation ratio (R) of 15 % (p=0.007) and a prolongation of T1/2 from 12.7 to 15.9 h (p=0.002). The T1/2 changes observed were outside (1.31) the FDA bioequivalence range (0.80–1.25). No significant differences were noted in other PK parameters between ATV/RTV alone and ATV/RTV with CEP-1347. The concentration versus time profiles of RTV and ATV are summarized in Figs. 1 and 2, respectively.
Table 2. Ritonavir (RTV) pharmacokinetics before and after the addition of CEP-1347.
| RTV | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Visit 1 | Visit 2 | GMR (90 % CI) | p |
| AUC0–24 (h μg/mL) | 14.2 (10.9) | 13.2 (7.2) | 1.12 (0.90, 1.39) | 0.387 |
| Ra | 1.12 (0.20) | 1.16 (0.38) | 1.05 (0.94, 1.16) | 0.465 |
| Clss/F (L/h) | 10.9 (6.7) | 11.0 (10.5) | 0.90 (0.72, 1.11) | 0.390 |
| Cmax (μg/mL) | 1.40 (0.98) | 1.27 (0.69) | 1.07 (0.85, 1.34) | 0.628 |
| Tmax (h) | 3 (0–6) | 3 (0–6) | 0.89 (0.79, 1.15) | 0.445 |
| T1/2 (h) | 5.9 (1.8–21.8) | 5.8 (2.2–33.9) | 1.20 (0.92, 1.56) | 0.246 |
Note: Values are the arithmetic mean (standard deviation) for 19 of the 20 available patients who participated in the present study, except T1/2 and Tmax are given as median (range). Two-sided paired t test of logarithmically transformed data was used for comparison
GMR Geometric mean ratio=(Geometric mean for visit 2)/(Geometric mean for visit 1), CI confidence interval
Note that R is the only parameter that meets the FDA definition of bioequivalence, based on the 90 % CI for the GMR falling completely within the bioequivalence region (0.80, 1.25). There is insufficient evidence that the other parameters are either bioequivalent (p>0.1 for all) or different (p>0.1 for all) based on this data with n=19
Table 3. Atazanavir (ATV) pharmacokinetics before and after the addition of CEP-1347.
| ATV | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Visit 1 | Visit 2 | GMR (90 % CI) | p |
| AUC0–24 (h μg/mL) | 40.2 (26.1) | 49.6 (24.0) | 1.30 (0.92–1.85) | 0.207 |
| Ra | 1.4 (0.3) | 1.6 (0.3) | 1.12 (1.05–1.20) | 0.007 |
| Clss/F (L/h) | 12.0 (10.3) | 9.3 (10.7) | 0.77 (0.54–1.09) | 0.212 |
| Cmax (μg/mL) | 4.0 (2.1) | 4.5 (1.9) | 1.14 (0.79–1.67) | 0.542 |
| Tmax (h) | 2.0 (1.0–6.0) | 2.0 (0.5–4.0) | 0.86 (0.67–1.11) | 0.314 |
| T1/2 (h)b | 12.7 (3.7–27.2) | 15.9 (6.8–31.48) | 1.31 (1.15–1.49) | 0.002 |
Note: Values are the arithmetic mean (standard deviation) for 19 of 20 available patients who participated in the present study, except T1/2 and Tmax are given as median (range). Two-sided paired t test of logarithmically transformed data was used for comparison
GMR Geometric mean ratio=(Geometric mean for visit 2)/(Geometric mean for visit 1), CI confidence interval
Note that R is both bioequivalent, with 90 % CI for the GMR within the FDA bioequivalence region of (0.80, 1.25), and statistically significantly increased (p=0.007), suggesting that the 12 % (5–20 %) increase is not necessarily clinically significant. There is insufficient evidence of bioequivalence for the other parameters (p>0.1 for all)
GMR for T1/2 (in hour)=1.31 (1.15–1.49), indicating a statistically significantly 31 % increase (p=0.002); and there is insufficient evidence of bioequivalence (p>0.1)
Fig. 1.
Effect of CEP-1347 on the time versus concentration curves of ritonavir (mean±SEM). Shown are individual ritonavir (100 mg once daily) concentrations in plasma at each time point from visit 1 (before) to 2 (after) in study participants taking CEP-1347 (50 mg twice daily)
Fig. 2.
Effect of CEP-1347 on the concentration versus time profile of atazanavir (mean±SEM). Shown are individual atazanavir (300 mg once daily) concentrations in plasma at each time point from visit 1 and 2 in study participants taking CEP-1347 (50 mg twice daily)
CEP-1347 pharmacokinetics
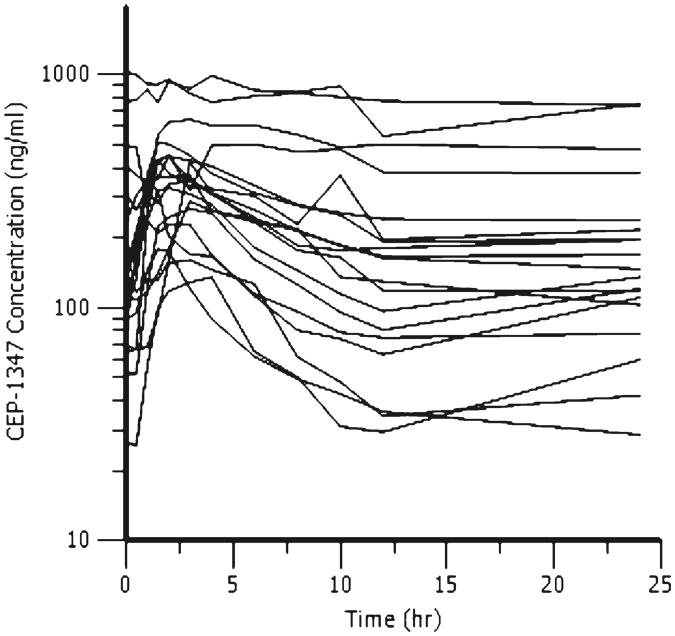
Considerable inter-patient variability was noted for CEP-1347 plasma concentrations in ATV/RTV-maintained patients. PK results for CEP-1347 are summarized in Table 4 and Fig. 3. The mean (standard deviation) of the CEP-1347 AUCτ, R, Clss/F, and Cmax were 3,559 (2,697) h ng/mL, 5.1 (1.5), 22.3 (15.3) L/h, 427.4 (245.3) ng/mL, respectively.
Table 4. CEP-1347 pharmacokinetics during co-administration of ATV/r.
| CEP-1347 | |
|---|---|
|
| |
| Parameter | Present study |
| AUCτ (h ng/mL) | 3,559 (2,697) |
| AUC12–24 (h ng/mL) | 2,634 (2,482) |
| R (accumulation ratio) | 5.1 (1.5) |
| Clss/F (L/h) | 22.3 (15.3) |
| Cmax (ng/mL) | 427.4 (245.3) |
| Tmax (h) | 3.0 (0.0–6.0) |
| T1/2 (h) | 35.2 (21.0–60.9) |
Note: Values are the arithmetic mean (standard deviation) for 19 of the 20 available patients who participated in the present study, except T1/2 and Tmax are given as median (range). Reference values are from manufacturer's data on file obtained from a pharmacokinetic study in ten healthy volunteers
Fig. 3. CEP-1347 concentration versus time profiles. Shown are individual CEP-1347 (50 mg twice daily) concentrations in plasma at each time point in study participants taking atazanavir/r 300/100 mg once daily.
Discussion
CEP-1347 has been reported to be a competitive inhibitor of CYP3A4/5, CYP2C8, CYP2D6, CYP2C9, CYP1A2, and CYP2C19 in in vitro studies. In the present study, CEP-1347 had minimal effect on ritonavir pharmacokinetics; however, did have a significant effect on the pharmacokinetics of ATV, including accumulation ratio and T1/2. The change in T1/2 was outside the FDA bioequivalence region, suggesting that the effect might be clinically relevant. ATV and RTV pharmacokinetic parameters obtained in this study in the presence of CEP-1347 were comparable to values that have been previously reported. The estimated mean clearance of atazanavir (12.0 and 9.3 L/h for visits 1 and 2) in the present study was higher than that previously reported in patients (8.5 L/h) (Haberl et al. 2010), although a population analysis reported 12.9 L/h in patients given ATV/r at 300/100 mg/day, similar to our present study (Colombo et al. 2006). For mean AUC0-24 and Cmax, estimates ranging from 40.2 to 49.6 h μg/mL and 4.0 to 4.5 μg/mL, respectively, were consistent with literature (Wang et al. 2011). These data together indicate that CEP-1347 does not substantially alter atazanavir or ritonavir pharmacokinetics.
The considerable inter-patient variability noted for CEP-1347 plasma concentrations and the absence of a control group of patients on CEP-1347 but not receiving ATV/RTV prevents definite conclusions on the effect of ATV/RTV on CEP-1347 pharmacokinetics. A comparison with historical data from healthy volunteers (data not shown) would suggest reduced CEP-1347 metabolism in the context of ATV/r co-administration compared with CEP-1347 administration alone. However, even in the case that such decreased metabolism could be confirmed, dose adjustment for CEP-1347 with concurrent use of protease inhibitors may not be necessary given that the drug was well tolerated and the aim is to achieve high CNS concentrations. The co-administration of CEP-1347 had a limited effect on ATV and RTV pharmacokinetics. These data suggest that no dose adjustment is needed for ATV/RTV with concurrent use of CEP-1347. In fact, during the 21-day study period, the average viral load in these patients decreased from 3.80±3.70 to 2.41±2.03 log copies/mL as a result of antiretroviral therapy with concurrent CEP-1347.
Our analysis has a number of strengths and limitations. Strengths included the first-in-patient clinical investigation and a systematic pharmacokinetic analysis of drug interactions between CEP-1347 and ATV/RTV in patients with HIV infection. However, the study was in general of small sample size and was not adequately powered to evaluate neurocognitive benefits. Although the effectiveness of CEP-1347 with regards to neurocognitive function improvement among HIV-infected patients remains to be determined, the findings from this analysis represents a potential challenge in new drug development for this indication as significant interactions with antiretroviral therapy might be clearly predictable from the drug metabolism profiles.
In conclusion, co-administration of CEP-1347 with atazanavir/ritonavir in HIV-infected patients might result in a limited impact on ATV but not RTV pharmacokinetics.
Methods
Clinical study
This was a prospective, open-label, controlled, multiple-dose study with 20 HIV-1-infected patients. The protocol was approved by the Institutional Review Board at the Universities of Rochester and Buffalo. All participants gave written informed consent. Inclusion criteria were as follows: (1) seropositive for HIV based on self-report, confirmed by ELISA and Western blot or detectable HIV RNA; (2) capable of giving informed consent; (3) stable antiretroviral regimen including ATV/RTV (Bristol-Myers Squibb, New York, NY) and tenofovir/emtricitabine (TDF/FTC, Gilead, Foster City, CA) for at least 4 weeks prior to enrollment and no antiretroviral regimen change for the duration of the study; (4) age 21 years and older; and women of childbearing potential must be using an adequate method of contraception to avoid pregnancy throughout the study and for up to 4 weeks after the study in such a manner that the risk of pregnancy is minimized. Exclusion criteria included: (1) active opportunistic infection and neoplasms; (2) patients taking efavirenz or any other nonnucleoside reverse transcriptase inhibitors; (3) current participation in other drug studies or receiving other investigational drugs within the previous 30 days of this trial; (4) women who are pregnant or nursing; (5) patients currently taking medications, over-the-counter (OTC) or herbal preparations known to interfere with drugs metabolized by the cytochrome P450 isoenzyme system including but not limited to ketoconazole, itraconazole, rifampin and erythromycin; (6) active liver disease or evidence of a significant decrease in liver function (LFT's >3 times upper limit of normal laboratory values at the University of Rochester Medical Center); (7) patients with pancreatitis (serum amylase must be <1.5 upper limit of normal (ULN), if >1.5 ULN then lipase must be <1.5 ULN); (8) current or recent (within 1 month) history of gastrointestinal disease or history of any GI surgery that could impact the absorption of CEP-1347; (9) ingestion of CEP-1347 within 30 days of screening; (10) history of recent (within 6 months) drug or alcohol abuse; (11) evidence of any clinical or laboratory determinations that in the opinion of the investigator would preclude the subject from safely participating in the study.
On day 1, the subjects were instructed to fast prior to receiving atazanavir/ritonavir (ATV/RTV, 300/100 mg, once daily) with a breakfast provided by dieticians as part of standard diet. Whole blood samples were collected at pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h post dose. Caffeine and citrus product consumption was restricted during the pharmacokinetic sampling. From days 2 to 21, participants then received 50 mg CEP-1347 (Cephalon, Frazer, PA) twice daily in addition to ATV/RTV with meals. Study drug adherence was assessed on days 2, 14, and 21 by pill count. The second 24-h pharmacokinetic evaluation was performed on day 21 following the standard procedures.
CEP-1347 assay
CEP-1347 was quantified utilizing a reverse phase high-performance liquid chromatography (RP-HPLC) method provided by the manufacture and validated in our laboratory. In brief, plasma samples were prepared for RP-HPLC assay using protein precipitation. After the addition of internal standard solution, 150 μL of acetonitrile was added to 100 μL of sample followed by vortexing and centrifugation. The resulting supernatant was filtered and 35 μL was injected onto the HPLC system. Analytical grade powder of CEP-1347 and the internal standard were provided by Cephalon on a material transfer agreement with the University at Buffalo. Calibration standards were linear from 0.500 to 200 ng/mL. Plasma quality controls were prepared at three different levels (160, 16.0, and 1.50 ng/mL) and were assayed in conjunction with patient samples to monitor precision and accuracy throughout the analysis. Intra-assay variation (coefficient of variation) ranged from 2.60 to 7.02 %, while the inter-assay variation ranged from 0.125 to 2.16 %. The lower limit of quantitation (LLOQ) value for CEP-1347 was 0.500 ng/mL.
ATV and RTV assay
Both antiretrovirals were quantified using a previously published simultaneous RP-HPLC assay with modification. The method involved a liquid–liquid extraction of the plasma sample, followed by separation on a C8 Symmetry™ column and detection at 248 nm for ATV and RTV, using a Model 996 photodiode array detector to assure specificity. Analytical grade powders were provided by NIH AIDS Research and Reference Reagent Program. Quality control concentrations were 300, 600, 3,200, and 12,800 ng/mL for atazanavir and 600, 3,200, and 12,800 ng/mL for ritonavir. The LLOQ values for atazanavir and ritonavir were 100 and 200 ng/mL, respectively. The coefficient of variation for the analytes was ≤9.32 % at all quality control levels. During the course of assay, inter-assay variation for atazanavir and ritonavir was ≤4.94 %. There were no interferences observed between CEP-1347 and atazanavir or ritonavir assays.
Pharmacokinetics analysis
Steady-state pharmacokinetic parameters for CEP-1347, atazanavir and ritonavir were determined using standard non-compartmental methods. These parameters included the area under the concentration–time curve (AUC0-24,), maximum plasma concentration (Cmax), time of Cmax (Tmax), elimination half-life (T1/2), accumulation ratio (R), and oral clearance (Clss/F), where F is the bioavailability. The maximum plasma concentration (Cmax) and Tmax were estimated by inspection of the individual patient profiles.
Statistical methods
Two-sided paired 0.05 level t tests were applied to the logarithmically transformed PK parameter data from visits 1–2 to test for changes. The magnitude of each change was estimated by its Geometric Mean Ratio (GMR), equivalent to exponentiating the difference in arithmetic means of the logarithmically transformed data, or equivalently computing the ratio of the geometric means. Ninety percent (90 %) confidence intervals (CI) were computed for each GMR and compared with the FDA bioequivalence region of (0.80, 1.25) to formally test bioequivalence of each PK parameter using the standard 0.10 level two-sided bioequivalence test suggested by the FDA. Demographic and clinical characteristics, as well as the values at each visit were summarized by the common arithmetic mean and standard deviation, proportions for categorical variables, or by the median and range for temporal parameters Tmax (in hour) and T1/2.
Study highlights.
CEP-1347 is a potent inhibitor of mixed lineage kinase (MLK) inhibitors and was investigated for ameliorating HIV-associated neurocognitive disorders. It is largely unknown if CEP-1347 interacts with current antiretroviral therapy.
The objective of the study was to investigate the potential drug interactions between CEP-1347 and commonly prescribed protease inhibitors, atazanavir, and ritonavir.
Co-administration of CEP-1347 with atazanavir and ritonavir in HIV-infected patients might result in a limited impact on atazanavir but not ritonavir pharmacokinetics.
These findings can be used to facilitate new drug development for HIV-infected population.
Acknowledgments
The dedication of the clinical research staff of the Center for Human Experimental Therapeutics and the Clinical and Translational Science Institute at University of Rochester, and the Translational Pharmacology Research Core at the New York State Center of Excellence in Bioinformatics and Life Sciences, University at Buffalo, is appreciated. This project was supported in part by grant P01MH064570 from the National Institute of Mental Health. Dr. Qing Ma is currently supported by grant K08MH098794.
Footnotes
Conflict of interest The authors declared no conflict of interest.
Contributor Information
Qing Ma, Center for Human Experimental Therapeutics, Clinical and Translational Sciences Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Translational Pharmacology Research Core, New York State Center of Excellence in Bioinformatics and Life Sciences, Buffalo, NY, USA, Department of Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, University at Buffalo, Buffalo, NY, USA.
Harris A. Gelbard, Department of Neurology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Department of Microbiology and Immunology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Center for Neural Development and Disease, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
Sanjay B. Maggirwar, Department of Microbiology and Immunology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
Stephen Dewhurst, Department of Microbiology and Immunology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA.
Howard E. Gendelman, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USADepartment of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA
Derick R. Peterson, Department of Biostatistics and Computational Biology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
Robin DiFrancesco, Translational Pharmacology Research Core, New York State Center of Excellence in Bioinformatics and Life Sciences, Buffalo, NY, USA.
Jill S. Hochreiter, Translational Pharmacology Research Core, New York State Center of Excellence in Bioinformatics and Life Sciences, Buffalo, NY, USA
Gene D. Morse, Department of Pharmacology and Physiology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Center for Human Experimental Therapeutics, Clinical and Translational Sciences Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Translational Pharmacology Research Core, New York State Center of Excellence in Bioinformatics and Life Sciences, Buffalo, NY, USA, Department of Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, University at Buffalo, Buffalo, NY, USA
Giovanni Schifitto, Email: giovanni.schifitto@rochester.edu, Department of Neurology, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Department of Imaging Sciences, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Clinical Research Center, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA, Department of Neurology, Movement and Inherited Neurologic Disorders, Clinical Trials Coordination Center, 1351 Mount Hope Avenue, Suite 223, Rochester, NY 14620, USA.
References
- Bodner A, et al. Mixed lineage kinase 3 mediates gp120IIIB-induced neurotoxicity. J Neurochem. 2002;82:1424–1434. doi: 10.1046/j.1471-4159.2002.01088.x. [DOI] [PubMed] [Google Scholar]
- Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188:246–253. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, et al. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. J Neurochem. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Colombo S, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50:3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, et al. Neuroprotective activities of CEP-1347 in models of neuroAIDS. J Immunol. 2010;184:746–756. doi: 10.4049/jimmunol.0902962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, et al. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Haberl A, et al. Atazanavir plasma concentrations are impaired in HIV-1-infected adults simultaneously taking a methadone oral solution in a once-daily observed therapy setting. Eur J Clin Pharmacol. 2010;66:375–381. doi: 10.1007/s00228-009-0767-8. [DOI] [PubMed] [Google Scholar]
- Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- Harris C, Maroney AC, Johnson EM., Jr Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis. J Neurochem. 2002;83:992–1001. doi: 10.1046/j.1471-4159.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Kaneko M, et al. Neurotrophic 3,9-bis[(alkylthio)methyl]-and-bis(alkoxymethyl)-K-252a derivatives. J Med Chem. 1997;40:1863–1869. doi: 10.1021/jm970031d. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, et al. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Lund S, et al. Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. J Neurochem. 2005;92:1439–1451. doi: 10.1111/j.1471-4159.2005.03014.x. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. Protein kinase C alpha requirement in the activation of p38 mitogen-activated protein kinase, which is linked to the induction of tumor necrosis factor alpha in lipopolysaccharide-stimulated microglia. Neurochem Int. 2004;44:205–214. doi: 10.1016/s0197-0186(03)00163-3. [DOI] [PubMed] [Google Scholar]
- Namgung U, Xia Z. Arsenite-induced apoptosis in cortical neurons is mediated by c-Jun N-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J Neurosci. 2000;20:6442–6451. doi: 10.1523/JNEUROSCI.20-17-06442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273:17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- Perry SW, et al. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, et al. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, et al. Preservation of cholinergic activity and prevention of neuron death by CEP-1347/KT-7515 following excitotoxic injury of the nucleus basalis magnocellularis. Neuroscience. 1998;86:461–472. doi: 10.1016/s0306-4522(98)00059-1. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J Pharmacol Exp Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- Saporito MS, Thomas BA, Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- Sui Z, et al. Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol. 2006;177:702–711. doi: 10.4049/jimmunol.177.1.702. [DOI] [PubMed] [Google Scholar]
- Suzuki T, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley AK, et al. Tumor necrosis factor alpha-induced apoptosis in human neuronal cells: protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol Cell Biol. 1995;15:2359–2366. doi: 10.1128/mcb.15.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter L, et al. Mitogen-activated protein kinase kinase 7 is activated during low potassium-induced apoptosis in rat cerebellar granule neurons. Neurosci Lett. 2002;320:29–32. doi: 10.1016/s0304-3940(02)00005-8. [DOI] [PubMed] [Google Scholar]
- Troy CM, et al. beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- Wang LH, Besirli CG, Johnson EM., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu Rev Pharmacol Toxicol. 2004;44:451–474. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Effects of the H2-receptor antagonist famotidine on the pharmacokinetics of atazanavir-ritonavir with or without tenofovir in HIV-infected patients. AIDS Patient Care STDS. 2011;25:509–515. doi: 10.1089/apc.2011.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, et al. HIV-1-mediated apoptosis of neuronal cells: proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc Natl Acad Sci U S A. 2004;101:7070–7075. doi: 10.1073/pnas.0304859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Zhang K, et al. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]