Abstract
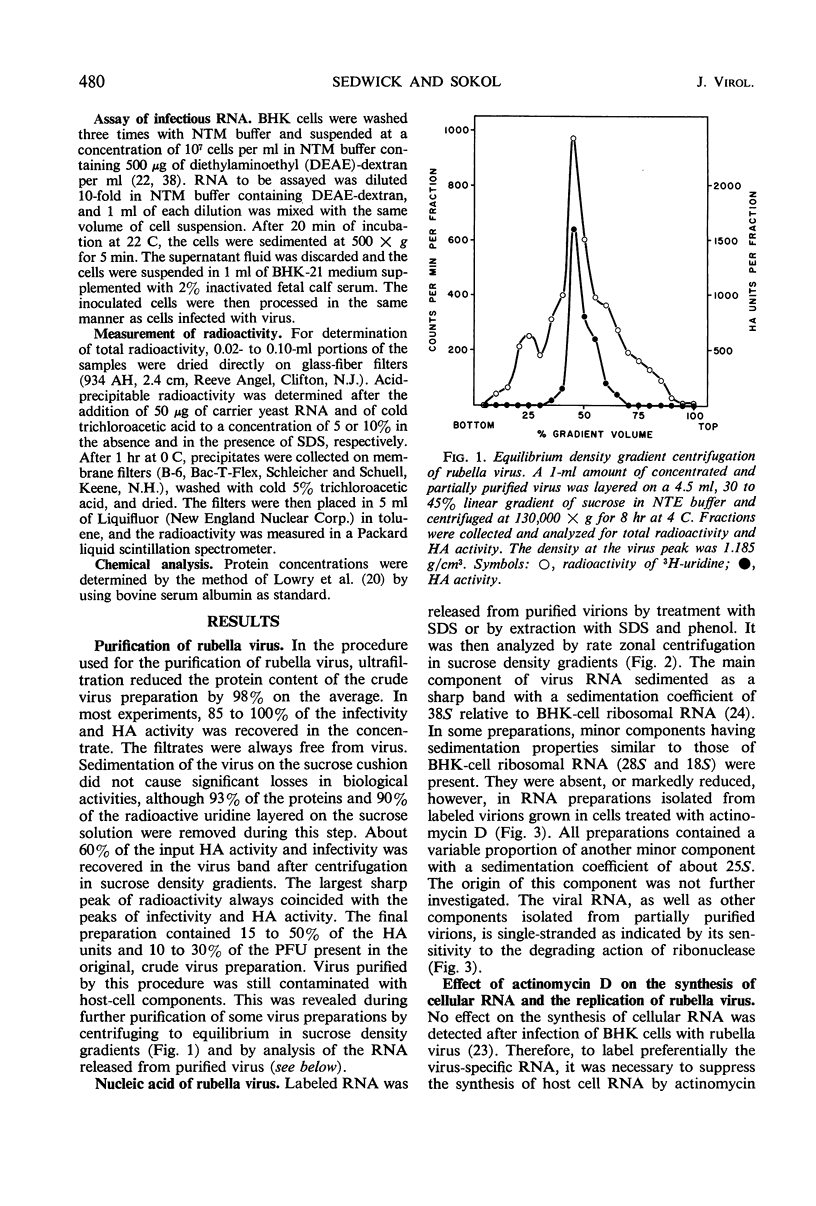
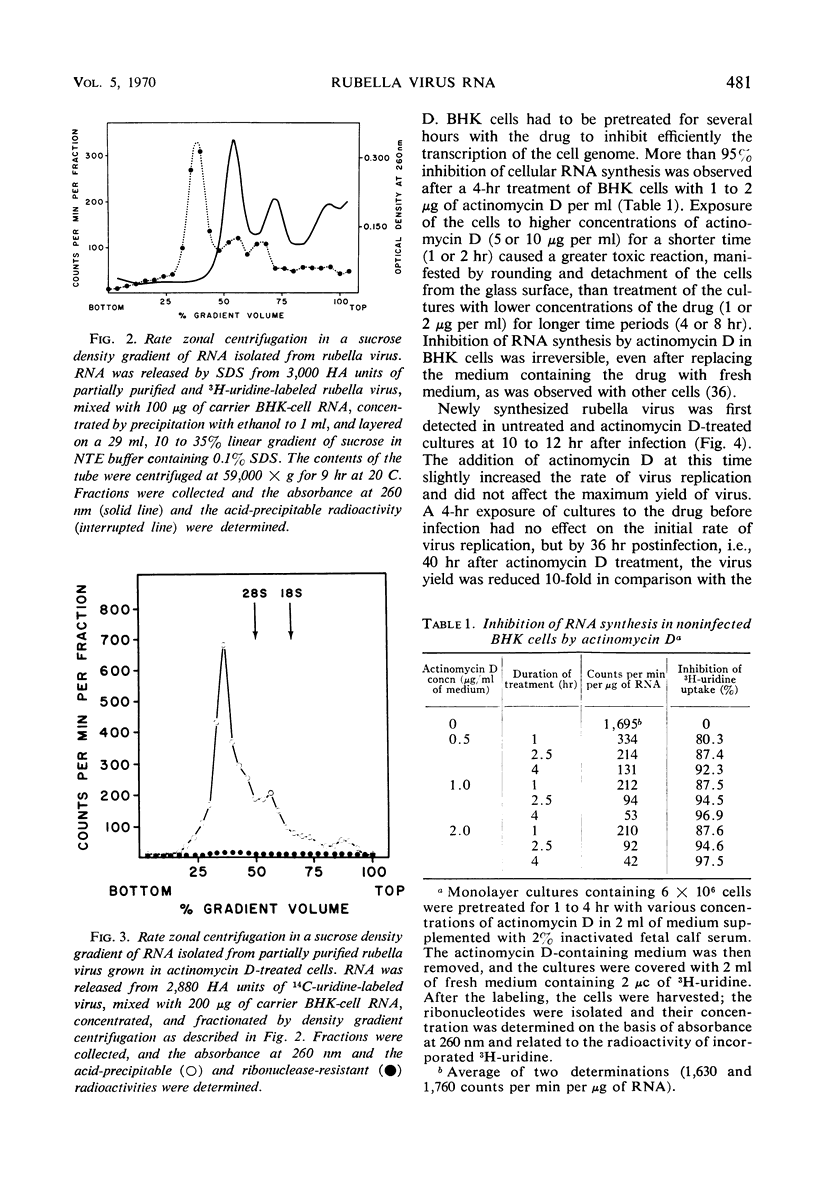
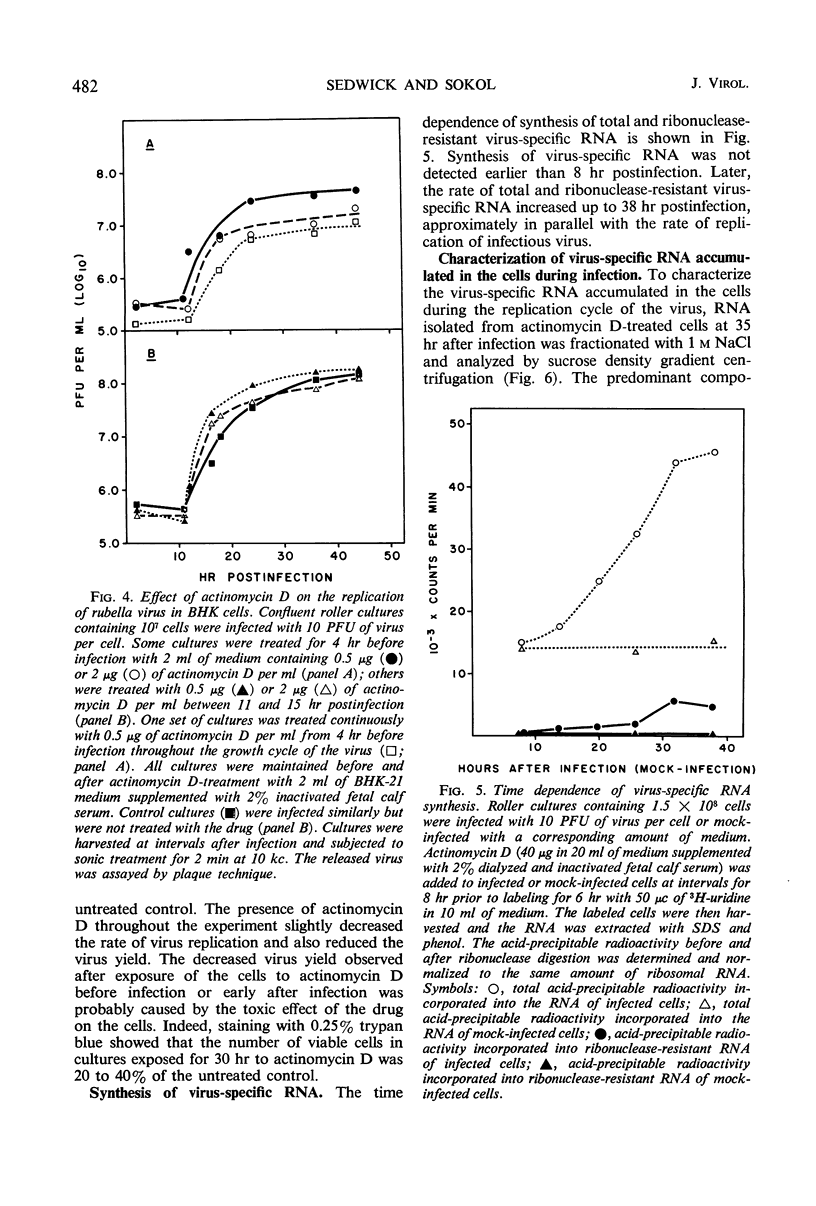
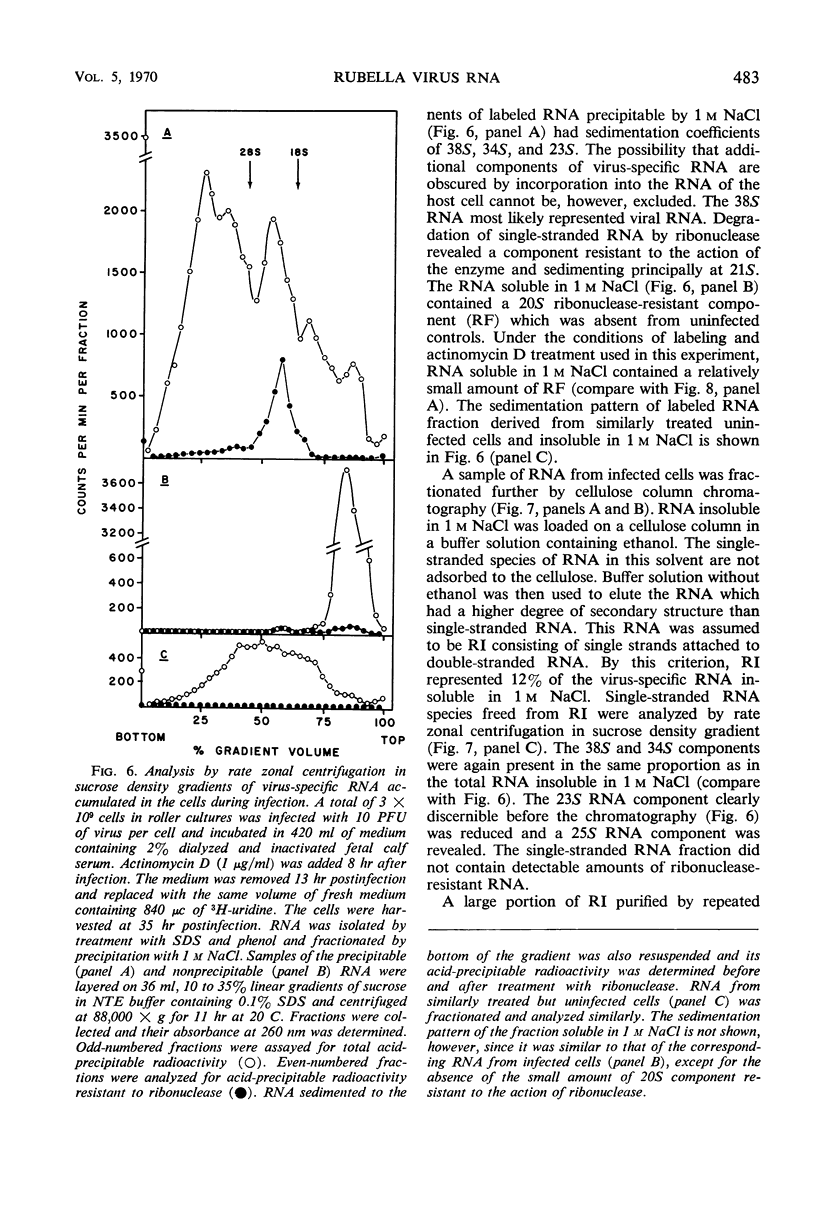
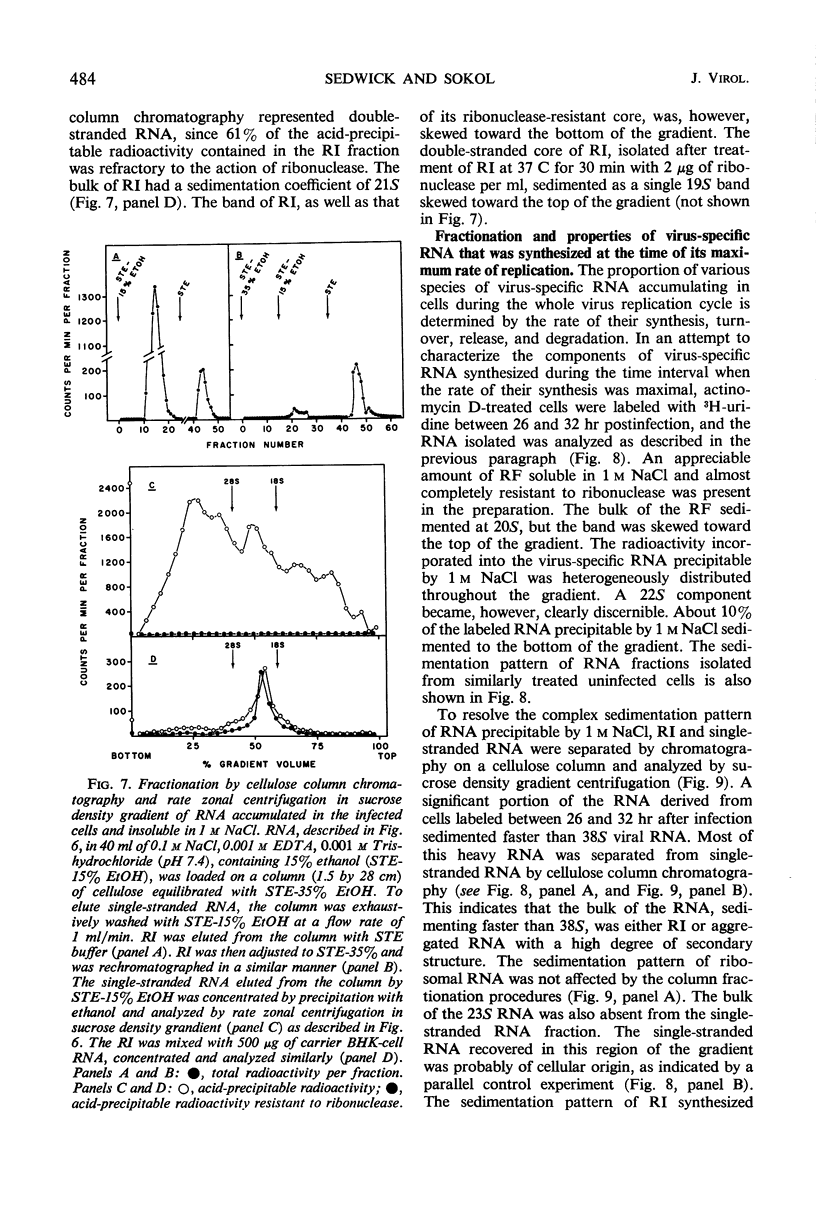
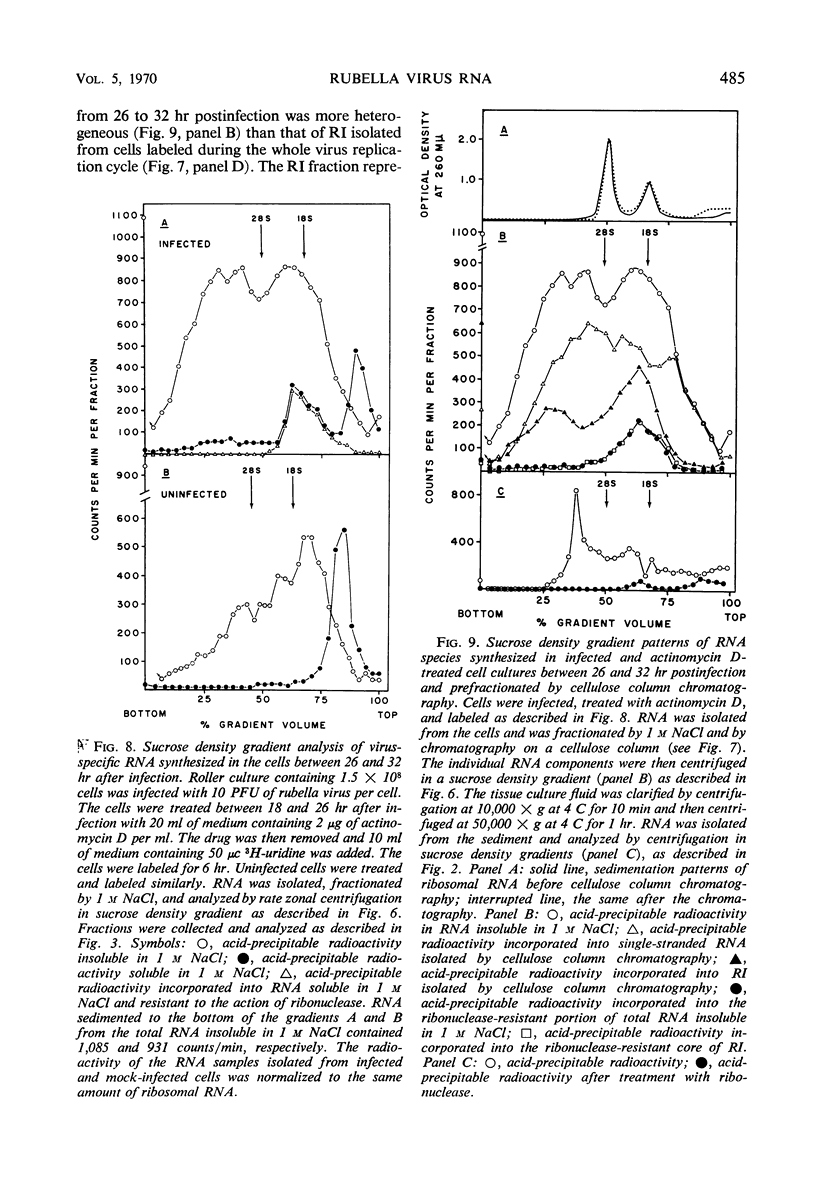
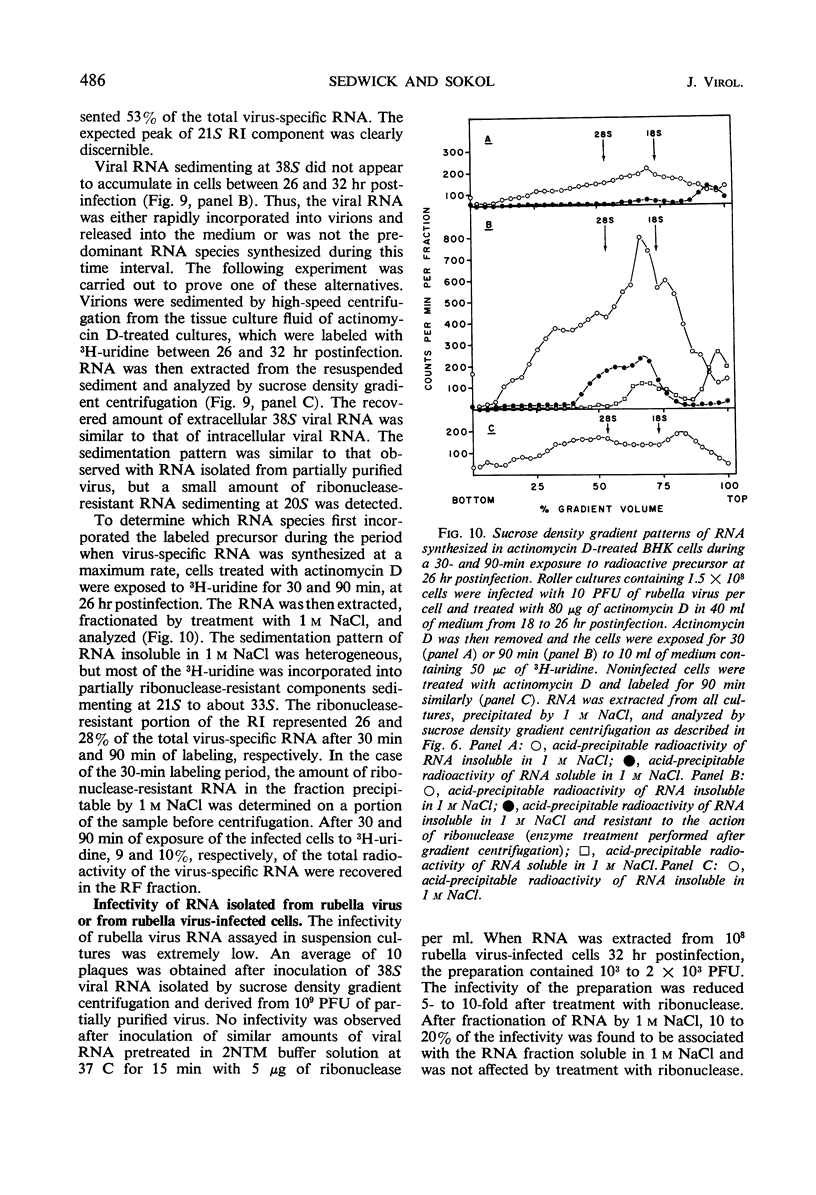
Ribonucleic acid (RNA) has been isolated from partially purified rubella virus preparations and fractionated by rate zonal centrifugation in sucrose density gradients. The bulk of the RNA sedimented as a sharp band with a sedimentation coefficient of 38S. Rubella virus RNA appears to be single-stranded on the basis of its sensitivity to the degrading action of ribonuclease. Fractionation by precipitation with 1 m NaCl, followed by chromatography on cellulose columns, and by rate zonal centrifugation in sucrose density gradients of labeled RNA isolated from actinomycin D-treated and infected baby hamster kidney cells revealed the presence of the following virus-specific types of RNA: (i) single-stranded RNA with a heterogeneous sedimentation pattern, the 38S viral RNA becoming the predominant species only after long periods of labeling late after infection; (ii) double-stranded RNA with a sedimentation coefficient of 20S; (iii) RNA apparently composed of 20S double-stranded RNA and single-stranded branches. On the basis of their properties, the last two species were tentatively identified as the replicative form and the replicative intermediate of rubella virus RNA. Rubella virus RNA was infectious.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Girard M. An intermediate in the synthesis of poliovirus RNA. Proc Natl Acad Sci U S A. 1966 Aug;56(2):741–748. doi: 10.1073/pnas.56.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Infectious replicative intermediate of poliovirus: purification and characterization. Virology. 1969 Apr;37(4):521–534. doi: 10.1016/0042-6822(69)90270-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G. Purification and characterization of poliovirus-induced infectious double-stranded ribonucleic acid. J Biol Chem. 1967 Apr 25;242(8):1736–1743. [PubMed] [Google Scholar]
- Brodersen M., Thomssen R. Einbau von 3H-Uridin in Rubellaviruspartikel. Arch Gesamte Virusforsch. 1969;26(1):118–126. [PubMed] [Google Scholar]
- Edwards M. R., Cohen S. M., Bruno M., Deibel R. Micromorphological aspects of the development of rubella virus in BHK-21 cells. J Virol. 1969 Apr;3(4):439–444. doi: 10.1128/jvi.3.4.439-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., ERIKSON R. L., FRANKLIN R. M. REPLICATION OF THE RNA OF BACTERIOPHAGE R17. Science. 1964 Oct 23;146(3643):527–530. doi: 10.1126/science.146.3643.527. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Berezesky I. K. Cytoplasmic fractions associated with Semliki Forest virus ribonucleic acid replication. J Virol. 1967 Apr;1(2):374–383. doi: 10.1128/jvi.1.2.374-383.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Replicative intermediate of an arbovirus. J Virol. 1968 Jun;2(6):547–552. doi: 10.1128/jvi.2.6.547-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamvas J. J., Ugovsek S., Iwakata S., Labzoffsky N. A. Virus particles in rubella infected tissue cultures. (brief report). Arch Gesamte Virusforsch. 1969;26(3):287–294. doi: 10.1007/BF01242381. [DOI] [PubMed] [Google Scholar]
- Holmes I. H., Wark M. C., Warburton M. F. Is rubella an arbovirus? II. Ultrastructural morphology and development. Virology. 1969 Jan;37(1):15–25. doi: 10.1016/0042-6822(69)90301-8. [DOI] [PubMed] [Google Scholar]
- KELLY R. B., GOULD J. L., SINSHEIMER R. L. THE REPLICATION OF BACTERIOPHAGE MS2. IV. RNA COMPONENTS SPECIFICALLY ASSOCIATED WITH INFECTION. J Mol Biol. 1965 Mar;11:562–575. doi: 10.1016/s0022-2836(65)80011-0. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. Ribonucleic acids. II. Improved preparation of rat-liver ribonucleic acid. Biochim Biophys Acta. 1962 Apr 2;55:545–546. doi: 10.1016/0006-3002(62)90988-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAASSAB H. F., COCHRAN K. W. INFLUENCE OF 5-FLUORODEOXYURIDINE ON GROWTH CHARACTERISTICS OF RUBELLA VIRUS. Proc Soc Exp Biol Med. 1964 Nov;117:410–413. doi: 10.3181/00379727-117-29595. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- Maes R., Vaheri A., Sedwick D., Plotkin S. Synthesis of virus and macromolecules by rubella-infected cells. Nature. 1966 Apr 23;210(5034):384–385. doi: 10.1038/210384a0. [DOI] [PubMed] [Google Scholar]
- Mettler N. E., Petrelli R. L., Casals J. Absence of antigenic cross-reactions between rubella virus and arbouviruses. Virology. 1968 Nov;36(3):503–504. doi: 10.1016/0042-6822(68)90175-x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Halonen P. E., Harrison A. K. Electron microscopy of the development of rubella virus in BHK-21 cells. J Virol. 1968 Oct;2(10):1223–1227. doi: 10.1128/jvi.2.10.1223-1227.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKMAN P. D. BIOLOGICAL CHARACTERISTICS OF RUBELLA VIRUS. Arch Gesamte Virusforsch. 1965;16:401–411. doi: 10.1007/BF01253846. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Cornfeld D., Ingalls T. H. Studies of immunization with living rubella virus. Trials in children with a strain cultured from an aborted fetus. Am J Dis Child. 1965 Oct;110(4):381–389. doi: 10.1001/archpedi.1965.02090030401007. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Hobbins T. E. Physical characteristics of rubella virus. J Immunol. 1969 Apr;102(4):1016–1023. [PubMed] [Google Scholar]
- Spiegelman S., Pace N. R., Mills D. R., Levisohn R., Eikhom T. S., Taylor M. M., Peterson R. L., Bishop D. H. The mechanism of RNA replication. Cold Spring Harb Symp Quant Biol. 1968;33:101–124. doi: 10.1101/sqb.1968.033.01.015. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr, Dodson M. L., Jr, Hartman K. A. Replication of Western equine encephalomyelitis virus. I. Some chemical and physical characteristics of viral ribonucleic acid. J Virol. 1968 Jun;2(6):558–566. doi: 10.1128/jvi.2.6.558-566.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr Heterogeneous RNA's occurring during the replication of Western equine encephalomyelitis virus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):974–981. doi: 10.1073/pnas.55.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Pagano J. S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965 Nov;27(3):434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A. Growth of rubella virus in BHK21 cells. II. Enhancing effect of DEAE-dextran, semicarbazide and low doses of metabolic inhibitors. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1092–1098. doi: 10.3181/00379727-125-32284. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Walder R., Munz K. Propiedades físicas y microscopia electrónica del virus rubeola. Acta Cient Venez. 1968;19(6):212–217. [PubMed] [Google Scholar]
- Weissmann C., Feix G., Slor H. In vitro synthesis of phage RNA: the nature of the intermediates. Cold Spring Harb Symp Quant Biol. 1968;33:83–100. doi: 10.1101/sqb.1968.033.01.014. [DOI] [PubMed] [Google Scholar]



