Abstract
This study was designed to identify TGF-β signaling pathway-related serum microRNAs (miRNAs) as predictors of survival in advanced non-small cell lung cancer (NSCLC). Serum samples from 391 patients with advanced NSCLC were collected prior to treatment. Global miRNA microarray expression profiling based on sera from four patients with good survival (>24 months) and four patients with poor survival (<6 months) was used to identify 140 highly expressed serum miRNAs, among which 35 miRNAs had binding sites within the 3’-untranslated regions of a panel of 11 genes in the TGF-β signaling pathway and were assayed by quantitative RT-PCR for their associations with survival in a training (n=192) and testing set (n=191). Out of the 35 miRNAs, survival analysis using Cox regression model identified 17 miRNAs significantly associated with 2-year patient survival. MiR-16 exhibited the most statistically significant association: high expression of miR-16 was associated with a significantly better survival (adjusted hazard ratio = 0.4, 95% confidence interval: 0.3–0.5). A combined 17-miRNA risk score was created that was able to identify patients at the highest risk of death. Those with a high risk score had a 2.5-fold increased risk of death compared to those with a low risk score (95% CI=1.8–3.4, P=1.1×10−7). This increase in risk of death was corresponding to an 7.8 month decrease in median survival time (P=9.5×10−14). Our results suggest that serum miRNAs could serve as predictors of survival for advanced NSCLC.
Keywords: serum miRNA, TGF-β, survival, NSCLC
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers, and its overall 5-year survival rate is only 17% (1). Approximately 65–75% of patients with NSCLC possess unresectable advanced or metastatic disease at diagnosis (2). The overall response rate for platinum-based therapy, which is the standard of care for these patients, ranges between 17% and 32% (3), with a median survival time of only 7–9 months (4). The current challenge posed by the rather short survival time in patients with advanced NSCLC demands the discovery of new biomarkers that enable prediction of survival time and categorization of different prognostic groups, thereby enabling health care providers to personalize treatment and helping patients to manage expectations effectively.
Alterations in miRNA expression have been known to affect multiple cellular processes, including cell differentiation, cell cycle progression, and apoptosis (5–7). It has been clearly demonstrated that miRNAs are of potential clinical relevance and possess enormous promise to serve as novel biomarkers in cancer diagnosis and prognosis (8–10). Stable existence of cell-free miRNA in plasma/serum has been repeatedly demonstrated in numerous studies (11–13), and alterations in miRNA profiles or expression levels are potentially indicative of physiological and pathological change. A number of studies have demonstrated the potential of miRNAs in plasma/serum as biomarkers for specific cancer diagnosis and prognosis (14). In this study, we aimed to identify potential serum miRNA biomarkers for predicting patient survival in advanced-stage NSCLC. We demonstrate that expression levels of selected serum miRNAs are significantly associated with patient survival.
To focus on serum miRNAs associated with the cancer signaling network, we utilized a pathway-based miRNA profiling approach, specifically focusing on the transforming growth factor-beta (TGF-β) pathway. This pathway plays critical roles in control of cell proliferation, differentiation, apoptosis, adhesion, invasion, and the cellular microenvironment (15). TGF-β signaling has tumor suppressor effects during the early phase of tumor progression and, in contrast, assists in tumor progression by promoting tumor cell invasion, dissemination, and immune evasion in late-stage disease (16).
Materials and Methods
Patient Population and Clinical Data Collection
This study was conducted in 391 Caucasian patients with newly diagnosed, histologically confirmed NSCLC recruited at the University of Texas MD Anderson Cancer Center between January 2002 and January 2009, which is part of an ongoing lung cancer study initiated in 1991. None of the patients had undergone treatment prior to study enrollment. All study participants signed informed consent and underwent a 45-min in-person interview by trained MD Anderson staff with a structured questionnaire that elicited information on demographic characteristics, medical history and smoking history. Immediately after each interview, a 40-mL peripheral blood sample was drawn. About 10 ml was drawn into a gold top serum-separating tube, processed for serum extraction within two hours, and then transferred into liquid nitrogen tanks for long term storage. Clinical and follow-up data were abstracted from medical charts. Serum samples were selected retrospectively at the time of analysis according to the following requirements: 1) the patient had been diagnosed with stage III-IV NSCLC, 2) serum was available in sufficient volume for RNA isolation, and 3) demographic, clinical, and follow-up data for the patient were available. There were a total of 1,060 advanced NSCLC Caucasian patients recruited during the study period. We excluded 12 patients without sufficient serum volume, one patient with >10 years of serum storage, 17 patients with serum samples collected after treatment started, 201 newly recruited patients with demographic and clinical data yet to be input into database at the time of sample selection, and 22 patients lost to follow-up prior to 2 years. Among the remaining 807 patients, 484 patients had their serum samples stored in Cryo vials and the other 323 serum samples were stored in MAPI straws (Cryo Bio System). To minimize the effect of serum storage condition on miRNA expression, we only used the 484 samples stored in Cryo vials. After excluding 29 patients with hemolyzed serum samples upon visual determination and 64 patients without sufficient serum RNA concentration after isolation, 391 patients were included in our final dataset. Comparisons of the patients included in this study to the overall study population of 1,060 showed no differences by basic demographic and clinical data distribution including age, gender, smoking status, disease stage, histology, grade, performance status and treatment regimens, except that patients included in this study had a slightly high death rate. This would be expected given that the majority of excluded patients were recently recruited patients who were still alive and did not have sufficient follow-up time. Study approval was obtained from the MD Anderson Institutional Review Board.
The initial global miRNA screening included eight Caucasian patients, four with good survival (survival time >24 months) and four with poor survival (survival time <6 months), matched by age, sex, histological stage, and smoking status (Figure 1). Assignment of the 383 samples to the training and testing populations was performed by sorting the samples by enrollment date and then distributing in an alternating manner (Table 1).
Figure 1.
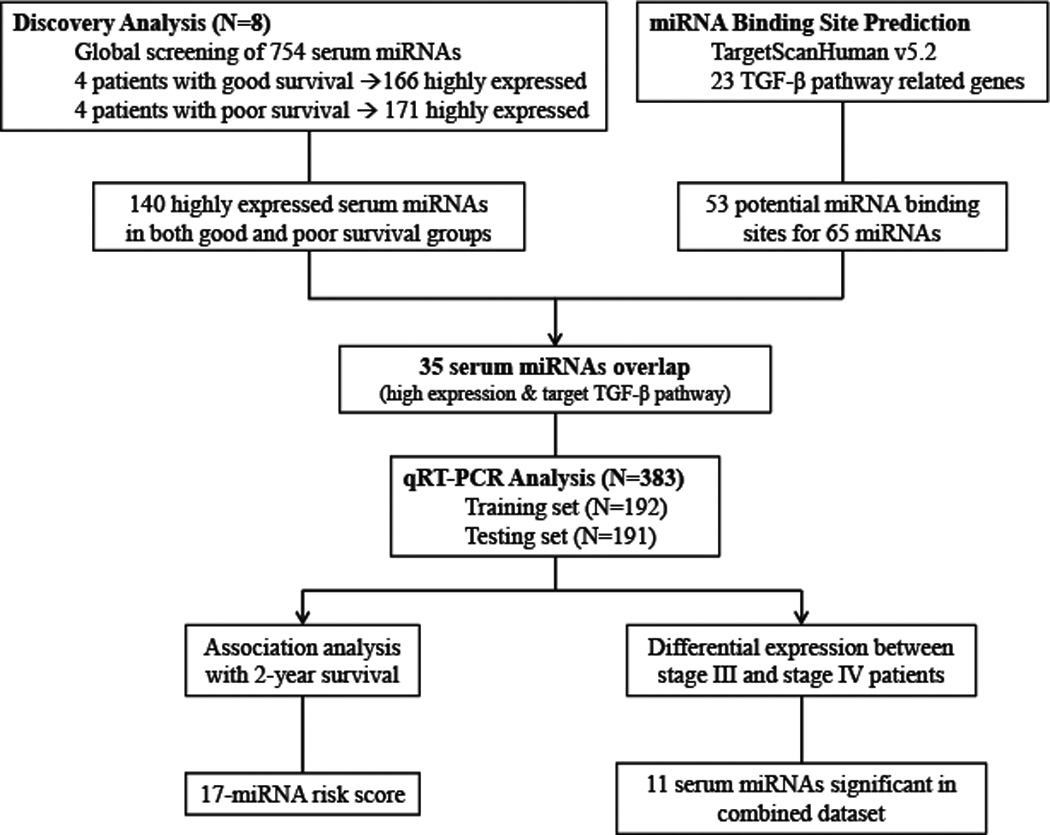
Schematic of study design.
Table 1.
Selected characteristics of NSCLC patient population recruited at the University of Texas MD Anderson Cancer Center between 2002 and 2009.
| Characteristics | Study population (N=391) |
Training set (N=192) |
Testing set (N=191) |
|---|---|---|---|
| Vital status, n (%) | |||
| Alive | 57(14.6) | 33(17.2) | 24(12.6) |
| Dead | 334(85.4) | 159(82.8) | 167(87.4) |
| Mean age, years (SD) | 62.05(11.3) | 63.41(11.3) | 60.9(11.3) |
| Sex | |||
| Male | 203(51.9) | 107(55.7) | 92(48.2) |
| Female | 188(48.1) | 85(44.3) | 99(51.8) |
| Smoking status, n (%) | |||
| Never | 80(20.5) | 34(17.7) | 45(23.6) |
| Former | 165(42.2) | 91(47.4) | 70(36.7) |
| Current | 146(37.3) | 67(34.9) | 76(39.8) |
| TNM classification, n (%) | |||
| IIIA | 58(14.8) | 31(16.2) | 27(14.1) |
| IIIB (dry) | 63(16.1) | 31(16.2) | 30(15.7) |
| IIIB (wet) | 28(7.2) | 12(6.3) | 14(7.3) |
| IV | 242(61.9) | 118(61.5) | 120(62.8) |
| Histology | |||
| Adenocarcinoma | 214(54.7) | 97(50.5) | 114(59.7) |
| Squamous | 87(22.3) | 44(22.9) | 39(20.4) |
| Other | 12(3.1) | 51(26.6) | 38(19.9) |
| Performance status, n (%) | |||
| 0 | 82(21.0) | 43(22.4) | 39(20.4) |
| 1 | 186(47.6) | 95(49.5) | 85(44.5) |
| 2–4 | 76(19.4) | 34(17.7) | 40(20.9) |
| Unknown | 47(12.0) | 20(10.4) | 27(14.1) |
| Histology grade, n (%) | |||
| Well differentiated | 20(5.1) | 8(4.2) | 12(6.3) |
| Moderately differentiated | 60(15.4) | 26(13.5) | 34(17.8) |
| Poorly differentiated | 141(36.1) | 70(36.5) | 68(35.6) |
| Undifferentiated | 5(1.3) | 5(2.6) | 0(0.0) |
| Unknown | 165(42.2) | 83(43.2) | 77(40.3) |
| Treatment regimena, n (%) | |||
| Chemotherapy | 100(25.6) | 43(22.4) | 52(27.2) |
| Chemoradiotherapya | 114(29.2) | 61(31.8) | 50(26.2) |
| Chemotherapy+Surgery | 18(4.6) | 8(4.2) | 10(5.2) |
| Chemoradiotherapya+Surgery | 17(4.4) | 10(5.2) | 7(3.7) |
| Surgery | 15(3.8) | 3(1.6) | 12(6.3) |
| Raidation | 53(13.6) | 30(15.6) | 23(12) |
| Radiation+Surgery | 5(1.3) | 1(0.5) | 4(2.1) |
| No treatment | 69(17.7) | 36(18.8) | 33(17.3) |
| MSTb, months | 10.3 | 10.3 | 10.0 |
| MFTc, months (range) | 10.3(1.0–85.7) | 10.4(1.0–76.2) | 10.0(1.6–85.7) |
Combined chemotherapy and radiation concurrently or consequentially;
MST=median survival time;
MFT=median follow-up time.
Total RNA Isolation
Total RNA was isolated from serum samples using the miRNeasy Mini Kit (Qiagen) following the manufacturer’s protocol. For whole-genome miRNA profiling, 700 µL serum was used for RNA isolation, and for qRT-PCR assay, the serum amount was reduced to 400 µL. Synthetic cel-miR-39 and cel-miR-54 were added to each sample as internal controls for evaluation of successful extraction. RNAs were eluted twice with 30 µL of water. The total concentration of small RNA molecules was quantified by NanoDrop ND-1000 spectrometer (Thermo Scientific, DE). To minimize any potential inaccuracies generated by the Nanodrop measurements, we carefully kept consistent the amount of serum samples and spike-in miRNAs prepared from the same batch for isolation - throughout all experiments. In addition, the raw Ct value for each miRNA was normalized to the raw Ct value for spike-in miRNAs obtained from each individual sample to eliminate potential variations introduced from the isolation process and RNA quantification. To eliminate potential hemolysis-introduced miRNA bias, 30 serum samples were random selected and tested for free hemoglobin levels on VITROS® Fusion 5.1 Chemistry System (Ortho Clinical Diagnostics) at the University of Texas MD Anderson Cancer Center Core Laboratory by standard protocol.
Selection of TGF-β Signaling Pathway Related Serum MiRNAs
Whole-genome serum miRNA profiling was performed on the eight serum samples using the TaqMan Human MicroRNA Microarray Card Set v3.0 (Applied Biosystems, CA). In brief, purified serum RNA samples were reverse-transcribed using Megaplex RT Primers followed by a pre-amplification step using Megaplex PreAmp Primers for maximum sensitivity of detection. For final quantification, the TaqMan Universal Master Mix II was added, and each sample was loaded on the MicroRNA Microarray Cards. Quantitative PCR was performed on the 7900HT Fast Real-Time PCR System (Applied Biosystems). The expression threshold for each miRNA detector was automatically determined. A miRNA candidate was considered highly expressed when over 75% of samples in both study groups generated detectable Ct values of less than 35.
To identify miRNA candidates associated with the TGF-β signaling pathway, a total of 23 candidate genes were selected utilizing Gene Ontology (http://www.geneontology.org) and the National Center for Biotechnology Information (NCBI) PubMed (http://www.ncbi.nlm.nih.gov) databases as previously described (17). MiRNAs potentially binding these candidate genes were predicted by TargetScanHuman v5.2 (www.targetscan.org) (18), and miRNAs associated with the binding sites broadly conserved among vertebrates with higher probability of preferential scores (19) were considered for further analysis.
Quantitative Real-Time PCR Assay
Selected miRNAs were tested using individual TaqMan MiRNA Assays (Applied Biosystems) to quantitatively measure the difference in their expression levels between patient groups with varied survival times. Each assay was tested in duplicates. Data points that generated duplicated Ct values with over one cycle variance were excluded from analysis. The mean Ct value obtained from each sample was normalized to the averaged expression of spike-in miRNAs and then subjected to analysis with the 2−ΔΔCt method (20).
Statistical Analysis
Statistical analyses were performed using Intercooled STATA software, version 10 (College Station, TX). χ2 analysis was used to evaluate differences in patient characteristics. Survival time was calculated from the date of study enrollment to the date of death or last patient follow-up. The median time between diagnosis of NSCLC and study enrollment (date of blood draw) was 24 days. Rank sum test was used to assess differences in median miRNA expression levels between stage III and stage IV patients. The associations between 2-year survival and serum miRNA expression levels were estimated as hazard ratios (HRs) and 95% confidence intervals (CIs) for each miRNA using the Cox proportional hazards model, adjusted for age, sex, smoking status, clinical stage, and treatment regimen. Patients with survival times over 24 months were censored at 24 months in Cox regression analysis. In all statistical analyses, a P value ≤ 0.05 was considered significant. The combined 17-miRNA risk score for each patient was derived by linear combination of the product of reference-normalized expression level of each miRNA by its Cox regression corresponding coefficient (21). All patients were dichotomized by the median risk score, and individuals with a risk score higher or lower than the median were classified as high or low risk groups, respectively. The optimized statistical method, C-index was utilized to evaluate predictive accuracy of 2-year survival (22).
Results
Patient Characteristics
A total of 391 Caucasian patients with advanced NSCLC were included in the analysis that covered a broad range of follow-up and survival times (Table 1). For the initial global miRNA screening using eight samples, survival times in the poor- and good-survival groups ranged from 2.47 to 3.82 months and from 24.61 to 65.23 months, respectively. For the overall patient study population, there was no significant difference between the training set and testing set with respect to vital status, sex, smoking status, performance status, TNM stage, or histology distribution (Table 1). A slight difference was seen between the two populations in terms of age, which however, was adjusted in subsequent analysis. At the time of analysis, 326 (85.1%) patients had died and 57 (14.9%) were still alive, all of which had survived for over two years.
Whole-Genome Serum MiRNA Profiling
In the combined pool of 754 human miRNAs, excluding endogenous controls and low-quality data points, 166 and 171 serum miRNAs were identified to be highly expressed in the poor- and good-survival groups, respectively, with 140 serum miRNAs were highly expressed in both groups. MiRNA target prediction using the TargetScan database identified 53 potential binding sites for 65 miRNAs in the 3’-UTR of 11 TGF-β pathway genes (Supplementary Data: Table S1). Cross referencing of this list of miRNAs with the 140 highly expressed serum miRNAs identified 35 candidate miRNAs for further analysis (Figure 1). These 35 miRNA candidates included members in families associated with cancer progression processes, such as the let-7 family (23), the miR-17~92 cluster (24), and the miR-200 family (25, 26) (Supplementary material, Table S2). In addition, it was noteworthy that one of the candidates, miR-30d, was one of the four biomarkers previously identified to be predictive of survival in early-stage NSCLC (27).
Association of Serum MiRNA Expression with NSCLC Survival
The 35 candidate miRNAs were assessed for association with survival duration. In the training set, expression levels of 25 serum miRNAs were significantly associated with 2-year survival using the median expression levels as analysis cutoff (data not shown). When the same cutoffs were applied to the testing set, 17 out of 25 miRNAs remained significant with the same direction of effect (Table 2). The results showed that higher expression levels (above the median value) of these 17 serum miRNAs were significantly associated with higher probability of 2-year survival in both the training and testing sets using the same cutoff points. MiR-16 presented the most significant result: in the combined dataset analysis, the 2-year survival rate in the high-expression group reached 37.9%, whereas only 3 of 174 patients (1.7%) in the low-expression group survived over 2 years, with an adjusted HR of 0.4 (95% CI: 0.3–0.5), and a P value of 1.7×10−10. The MST advantage in the high-expression group compared with the low-expression group ranged from 2.8 months to 11.5 months among these 17 serum miRNAs (Figure 2A).
Table 2.
Serum miRNA expression associated with 2-year survival in patients with advanced NSCLC from the study population recruited at the University of Texas MD Anderson Cancer Center between 2002 and 2009.
| Expression levela | Training set | Testing set | Combined set | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-year survival | 2-year survival | 2-year survival | ||||||||||||||
| N (No) | N (Yes) | Adjusted HRb (95% CI) |
P | MST | N (No) | N (Yes) | Adjusted HRb (95% CI) |
P | MST | N (No) | N (Yes) | Adjusted HRb (95% CI) |
P | MST | ||
| n% | n% | n% | n% | n% | n% | |||||||||||
| miR-16 | <0.71 | 83(97.7) | 2(2.4) | 1(reference) | 7.9 | 88(98.9) | 1(1.1) | 1(reference) | 9.0 | 171(98.3) | 3(1.7) | 1(reference) | 8.5 | |||
| >0.71 | 51(60.7) | 33(39.3) | 0.44(0.29–0.68) | 1.9×10−04 | 16.7 | 54(63.5) | 31(36.5) | 0.37(0.25–0.55) | 1.3×10−06 | 19.0 | 105(62.1) | 64(37.9) | 0.39(0.29–0.52) | 1.7×10−10 | 18.0 | |
| miR-30e-3p | <0.58 | 86(100) | 0(0) | 1(reference) | 8.3 | 89(98.9) | 1(1.1) | 1(reference) | 9.2 | 175(99.4) | 1(0.6) | 1(reference) | 8.7 | |||
| >0.58 | 48(56.5) | 37(43.5) | 0.45(0.29–0.69) | 3.1×10−04 | 20.4 | 54(62.8) | 32(37.2) | 0.38(0.25–0.58) | 5.6×10−06 | 19.0 | 102(59.7) | 69(40.4) | 0.4(0.30–0.54) | 7.8×10−10 | 19.8 | |
| miR-106a | <0.68 | 80(92.0) | 7(8.1) | 1(reference) | 7.6 | 83(98.8) | 1(1.2) | 1(reference) | 8.6 | 163(95.3) | 8(4.7) | 1(reference) | 8.5 | |||
| >0.68 | 56(64.4) | 31(35.6) | 0.57(0.39–0.85) | 5.7×10−03 | 15.7 | 60(65.2) | 32(34.8) | 0.40(0.28–0.59) | 2.8×10−06 | 16.0 | 116(64.8) | 63(35.2) | 0.47(0.36–0.61) | 2.0×10−08 | 16.0 | |
| miR-148b | <0.72 | 78(96.3) | 3(3.7) | 1(reference) | 7.8 | 78(96.3) | 3(3.7) | 1(reference) | 8.8 | 156(96.3) | 6(3.7) | 1(reference) | 8.3 | |||
| >0.72 | 51(63.0) | 30(37.0) | 0.49(0.33–0.74) | 5.9×10−04 | 16.4 | 57(67.1) | 28(32.9) | 0.51(0.35–0.75) | 5.3×10−04 | 14.4 | 108(65.1) | 58(34.9) | 0.5(0.38–0.65) | 3.4×10−07 | 15.5 | |
| miR-26a | <0.68 | 78(89.7) | 9(10.3) | 1(reference) | 8.5 | 77(96.3) | 3(3.8) | 1(reference) | 9.0 | 155(92.8) | 12(7.2) | 1(reference) | 8.7 | |||
| >0.68 | 57(66.3) | 29(33.7) | 0.65(0.44–0.95) | 2.8×10−02 | 15.7 | 65(68.4) | 30(31.6) | 0.45(0.30–0.66) | 5.8×10−05 | 15.5 | 122(67.4) | 59(32.6) | 0.51(0.39–0.67) | 7.5×10−07 | 15.7 | |
| miR-27a | <0.67 | 81(93.1) | 6(6.9) | 1(reference) | 8.6 | 79(97.5) | 2(2.5) | 1(reference) | 9.0 | 160(95.2) | 8(4.8) | 1(reference) | 8.7 | |||
| >0.67 | 55(64.0) | 31(36.1) | 0.59(0.4–0.87) | 7.3×10−03 | 16.4 | 64(67.4) | 31(32.6) | 0.47(0.32–0.71) | 2.3×10−04 | 16.0 | 119(65.8) | 62(34.3) | 0.51(0.39–0.67) | 9.3×10−07 | 16.2 | |
| miR-106b | <0.70 | 81(93.1) | 6(6.9) | 1(reference) | 7.2 | 85(95.5) | 4(4.5) | 1(reference) | 8.9 | 166(94.3) | 10(5.7) | 1(reference) | 8.3 | |||
| >0.70 | 55(64.0) | 31(36.1) | 0.52(0.35–0.77) | 1.1×10−03 | 16.4 | 58(66.7) | 29(33.3) | 0.5(0.34–0.72) | 2.8×10−04 | 15.5 | 113(65.3) | 60(34.7) | 0.53(0.41–0.68) | 9.4×10−07 | 16.0 | |
| miR-301a | <1.16 | 71(92.2) | 6(7.8) | 1(reference) | 7.6 | 73(93.6) | 5(6.4) | 1(reference) | 8.3 | 144(92.9) | 11(7.1) | 1(reference) | 7.9 | |||
| >1.16 | 50(65.8) | 26(34.2) | 0.53(0.36–0.8) | 2.1×10−03 | 14.4 | 51(66.2) | 26(33.8) | 0.54(0.35–0.82) | 3.7×10−03 | 16.0 | 101(66.0) | 52(34.0) | 0.53(0.40–0.70) | 6.7×10−06 | 15.8 | |
| miR-27b | <0.72 | 79(91.9) | 7(8.1) | 1(reference) | 7.1 | 75(93.8) | 5(6.3) | 1(reference) | 9.0 | 154(92.8) | 12(7.2) | 1(reference) | 8.2 | |||
| >0.72 | 56(65.9) | 29(34.1) | 0.53(0.36–0.79) | 1.6×10−03 | 14.8 | 66(74.2) | 23(25.8) | 0.66(0.46–0.93) | 1.9×10−02 | 11.1 | 122(70.1) | 52(29.9) | 0.58(0.45–0.75) | 2.2×10−05 | 13.2 | |
| miR-152 | <0.68 | 77(91.7) | 7(8.3) | 1(reference) | 7.8 | 82(92.1) | 7(7.9) | 1(reference) | 8.6 | 159(91.9) | 14(8.1) | 1(reference) | 8.4 | |||
| >0.68 | 55(66.3) | 28(33.7) | 0.6(0.41–0.87) | 7.2×10−03 | 14.4 | 56(70) | 24(30.0) | 0.60(0.42–0.86) | 5.3×10−03 | 12.7 | 111(68.1) | 52(31.9) | 0.59(0.46–0.75) | 3.3×10−05 | 14.1 | |
| miR-20a | <0.67 | 79(92.9) | 6(7.1) | 1(reference) | 7.8 | 76(92.7) | 6(7.3) | 1(reference) | 9.0 | 155(92.8) | 12(7.2) | 1(reference) | 8.5 | |||
| >0.67 | 55(65.5) | 29(34.5) | 0.63(0.42–0.95) | 2.7×10−02 | 14.3 | 66(71.0) | 27(29.0) | 0.58(0.41–0.83) | 2.5×10−03 | 12.9 | 121(68.4) | 56(31.6) | 0.62(0.48–0.79) | 1.5×10−04 | 14.1 | |
| miR-30a-5p | <0.61 | 75(89.3) | 9(10.7) | 1(reference) | 8.7 | 76(90.5) | 8(9.5) | 1(reference) | 8.6 | 151(89.9) | 17(10.1) | 1(reference) | 8.7 | |||
| >0.61 | 55(65.5) | 29(34.5) | 0.69(0.48–1.01) | 5.5×10−02 | 9.6 | 66(72.5) | 25(27.5) | 0.58(0.41–0.83) | 2.6×10−03 | 11.6 | 121(69.1) | 54(30.9) | 0.63(0.49–0.81) | 3.2×10−04 | 11.5 | |
| miR-148a | <0.74 | 79(92.9) | 6(7.1) | 1(reference) | 8.2 | 80(90.9) | 8(9.1) | 1(reference) | 9.6 | 159(91.9) | 14(8.1) | 1(reference) | 8.8 | |||
| >0.74 | 55(64.7) | 30(35.3) | 0.57(0.39–0.84) | 3.8×10−03 | 14.3 | 59(70.2) | 25(29.8) | 0.72(0.49–1.04) | 8.3×10−02 | 12.9 | 114(67.5) | 55(32.5) | 0.63(0.49–0.81) | 4.1×10−04 | 14.1 | |
| miR-145 | <0.62 | 77(91.7) | 7(8.3) | 1(reference) | 8.2 | 73(90.1) | 8(9.9) | 1(reference) | 9.6 | 150(90.9) | 15(9.1) | 1(reference) | 8.8 | |||
| >0.62 | 54(65.1) | 29(34.9) | 0.62(0.42–0.92) | 1.7×10−02 | 15.7 | 67(72.0) | 26(28.0) | 0.70(0.49–0.99) | 4.3×10−02 | 12.4 | 121(68.8) | 55(31.3) | 0.64(0.49–0.82) | 4.4×10−04 | 12.7 | |
| miR-92a | <0.63 | 77(88.5) | 10(11.5) | 1(reference) | 8.6 | 73(91.3) | 7(8.8) | 1(reference) | 8.9 | 150(89.8) | 17(10.2) | 1(reference) | 8.7 | |||
| >0.63 | 59(67.8) | 28(32.2) | 0.70(0.48–1.04) | 7.6×10−02 | 14.1 | 70(72.9) | 26(27.1) | 0.66(0.47–0.92) | 1.6×10−02 | 11.1 | 129(70.5) | 54(29.5) | 0.67(0.53–0.86) | 1.6×10−03 | 12.4 | |
| let-7c | <0.66 | 74(89.2) | 9(10.8) | 1(reference) | 8.4 | 66(88.0) | 9(12.0) | 1(reference) | 9.2 | 140(88.6) | 18(11.4) | 1(reference) | 8.6 | |||
| >0.66 | 56(68.3) | 26(31.7) | 0.65(0.44–0.94) | 2.4×10−02 | 14.3 | 68(73.9) | 24(26.1) | 0.72(0.50–1.04) | 7.7×10−02 | 11.6 | 124(71.3) | 50(28.7) | 0.68(0.53–0.87) | 2.6×10−03 | 13.4 | |
| let-7b | <0.60 | 78(90.7) | 8(9.3) | 1(reference) | 8.6 | 89(92.7) | 7(7.3) | 1(reference) | 9.3 | 167(91.8) | 15(8.2) | 1(reference) | 9.0 | |||
| >0.60 | 55(64.7) | 30(35.3) | 0.65(0.44–0.97) | 3.5×10−02 | 11.3 | 53(67.1) | 26(32.9) | 0.67(0.46–0.98) | 3.9×10−02 | 13.7 | 108(65.9) | 56(34.2) | 0.69(0.53–0.89) | 4.5×10−03 | 12.7 | |
Expression levels were calculated using the 2−ΔΔCt method. ΔCt was defined as the raw Ct value for a specific miRNA (or spike-in control miRNAs) in an individual sample minus the mean raw Ct value of this miRNA (or spike-in control miRNAs) in all the tested samples. ΔΔCt was defined as the difference between ΔCt of the specific miRNA and ΔCt of the spike-in control miRNAs.
Adjusted for age, sex, smoking status, clinical stage, and treatment regime
Figure 2.
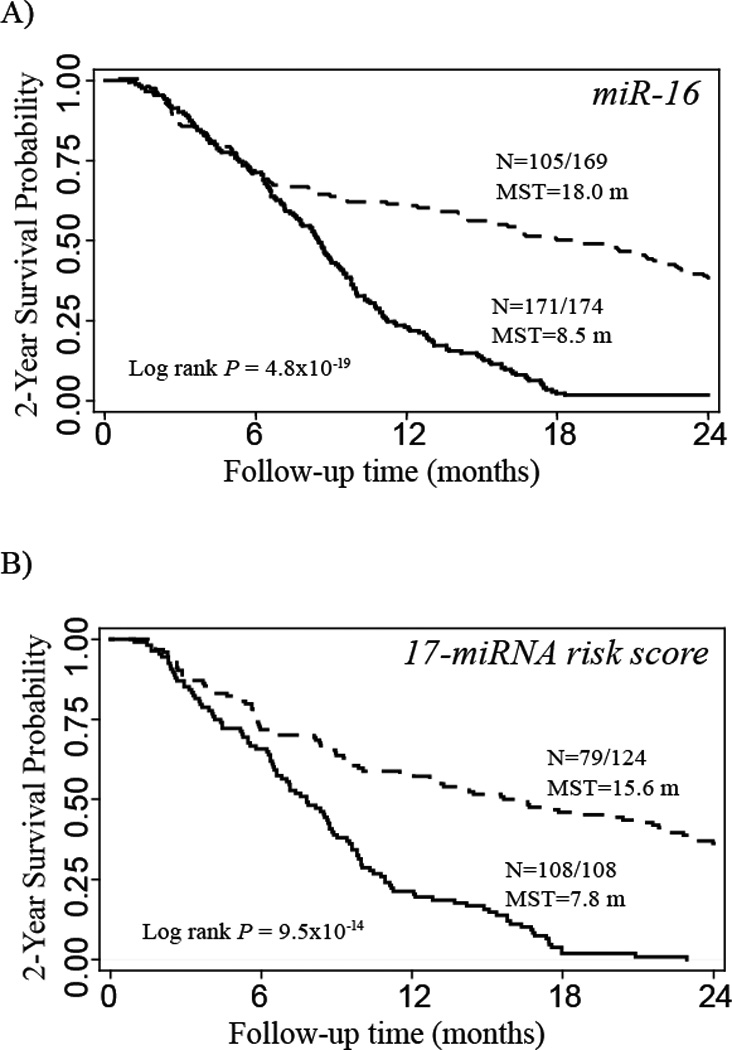
Kaplan-Meier 2-year survival curves for advanced NSCLC patients grouped by low (solid line) and high (dashed line) expression of serum miR-16 (A), and high (solid line) and low (dashed line) risk scores (B). N = number of patients with an event (death) at 2 years / total number of patients in the dataset. MST=median survival time.
Association of Serum MiRNA Expression with NSCLC Stage
As clinical stage is an established prognostic factor for NSCLC, serum miRNA expression was compared between stage III and stage IV patients. Among the 35 miRNAs analyzed, the expression levels of 11 miRNAs were significantly different between stage III and stage IV (Supplementary material, Table S3). Among these 11 miRNAs, 9 were overlapping with the significant miRNAs associated with survival (Table 2). We included stage as an adjustment factor in our analysis of 2-year survival.
Prediction of NSCLC Survival by 17-miRNA Risk Score
The combined effects of these single serum miRNAs significantly associated with survival were investigated by calculating a risk score using the linear combination of expression levels of these 17 miRNAs for each individual on the training data set (Table 3). When the same cutoff was applied to the testing set, similar to the training set, patients with high-risk scores exhibited significantly increased risk of death compared to those with low-risk scores (HR=2.71, 95% CI, 1.65–4.45) (Table 3). The corresponding risk of death in the combined dataset was 2.46 (95% CI, 1.77–3.44). Kaplan-Meier 2-year survival curve showed that patients with the high risk scores had a MST of 7.8 months compared to 15.6 months in the low risk group (Figure 2B).
Table 3.
Serum miRNA 17-marker risk score associated with 2-year survival in patients with advanced NSCLC from the study population recruited at the University of Texas MD Anderson Cancer Center between 2002 and 2009.
| Risk score | 2-Year Death, n% | 2-Year Survival, n% | Adjusted HRa (95% CI) | P | MST | |
|---|---|---|---|---|---|---|
| Training Set | Low | 37 (64.9) | 20 (35.1) | 1(reference) | 0.002 | 14.3 |
| High | 56 (100.0) | 0 (0.0) | 2.18 (1.32–3.59) | 7.8 | ||
| Testing Set | Low | 42 (62.7) | 25 (37.3) | 1(reference) | 8.0 × 10−5 | 17.4 |
| High | 52 (100.0) | 0 (0.0) | 2.71 (1.65–4.45) | 7.6 | ||
| Combined Set | Low | 79 (63.7) | 45 (36.3) | 1(reference) | 1.1 × 10−7 | 15.6 |
| High | 108 (100.0) | 0 (0.0) | 2.46 (1.77–3.44) | 7.8 | ||
Adjusted for age, sex, smoking status, clinical stage, and treatment regimen.
The prognosis values of 2-year survival were expressed as C-index with 100 times of bootstrap in the training, testing and combined datasets (Table 4). In the combined dataset, the C-index was 0.57 (0.54–0.60) for the baseline model (age, gender, and smoking status), 0.64 (0.61–0.66) with addition of disease stage; and 0.69 (0.64–0.72) with further addition of the 17-miRNA risk score (Table 4).
Table 4.
C-index for prognosis models in prediction of 2-year survival among NSCLC study population recruited at the University of Texas MD Anderson Cancer Center between 2002 and 2009.
| Model | Training Set | Testing Set | Combined Set |
|---|---|---|---|
| Baseline* | 0.59 (0.55–0.64) | 0.56 (0.51–0.61) | 0.57 (0.54–0.60) |
| Baseline + Stage | 0.67 (0.62–0.71) | 0.61 (0.57–0.66) | 0.64 (0.61–0.66) |
| 17-miRNA risk score only | 0.63 (0.60–0.67) | 0.64 (0.58–0.68) | 0.64 (0.60–0.66) |
| Baseline + Stage + 17-miRNA risk score | 0.70 (0.65–0.74) | 0.68 (0.63–0.73) | 0.69 (0.64–0.72) |
Baseline model included age, gender and smoking status.
Discussion
It is well recognized that cancer patients of similar clinical characteristics vary considerably in prognosis, treatment response, and survival. Factors currently known to indicate poor prognosis of NSCLC patients include the presence of pulmonary symptoms, tumor size over 3 cm, non-squamous histology, metastases to multiple lymph nodes, and vascular invasion (28). Since these factors are largely qualitative and some of them require invasive procedures to assess, there has been an increasing demand for the discovery of non-invasive biomarkers that accurately predict survival in advanced NSCLC patients. This would assist in determining personalized treatment or follow-up regimens, and prospectively identify subgroups with unfavorable prognosis in order to better guide end-of-life management.
In recent years, endogenous circulating miRNAs have attracted extensive research interest because they remain stable and are well protected even under harsh conditions. A number of studies have investigated roles of circulating miRNAs in lung cancer, and most of them focused on identifying miRNA markers for disease screening and early detection (29–32). Only a few recent studies have looked into associations of circulating miRNA expression with NSCLC survival and clinical outcomes (27, 33–36). Hu et. al. (27) identified a serum miRNA signature for prediction of overall survival in early-stage NSCLC patients based on genome-wide profiling using RNA-seq in 30 pairs of good- and poor-survival patients, and subsequent qRT-PCR validation. Four serum miRNAs (miR-486, miR-30d, miR-1, miR-499) were reported to be associated with overall survival of early stage NSCLC. Several studies using a candidate gene approach have shown that high expression of miR-21 was a risk factor for poor chemosensitivity (36), poor survival (34), and may serve as an indicator of post-operative recurrence (35). A recent study detected cell-free miRNA profiles in pleural effusions from stage IV NSCLC patients and identified a 5-miRNA (miR-134, miR-151, miR-345, miR-93, miR-100) risk score as a prognosis marker for overall survival (33). In addition, several studies on associations between circulating miRNA levels and lung cancer stages have been reported (37–39). A study on plasma miRNA indicated that plasma miR-30e-3p was significantly higher in NSCLC patients with non-resectable tumor compared to those with resectable tumor; but in patients with resectable tumors only, high level of plasma miR-30e-3p was associated with a higher rate of disease free survival (30). Overall, the results of these studies have been inconsistent, due to small sample size and heterogeneous patient population.
Our current study was one of the largest in NSCLC and we focused on stage III and IV NSCLC patient. We adopted the pathway-based approach and focused our investigation on miRNAs related with genes in the TGF-β signaling pathway. Our study was designed to include an internal validation by distributing the entire population into a training set and a testing set, from where consistent results were obtained. For survival analysis, we identified serum miRNAs that predicted 2-year survival using Cox regression model (Table 2). Notable differences in survival times were identified for the patients. Having the ability to better predict prognosis – survival benefits greater than 9 months to a year – could be critical in treatment selection and end-of-life management for advanced-stage patients who are typically given a dismal prognosis. MiR-16 expression showed the most significant association with 2-year survival in our study. A recent study on resected stage I-III NSCLC tumors revealed the association of high level miR-16 expression with poor prognosis including disease-free survival and overall survival (40); Our current study was the first to report serum miR-16 in association with advanced stage NSCLC survival. We also showed an MST advantage of 11.1 months in patients with high serum levels of miR-30e-3p in contrast to those with low levels. These results were consistent with previously reported findings that plasma miR-30e-3p was associated with favorable disease-free survival (30). In addition, our results highlighted the significance of the let-7 family, one of the most studied tumor suppressor miRNA families involved in lung cancer, with consistent up-regulation observed in tissues for those with a favorable survival (10, 23, 41). Several other tissue-based investigations have also suggested miR-20 and miR-106a (41) as lung cancer prediction factors, which was consistent with our results in serum.
In addition to individual miRNA analysis, we also evaluated the collective prognosis value of multiple miRNAs by creating a risk score analysis for the 17 identified TGF-β pathway-enriched miRNAs that were significantly associated with 2-year survival. When the patient population was stratified by this combined risk score, a notable, highly significant MST advantage of 7.8 months was observed between the low risk group and the high risk group. To quantitatively present the prognosis power of the 17-miRNA risk score, the C-index for survival prediction of the study population was increased to 0.69 when the risk score was additionally included into the model, compared to 0.64 when only age, gender and smoking status and stage were included. Additional biomarkers are needed to further improve the prediction efficiency for clinical applications.
There were a few limitations in our study. First, the cellular pathways are redundant and are often co-regulated. It is possible that our results based on the TGF-β pathway and the miRNA-regulation of this pathway could have instead identified effects of serum miRNA expression on other cellular pathways. In addition, a majority of miRNAs have a suite of potential binding targets raising the possibility of pleiotropic effects. Second, we could not determine the miRNA releasing mechanisms and cells of origin. Our results indicated that at least some of the significant serum miRNAs could be derived from tumor cells since they are associated with both disease stage progression and patient survival. However, it is also possible that serum miRNAs come from other origins such as inflammatory cells, endothelium cells or blood cells. For example, previous studies on circulating miRNAs have suggested the effect of hemolysis on specific miRNA (e.g., miR-16) expression, which may limit the potential utility of these miRNAs in the clinical setting (42). To address this issue, we measured hemoglobin levels in 30 randomly selected serum samples (10 each from the low, medium and high survival groups). In all the samples tested, the hemoglobin levels were below 15 mg/dL, lower than the previously reported level of 25 mg/dL that started to affect the measurement of serum miRNA (42), suggesting the confounding effect of hemolysis in this current study is minimal. Third, we employed TargetScan to focus on prediction of the canonical 3’UTR miRNA binding; however, non-canonical miRNA binding sites in the coding and other regions are also capable of regulating gene expression (43–49). Future studies that include non-canonical miRNA binding sites would complement our current analysis. Finally, although we divided our sample set into a training and testing set, potential systematic biases still could be present in the sample set because the same overall cohort was used. Validation of our reported miRNA candidates in an independent external patient cohort is warranted.
In conclusion, our study was built on a large population of 391 advanced-stage NSCLC cases, which were distributed into three datasets for qualitative whole-genome serum miRNA profiling using a small-scale discovery set, followed by individual qRT-PCR assay evaluation on a training set and a testing set. Seventeen serum miRNAs were able to predict 2-year survival in an analysis using the continuous model, and a risk score was constructed based on these 17 miRNAs, which would potentially predict 2-year survival for late-stage NSCLC patients. If validated in an independent population, these serum miRNAs are promising to be utilized as non-invasive prognostic biomarkers in clinical applications, hence benefiting disease and treatment management for patients with advanced-stage NSCLC.
Supplementary Material
Acknowledgments
Financial support: Supported in part by National Cancer Institute (R01 CA111646, P50 CA070907, and R01 CA55769). Additional funding was provided by MD Anderson Research Trust and MD Anderson institutional support for the Center for Translational and Public Health Genomics.
Footnotes
Disclosure: None.
This study has been presented at the 103rd Annual Meeting of the American Association for Cancer research as a poster presentation on April 3rd, 2012 in Chicago, Illinois.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Esteban E, Casillas M, Cassinello A. Pemetrexed in first-line treatment of non-small cell lung cancer. Cancer Treat Rev. 2009;35:364–373. doi: 10.1016/j.ctrv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Gebbia V. Does an optimal therapeutic sequence exist in advanced non-small cell lung cancer? Expert Opin Pharmaco. 2008;9:1321–1337. doi: 10.1517/14656566.9.8.1321. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Calin G, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Ba Y, Ma LJ, Cai X, Yin Y, Wang KH, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 12.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. British Journal of Haematology. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, et al. Circulating microRNAs as stable bloodbased markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brase JC, Wuttig D, Kuner R, Sultmann H. Serum microRNAs as non-invasive biomarkers for cancer. Molecular Cancer. 2010;9 doi: 10.1186/1476-4598-9-306. -. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massague J. TGF beta in cancer. Cell. 2008;134:215–230. [Google Scholar]
- 16.Meulmeester E, ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 17.Lin MB, Stewart DJ, Spitz MR, Hildebrandt MAT, Lu C, Lin J, et al. Genetic variations in the transforming growth factor-beta pathway as predictors of survival in advanced non-small cell lung cancer. Carcinogenesis. 2011;32:1050–1056. doi: 10.1093/carcin/bgr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-DeltaDelta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Liu HS, Zhu L, Liu BY, Yang L, Meng XX, Zhang W, et al. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203. doi: 10.1016/j.canlet.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Zhou XH, Obuchowski N, McClish D. Thoughts on the ZOM/BWC controversy - replies. Acad Radiol. 2003;10:1179–1180. [Google Scholar]
- 23.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 24.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 25.Lin QF, Mao WD, Shu YQ, Lin F, Liu SP, Shen H, et al. A cluster of specified microRNAs in peripheral blood as biomarkers for metastatic non-small-cell lung cancer by stem-loop RT-PCR. J Cancer Res Clin. 2012;138:85–93. doi: 10.1007/s00432-011-1068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roybal JD, Zang Y, Ahn YH, Yang YN, Gibbons DL, Baird BN, et al. miR-200 Inhibits Lung Adenocarcinoma Cell Invasion and Metastasis by Targeting Flt1/VEGFR1. Mol Cancer Res. 2011;9:25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu ZB, Chen X, Zhao Y, Tian T, Jin GF, Shu YQ, et al. Serum MicroRNA Signatures Identified in a Genome-Wide Serum MicroRNA Expression Profiling Predict Survival of Non-Small-Cell Lung Cancer. J Clin Oncol. 2010;28:1721–1726. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 28.Health professional version. National Cancer Institute; 2011. Non-small cell lung cancer treatment (PDQ®) [PubMed] [Google Scholar]
- 29.Foss KM, Sima C, Ugolini D, Neri M, Allen KE, Weiss GJ. miR-1254 and miR-574-5p Serum-Based microRNA Biomarkers for Early-Stage Non-small Cell Lung Cancer. J Thorac Oncol. 2011;6:482–488. doi: 10.1097/JTO.0b013e318208c785. [DOI] [PubMed] [Google Scholar]
- 30.Silva J, Garcia V, Zaballos A, Provencio M, Lombardia L, Almonacid L, et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J. 2011;37:617–623. doi: 10.1183/09031936.00029610. [DOI] [PubMed] [Google Scholar]
- 31.Zhang CN, Wang C, Chen X, Yang CH, Li K, Wang JJ, et al. Expression Profile of MicroRNAs in Serum: A Fingerprint for Esophageal Squamous Cell Carcinoma. Clin Chem. 2010;56:1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 32.Zhang TF, Wang QM, Zhao D, Cui YL, Cao BR, Guo LP, et al. The oncogenetic role of microRNA-31 as a potential biomarker in oesophageal squamous cell carcinoma. Clin Sci. 2011;121:437–447. doi: 10.1042/CS20110207. [DOI] [PubMed] [Google Scholar]
- 33.Wang TT, Lv MM, Shen SN, Zhou S, Wang P, Chen YQ, et al. Cell-Free MicroRNA Expression Profiles in Malignant Effusion Associated with Patient Survival in Non-Small Cell Lung Cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic Significance of Serum miRNA-21 Expression in Human Non-Small Cell Lung Cancer. J Surg Oncol. 2011;104:847–851. doi: 10.1002/jso.22008. [DOI] [PubMed] [Google Scholar]
- 35.Le HB, Zhu WY, Chen DD, He JY, Huang YY, Liu XG, et al. Evaluation of dynamic change of serum miR-21 and miR-24 in pre- and post-operative lung carcinoma patients. Med Oncol. 2012;29:3190–3197. doi: 10.1007/s12032-012-0303-z. [DOI] [PubMed] [Google Scholar]
- 36.Wei J, Liu LK, Gao W, Zhu CJ, Liu YQ, Cheng T, et al. Reduction of plasma MicroRNA-21 is associated with chemotherapeutic response in patients with non-small cell lung cancer. Chinese J Cancer Res. 2011;23:123–128. doi: 10.1007/s11670-011-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng DL, Haddadin S, Wang Y, Gu LQ, Perry MC, Freter CE, et al. Plasma microRNAs as novel biomarkers for early detection of lung cancer. International Journal of Clinical and Experimental Pathology. 2011;4:575–586. [PMC free article] [PubMed] [Google Scholar]
- 38.Ma YX, Tian ZN, Zhang W. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J Cancer Res Clin. 2012;138:2045–2050. doi: 10.1007/s00432-012-1285-0. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Yang T, Li X, Yang Q, Liu R, Huang J, et al. Alteration of serum miR-206 and miR-133b is associated with lung carcinogenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Toxicol Appl Pharmacol. 2013;18:002. doi: 10.1016/j.taap.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Navarro A, Diaz T, Gallardo E, Vinolas N, Marrades RM, Gel B, et al. Prognostic Implications of miR-16 Expression Levels in Resected Non-Small-Cell Lung Cancer. Journal of Surgical Oncology. 2011;103:411–415. doi: 10.1002/jso.21847. [DOI] [PubMed] [Google Scholar]
- 41.Castillejo A, Rothman N, Murta-Nascimento C, Malats N, Garcia-Closas M, Gomez-Martinez A, et al. TGFB1 and TGFBR1 polymorphic variants in relationship to bladder cancer risk and prognosis. International Journal of Cancer. 2009;124:608–613. doi: 10.1002/ijc.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of Circulating MicroRNA: Preanalytical and Analytical Challenges. Clin Chem. 2011;57:833–840. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- 43.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, et al. TGF-beta Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raynal S, Nocentini S, Croisy A, Lawrence DA, Jullien P. Transforming growth factor-beta 1 enhances the lethal effects of DNA-damaging agents in a human lung-cancer cell line. International Journal of Cancer. 1997;72:356–361. doi: 10.1002/(sici)1097-0215(19970717)72:2<356::aid-ijc26>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 45.Didiano D, Hobert O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat Struct Mol Biol. 2006;13:849–851. doi: 10.1038/nsmb1138. [DOI] [PubMed] [Google Scholar]
- 46.Jeon HS, Jen J. TGF-beta Signaling and the Role of Inhibitory Smads in Non-small Cell Lung Cancer. J Thorac Oncol. 2010;5:417–419. doi: 10.1097/JTO.0b013e3181ce3afd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masui T, Wakefield LM, Lechner JF, Laveck MA, Sporn MB, Harris CC. Type-Beta Transforming Growth-Factor Is the Primary Differentiation-Inducing Serum Factor for Normal Human Bronchial Epithelial-Cells. P Natl Acad Sci USA. 1986;83:2438–2442. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masui T, Wakefield LM, Lechner JF, Laveck MA, Gerwin BI, Sporn MB, et al. Type-Beta Transforming Growth-Factor Induction of Squamous Differentiation of Normal Human Bronchial Epithelial-Cells Is Antagonized by Epinephrine and Cholera-Toxin. J Cell Biochem. 1986 118-. [Google Scholar]
- 49.Liu R, Zhang CN, Hu ZB, Li G, Wang C, Yang CH, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


