Abstract
Introduction
There is converging evidence supporting hyperactivity of the Hypothalamic-Pituitary-Adrenal (HPA) axis in schizophrenia spectrum disorders (SSD), such as schizotypal personality disorder (SPD), first-episode schizophrenia (FESZ) and chronic schizophrenia (CHSZ). Such an aberrant HPA activity might have volumetric consequences on the pituitary gland. However, previous magnetic resonance imaging (MRI) studies assessing pituitary volume (PV) in SSD are conflicting. The main objective of this study was to examine further PV in SSD.
Methods
PV were manually traced on structural MRIs in 137 subjects, including subjects with SPD (n=40), FESZ (n=15), CHSZ (n=15), and HC (n=67). We used an ANCOVA to test PV between groups and gender while controlling for inter-subject variability in age, years of education, socioeconomic status, and whole brain volume.
Results
Overall, women had larger PV than men, and within the male sample all SSD subjects had smaller PV than HC, statistically significant only for the SPD group. In addition, dose of medication, illness duration and age of onset were not associated with PV.
Conclusion
Chronic untreated HPA hyperactivity might account for smaller PV in SPD subjects, whereas the absence of PV changes in FESZ and CHSZ patients might be related to the normalizing effects of antipsychotics on PV. SPD studies offer a way to examine HPA related alterations in SSD without the potential confounds of medication effects.
Keywords: schizophrenia, first-episode, schizotypal, pituitary volume, MRI
1 INTRODUCTION
There is increasing evidence that suggests hyperactivity of the Hypothalamic-Pituitary-Adrenal (HPA) axis in schizophrenia spectrum disorders (SSD). More specifically, the HPA axis has been associated with stress responsivity, and there is also evidence that these two systems are closely related in schizophrenia (Kaneko et al., 1992; Walker and Diforio, 1997). Of note, schizotypal personality disorder (SPD) is genetically related to schizophrenia (Kendler et al., 1993), and shares clinical and biological features (Siever and Davis, 2004). Some studies have investigated HPA axis functioning in SPD, which might share HPA axis functioning with schizophrenia; SPD subjects might be better buffered against dopaminergic and HPA over activation in response to stress (Mitropoulou et al., 2004).
The HPA axis governs the release of cortisol, which enters the brain to coordinate components of the stress system and to control the excitability of neuronal networks (de Kloet et al., 1999). In response to stressors, corticotrophin-releasing hormone is released from the paraventricular nucleus of the hypothalamus. This triggers the secretion of adenocorticotropic hormone from the pituitary, which, in turn, leads to the secretion of glucocorticoids from the adrenal glands, in particular cortisol (Walker et al., 2008). Cortisol has an effect on brain function through specific receptors. That is, both fast and slow effects occur as a result of activating these receptors. Modulation of HPA activity is a crucial mechanism that enables the organism to meet changing demands of the environment. For example, animal studies have shown that, at heightened levels, GCs can induce regression of dendritic processes, inhibit neurogenesis, decrease neuronal survival following insults (Sapolsky, 2003), and contribute to neuronal death in brain areas like the hippocampus, that might be responsible for the volumetric changes reported in this area (Dickey et al., 2007).
HPA dysfunction in SSD has also been hypothesized to affect pituitary volumes. For example, Pariante et al. (Pariante et al., 2004) reported increased pituitary volume (PV) in medicated and neuroleptic-naïve patients with first episode psychosis, while smaller volumes where found in patients with schizophrenia. Upadhyaya et al. (Upadhyaya et al., 2007) studied PV in neuroleptic-naïve schizophrenia patients and found smaller volumes in patients compared to healthy individuals. In contrast, MacMaster et al. (MacMaster et al., 2007a) found increased volume after a 12 month follow up in patients with schizophrenia. Tournikioti et al. (Tournikioti et al., 2007), on the other hand, reported no volumetric difference between patients with chronic schizophrenia (CHSZ) and healthy controls. More recently, Takahashi et al. (Takahashi et al., 2009) reported that PVs were larger in treated SPD and schizophrenia than they were in a group of healthy controls.
As described, magnetic resonance imaging (MRI) studies assessing PV in schizophrenia spectrum disorders are limited and conflicting. The main objective of this study was to examine further pituitary gland volume in subjects diagnosed with SPD, FESZ, CHSZ, and healthy controls.
2 MATERIALS and METHODS
2.1 Subjects
One hundred and thirty seven subjects participated in this study, of which 70 were meeting DSM criteria for a schizophrenia spectrum disorder (SSD; mean age±SD = 30±10 years, 71% male) and 67 were healthy controls (HC; mean age±SD = 30±10 years; 73% male). Within the schizophrenia spectrum, 40 subjects were diagnosed with schizotypal personality disorder (SPD; mean age±SD = 29±8 years; 50% male), 15 with first-episode schizophrenia (FESZ; mean age±SD = 22±3 years; 100% male), and 15 with chronic schizophrenia (CHSZ; mean age±SD = 41±9 years; 100% male). All subjects were right handed. The project was approved by local institutional review boards at Harvard Medical School and at the VA Boston Healthcare System. Following a full description of the study to the participants, written informed consent was obtained.
All subjects were recruited and diagnosed as described in detail in previous studies (Anderson et al., 2002; Dickey et al., 1999; Dickey et al., 2007; Dickey et al., 2000; Hirayasu et al., 1998; Hirayasu et al., 2000b; Hirayasu et al., 2001; Kasai et al., 2003; Lee et al., 2002; Shenton et al., 1992). Briefly, SPD subjects were a community-based sample recruited at the VA Boston Healthcare System, Jamaica Plain Division, from newspaper advertisements in the local community. Patients with FESZ were recruited from inpatient units at McLean Hospital, and patients with CHSZ were recruited from the VA Boston Healthcare System, Brockton Division. Patients and SPD subjects were diagnosed based on the DSM III-R or DSM-IV criteria, using information from the Structured Clinical Interview for DSM-III-R, by trained interviewers. Consistent with the literature (Hirayasu et al., 2000a; Hirayasu et al., 1998; Hirayasu et al., 2001; Shenton et al., 1992) first episode was operationally defined as the first psychiatric hospitalization (DeLisi et al., 1991; DeLisi et al., 1997; Lieberman et al., 2001).
Healthy control subjects were recruited through newspaper advertisements in the community and no control subject had an Axis I or II psychiatric disorder or a first-degree relative with an Axis I psychiatric disorder with screening using the Structured Clinical Interview for DSM-III-R Non-Patient Edition (SCID-NP).
All participants met criteria for age (18–55 years), IQ above 75, a normal brain MRI as evaluated by a clinical neuroradiologist and a history negative for the following: seizures, head trauma with loss of consciousness, neurologic disorder, and any lifetime history of substance dependence.
2.2 MRI procedures
Participants were scanned at two locations (SPD and CHSZ at Brigham and Women’s Hospital, Boston, MA; FESZ at McLean Hospital, Belmont, MA; HC at both locations). All MR images were acquired on 1.5 Tesla General Electric Scanners (GE Medical Systems, Milwaukee, Wisconsin). As mentioned in PNL’s Technical Report, geometrically complicated ROI’s have no more inter-scan variation with the same rater than the intra-rater reliability on the same scan. (http://pnl.bwh.harvard.edu/pub/papers_html/TechnicalReport501.html) There were two MRI sequences that have been described in detail previously (Dickey et al., 1999; Dickey et al., 2000). Briefly, the first sequence was a coronal series of contiguous spoiled gradient-recalled acquisition (SPGR) images (TR=35msec, TE=5msec, flip angle=45°, FOV=24cm, and matrix size=256x256x124, voxel (volume of the pixel) dimensions=0.9375x0.9375x1.5 mm). The second acquisition was an axial series of contiguous double-echo (proton density and T2-weighted) images (TR=3000msec, TE=30 and 80 msec, FOV=24cm, and an interleaved acquisition with 3.0-mm slice thickness).
Scans were transferred to workstations for further image processing at the Psychiatry Neuroimaging Laboratory (Brigham and Women’s Hospital, Boston, MA; http://pnl.harvard.edu). First, the T2 scan was registered to the SPGR followed by an expectation-maximization (EM) technique (Bouix et al., 2007; Pohl et al., 2007) used to segment the brain into its major tissue classes: gray matter, white matter and cerebral spinal fluid. These tissue classes were merged into a single label map to measure the subject’s whole brain volume. The mask was checked by overlaying it onto the coregistered T2-scan to ensure that all extracranial and skull structures were removed and all intracranial structures fully preserved. The SPGR was then realigned using the line between the anterior and posterior commissures and the sagital sulcus to correct for head tilt, and then re-sampled into isotropic voxels of 0.9375mm3. The pituitary was traced on realigned and re-sampled SPGR images using 3DSlicer (Surgical Planning Laboratory, Brigham and Women’s Hospital, Boston, MA; http://www.slicer.org).
2.3 Tracing methods
One rater (FR) blind to subjects’ diagnoses, manually traced pituitary volumes on structural T1 sequence MRIs for all study subjects. On a random subset of 10 cases, high intra-rater (intraclass correlation coefficient r=0.81, p=0.01) and inter-rater reliability (FR and WSH; intraclass correlation coefficient r=0.97, p<0.001) were observed.
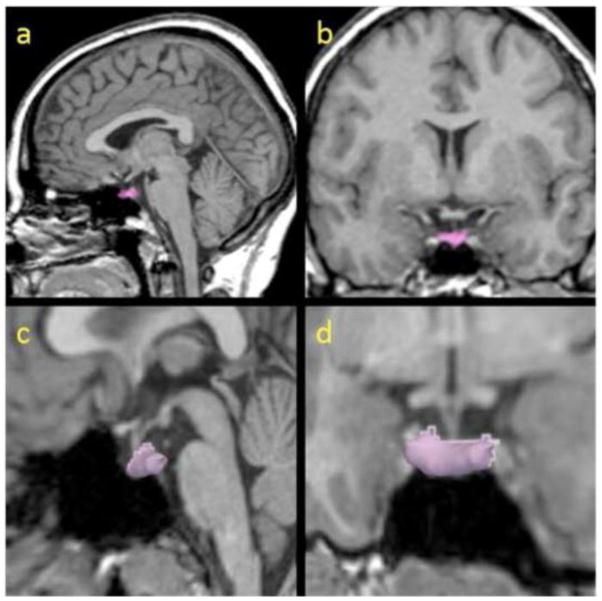
The tracing method was adapted from previously published studies (Pariante et al., 2004; Sassi et al., 2001; Upadhyaya et al., 2007). Briefly, each pituitary was first identified on the sagital plane where an anterior and posterior border was marked and used to define the number of coronal slices containing the pituitary. Then, the pituitary gland was traced in all coronal slices where it could be visualized following well-defined anatomical boundaries, i.e., the diaphragma sellae (superiorly), the sphenoid sinus (inferiorly), and the cavernous sinuses (bilaterally). Bright voxels corresponding to the posterior pituitary were included, as they are thought to be a reflection of vasopressin concentration (Fujisawa et al., 1987). The pituitary stalk was excluded (Figure 1).
Figure 1.
The pituitary gland manually traced (colored; a, b) and 3-dimensionally reconstructed (c, d) in sagital (a, c) and coronal (b, d) views.
2.4 Statistical analyses
Sociodemographic and clinical differences between groups were assessed by one-way analyses of variance (ANOVA), student t-test, or chi-square test. PV was analyzed using univariate analysis of covariance (ANCOVA). Four ANCOVA models were sequentially used. In the first model, all subjects were included. In the second model, females were excluded because there were no females in the FESZ and CHSZ group. In the third model, a model without CHSZ (and without females) was tested given the significant differences in sample characteristics (as described below in the results section) between CHSZ and the other groups, in particular age. Finally, in the fourth model, we directly compared PV between the SPD and HC sample given their similarities in sample characteristics, and to study further group and gender effects. Model covariates were used because they were different between groups or they were associated with PV. Scanner-site was used as a covariate in each model tested.
The relationship between PV and sample characteristics (age, years of education, SES, PSES, WAIS, MMSE, illness duration, symptom onset, chlorpromazine equivalence, PANSS, SANS, SAPS, whole brain volume) were examined with Spearman’s and Pearsons correlations as deemed appropriate. Post-hoc analyses were adjusted for multiple comparisons with Bonferroni correction. Two-tailed statistical significance level was set at p<0.05. All analyses were conducted with the PASW Statistics software version 17.0 (Chicago, IL, USA: SPSS Inc.).
3 RESULTS
3.1 Sample characteristics
Sample sociodemographic and clinical characteristics of the SPD, FESZ, CHSZ, and HC group are presented in Table 1. The FESZ were younger (p<0.05) and the CHSZ were older (p<0.001) than the other groups, whereas the SPD and HC group were not different in mean age (p=1.00). SPD and HC had more years of education than FESZ and CHSZ (p<0.05), but there were no differences in years of education between SPD and HC or between FESZ and CHSZ (p>0.05). CHSZ had lower (p=0.008), and FESZ had a trend (p=0.073) for lower socioeconomic status (SES) compared to HC, whereas FESZ had higher parental socioeconomic status (PSES) than all other groups (p<0.05). CHSZ had lower WAIS-III verbal IQ scores than SPD and HC (p<0.01), but there were no differences between SPD and HC (p=0.21). Further, CHSZ had lower WAIS-III vocabulary scaled scores than SPD and HC (p<0.01), but not FESZ (p=0.11), and there were no differences between SPD, FESZ, and HC (p>0.05). There were no significant differences between the groups in MMSE, whole brain volume, symptom onset, chlorpromazine equivalence, PANSS, SAPS or SANS scores.
Table 1.
Demographic and clinical characteristics of study groups.
| SPD | FESZ | CHSZ | HC | Statistical Analysis
|
|||
|---|---|---|---|---|---|---|---|
| F or t test | dfi | P-value | |||||
| N | 40 | 15 | 15 | 67 | |||
| Age, years a | 29.4 (7.8) | 21.5 (2.6) | 41.0 (9.0) | 30.2 (9.8) | 13.0 | 3, 133 | <0.001 |
| Gender M/F, %Maleb | 20/20 (50%) | 15/0 (100%) | 15/0 (100%) | 49/18 (73%) | |||
| Years of educationc | 14.8 (2.2) | 13.0 (2.5) | 12.2 (1.6) | 15.8 (2.3) | 14.9 | 3, 133 | <0.001 |
| Socioeconomic status (SES)d | 3.1 (1.3) | 3.6 (1.6) | 3.9 (1.0) | 2.7 (1.3) | 5.0 | 3, 132 | 0.003 |
| Parental SESe | 3.5 (1.4) | 1.3 (0.5) | 2.7 (1.2) | 2.8 (1.5) | 8.5 | 3, 133 | <0.001 |
| Handednessf | 0.7 (0.3) | 0.8 (0.3) | 0.8 (0.2) | 0.8 (0.2) | 2.7 | 3, 126 | 0.049 |
| WAIS-III Verbal IQg | 112.8 (14.5) | - | 96.1 (14.9) | 118.5 (14.4) | 13.7 | 2, 99 | <0.001 |
| WAIS-III Vocabulary scaled scoreh | 13.3 (2.6) | 12.6 (2.8) | 9.9 (3.0) | 13.7 (3.1) | 6.6 | 3, 84 | <0.001 |
| MMSE | - | 28.9 (1.3) | 29.1 (1.3) | 29.2 (0.8) | 0.4 | 2, 54 | 0.70 |
| Illness duration, years | - | - | 18.9 (9.5) | - | |||
| Symptom Onset, years | - | 21.5 (2.6) | 23.3 (4.7) | - | 1.3 | 26 | 0.22 |
| Medication dose (chlorpromazine equivalence in mg/day) | - | 214.8 (126.3) | 355.4 (317.6) | - | 1.4 | 23 | 0.17 |
| PANSS positive | - | 20.8 (5.9) | 21.8 (9.4) | - | 0.3 | 25 | 0.74 |
| PANSS negative | - | 16.8 (6.9) | 16.7 (5.5) | - | 0.0 | 25 | 0.97 |
| PANSS total score | - | 33.8 (7.9) | 36.8(12.3) | - | 0.7 | 25 | 0.48 |
| SANS | - | 9.2 (3.9) | 9.2 (5.8) | - | 0.0 | 25 | 0.97 |
| SAPS | - | 9.4 (5.1) | 9.3 (2.9) | - | 0.1 | 25 | 0.92 |
| Whole brain volume, mL | 1450.5 (152.9) | 1530.7 (107.0) | 1440.2 (104.5) | 1490.1 (132.7) | 1.9 | 3, 133 | 0.13 |
Values represent Mean (SD). Post-hoc analyses were adjusted for multiple comparisons with Bonferroni post-hoc t-tests.
Abbreviations: SPD, schizotypal personality disorder; FESZ, first-episode schizophrenia; CHSZ, chronic schizophrenia; HC, healthy control subjects; MMSE, Mini-Mental State Examination; PANSS positive and PANSS negative are subscales of the Positive and Negative Syndrome Scale (Kay et al., 1987) to measure severity of positive and negative symptoms, respectively; SANS, Scale for the Assessment of Negative Symptoms (Andreasen, 1989); SAPS, Scale for the Assessment of Positive Symptoms (Andreasen et al., 1995).
FESZ group was younger (p<0.05), whereas the CHSZ group was older (p<0.001) than the other groups. The SPD and HC group were not different in mean age (p=1.00).
Pearson’s Chi-square test showed a different gender ratio between groups (p<0.001). Females were underrepresented in the FESZ and CHSZ group.
SPD and HC had more years of education than FESZ and CHSZ (p<0.05). There were no differences in years of education between SPD and HC or between FESZ and CHSZ (p>0.05).
Higher numbers indicate lower socioeconomic status. Assessed using the Hollingshead Two-Factor measure (Hollingshead, 1965). CHSZ had lower socioeconomic status than HC (p=0.008). There was a trend difference between FESZ and HC (p=0.073). There were no differences for the other group comparisons.
FESZ had higher parental socioeconomic status than the other groups (p<0.05).
Evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971). Values > 0 indicate right-handedness. HC had stronger right hand dominance than SPD (p=0.042).
CHSZ had lower WAIS-III verbal IQ scores than SPD and HC (p<0.01). There were no differences between SPD and HC (p=0.21).
CHSZ had lower WAIS-III vocabulary scaled scores than SPD and HC (p<0.01), but not FESZ (p=0.11). There were no differences between SPD, FESZ, and HC (p>0.05).
Degrees of freedom differ among variables due to unavailability of data for some subjects.
All subjects from the SPD sample were antipsychotic naïve, whereas 13/15 FESZ (typical=1, atypical=12) and 12/15 CHSZ (typical=3, atypical=9) were receiving antipsychotic treatment. Information on medication status was unknown for 5 patients (FESZ=2, and CHSZ=3).
3.2 Pituitary volume
Correlational analyses did not reveal a significant association between PV and any sociodemographic, characteristic (p>0.05) or whole brain volume (r137= −0.002, p= 0.980), except for years of education (r137= −0.217, p=0.011). Also, clinical subtests for symptom severity, chlorpromazine equivalence, duration of illness, and age of symptom onset were not associated with pituitary volume in FESZ or CHSZ, except for PANSS negative in CHSZ (r15= −0.699, p=0.004). Women had significantly larger PV than men (ANCOVA with whole brain volume as covariate: F= 4.99, df= 1, 134, p= 0.027).
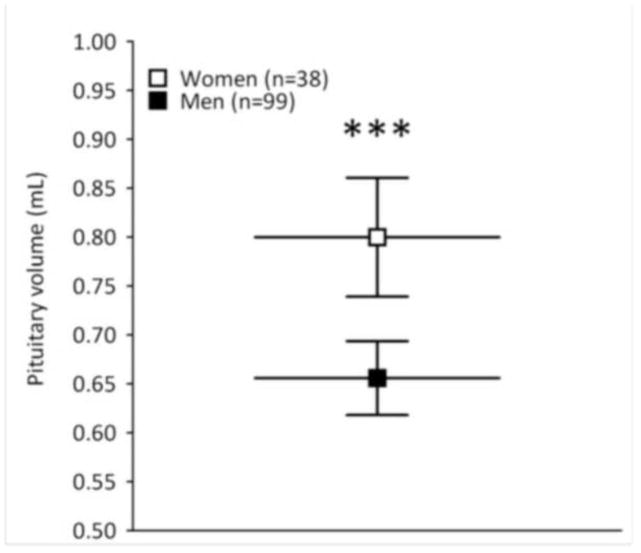
In the first ANCOVA model, which included all study subjects (n=137), PV was the dependent variable; group (i.e., SPD n=40, FESZ n=15, CHSZ n=15, HC n=67) and gender (male n=99, female n=38) were the between-subject factors; and age, years of education, SES, PSES, and whole brain volume were used as covariates. There was a main effect of gender (F= 12.96, df= 1, 126, p<0.001; covariate-adjusted pituitary volume mean±SD, female= 0.800±0.185, male= 0.656±0.189) (Figure 2a), but there was no group effect (F= 1.45, df= 3, 126, p= 0.232) or group by gender interaction (F= 2.28, df= 1, 126, p= 0.133). The significant main effect of gender with a non-significant group by gender interaction suggests that gender differences in pituitary volume are present in both SPD patients and controls.
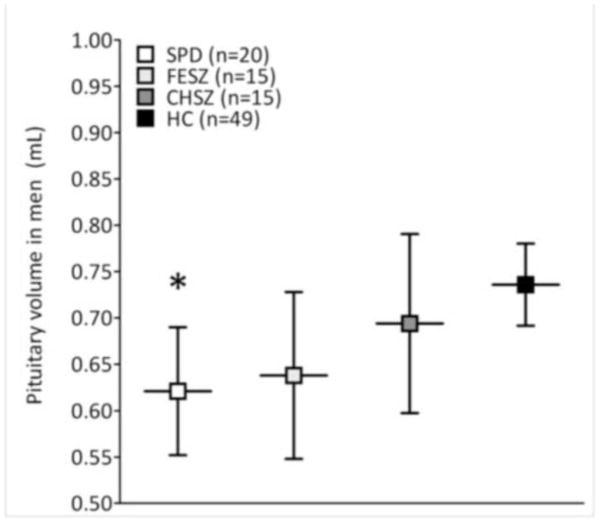
Figure 2.
Figure 2a–b. Pituitary volumes between genders (a) and across male groups (b) adjusted for age, years of education, (parental) socioeconomic status, and whole brain volume. Data points and horizontal bars represent group means; error bars represent 95% confidence interval of the mean. Figure 2a: Women (n=38) have significantly larger pituitary volumes than men (n=99) (p<0.001), but there is no group by gender interaction (p= 0.133), which suggests that gender differences in pituitary volume are present in both SPD patients and controls. Figure 2b: The ANCOVA model (n=99) without females is significant for group (p= 0.022). Male SPD have significantly smaller pituitary volume than male HC (p= 0.030) in Bonferroni-corrected post-hoc analyses. There are no statistically significant group differences for the other pair-wise comparisons (p>0.05). Abbreviations: SPD, schizotypal personality disorder; FESZ, first-episode schizophrenia; CHSZ, chronic schizophrenia; HC, healthy control subjects. *p<0.05, ***p<0.001.
The second ANCOVA model (n=99) without female, but with male pituitary volume as dependent variable, group (SPD n=20, FESZ n=15, CHSZ n=15, HC n=49) as between-subject factor, and age, years of education, SES, PSES, and whole brain volume as covariates revealed a significant main-effect for group (F= 3.38, df= 3, 90, p= 0.022). In Bonferroni-corrected post-hoc analyses, male SPD had significantly smaller PV than male HC (p= 0.030) (Table 2, Figure 2b), but there were no group differences for the other pair-wise comparisons (p>0.05).
Table 2.
Male pituitary volumes across groups
| SPD | FESZ | CHSZ | HC | |
|---|---|---|---|---|
| N | 20 | 15 | 15 | 49 |
| Pituitary gland, mL | 0.621 (0.148)a | 0.638 (0.164) | 0.694 (0.173) | 0.736 (0.151) |
Values represent covariate-adjusted mean (SD). Abbreviations: SPD, schizotypal personality disorder; FESZ, first-episode schizophrenia; CHSZ, chronic schizophrenia; HC, healthy control subjects.
ANCOVA followed by Bonferroni post-hoc t-tests were used. Age, years of education, (parental) socioeconomic status, and whole brain volume were used as covariates for pituitary volume.
p=0.030 compared to healthy controls (HC).
The SPD group-effect was replicated in the third ANCOVA model (n=84) without female and without CHSZ, but with male PV as dependent variable, group (SPD n=20, FESZ n=15, HC n=49) as between-subject factor, and age, years of education, SES, PSES, and whole brain volume as covariates (F= 5.21, df= 2, 76, p= 0.008). Male SPD had significantly smaller PV than male HC (p= 0.007). ANCOVA model 3 was also further tested without subjects from McLean hospital so that only subjects from Brigham and Women’s Hospital were included in the analyses, thus effectively eliminating the potential confounding effects of different scanners. In this additional analysis, the effects remained unchanged that is, we found a main-effect for group (F= 3.38, df= 2, 61, p= 0.041) with significantly smaller PVs for male SPD subjects compared to male controls (p=0.035).
Finally, to investigate further the SPD group effect and potential group by gender interaction, we performed a fourth ANCOVA model (ICV, years of education; n=107) directly comparing PV between the SPD group (n=40) and the healthy control group (n=67). Results showed a main-effect for gender (F= 10.81, df= 1, 101, p= 0.001) indicating larger PV for female subjects compared to male subjects. Furthermore, compared to controls, SPD subjects had a trend for smaller PV (F= 2.83, df= 1, 101, p= 0.095), but no group by gender interaction was observed (F= 1.78, df= 1, 101, p= 0.186), which suggests that the trend for smaller PV observed in the SPD sample is independent from gender.
In all ANCOVA models, the main-effects remained the same with scanner-site as additional covariate, and scanner-site was a non-significant independent predictor for PV volume in each model.
4 DISCUSSION
In this study, we compared PV of subjects in the schizophrenia spectrum, which revealed three main findings. First, male SPD subjects had smaller PV than male healthy control subjects. No volumetric changes in FESZ or CHSZ were found. Second, there was a gender-effect with women having larger volumes than men independent of group. Third, no effects of medication dose in terms of chlorpromazine equivalents on PV were found for the FESZ or CHSZ patients.
SPD shares clinical and neurobiological characteristics with schizophrenia (Raine, 2006), and it has been documented that abnormalities in stress response and HPA axis functioning are among the commonalities (Mitropoulou et al., 2004). It is thought that PV is influenced by underlying HPA functioning, but evidence is scarce. Takahashi et al. (Takahashi et al., 2009) were the first to report on PV in SPD (n=47, male=29), and found increased volumes compared to healthy controls, which is opposite to our findings in the male SPD group (n=20). Differences between the characteristics of the samples might help to explain this discrepancy in results. Our result was found in male SPD subjects who were on average five years older, neuroleptic naïve and recruited from the community, whereas the SPD group in the Takahashi study was a clinical sample, and most of them received low dose anti-psychotics and other medication. In a longitudinal study based on the same sample, Takahashi found that volumes in SPD subjects increased during the follow up period (mean=2.9yrs), and findings suggested that antipsychotics influence PV. (Takahashi et al., 2011)
Our results of no volume difference between FESZ and HC contrasts with two recent studies assessing PV in FE psychosis (Pariante et al., 2005; Pariante et al., 2004) where increased PV in FESZ was reported. Mondelli (Mondelli et al., 2008) and Pariante (Pariante, 2008), have proposed that PV may change depending on stage of psychosis, i.e., during the prodromal phase the PV increases (Garner et al., 2005), and after illness onset it tends to become smaller. Interestingly, in the study performed by Upadhyaya et al (Upadhyaya et al., 2007), PV in neuroleptic naïve SZ patients were significantly smaller than HC subjects. Differences remained significant when gender was considered as a covariate, but when comparing patients and controls by gender, there was no significant difference. In our study no women were included in FESZ and HC group comparison.
We also found no difference in PV between CHSZ and HC groups. Previous studies investigating PV in CHSZ have found smaller (Pariante et al., 2004; Upadhyaya et al., 2007) and larger volumes (Pariante et al., 2005; Takahashi et al., 2009) compared to controls, but our study did not replicate either of these findings. Our findings are consistent with those of Tournikioti et al. (Tournikioti et al., 2007) who reported no differences between CHSZ and controls in a sample comparable for age and gender to our sample. Our findings are also consistent with those of Mondelli et al (Mondelli et al., 2008), but their schizophrenia sample was seven years younger than our CHSZ group. Tournikioti et al. (Tournikioti et al., 2007) postulated the possibility that age differences (10 yrs) between SZ and HC could help explain the smaller pituitary volumes found in the SZ sample reported by Pariante (Pariante et al., 2004).
Furthermore, the interpretation of findings in this and other studies is complicated by the fact that patients often receive prolactin-increasing antipsychotics, which have been shown to be related to changes in PV (MacMaster et al., 2007a). This notion about the pharmacologic influence on PV has also been challenged with some of the studies reporting no effect of medication on PV (Pariante et al., 2005; Pariante et al., 2004; Takahashi et al., 2009; Tournikioti et al., 2007), suggesting that PV in the course of schizophrenia is associated with several mediating factors, including antipsychotics, illness duration, and illness stage. For example, in a recent one-year longitudinal study of a relatively young chronic sample, Takahashi et al. (2012) reported that CHSZ patients treated with atypical antipsychotics had significant pituitary reduction over time compared to those treated with typical antipsychotics (Takahashi et al., 2012). Although most FESZ and CHSZ patients in our sample received atypical antipsychotics, no effects of medication dose on PV were found in either group.
We found significant gender differences in PV with females having larger volumes than males regardless of diagnosis. Similar findings in PV have previously been reported (Takahashi et al., 2009). However, MacMaster et al. (MacMaster et al., 2007b) examined the effect of age and gender in a large sample of healthy controls (n=154, ages 7–35 years) and found females to have larger volumes at younger ages (age group 14 to 17 years), but not at older ages (age groups 18 through 35 years). In our sample, gender differences in both SPD and HC groups were significant even though the mean age was 29 yrs. Takahashi et al. (Takahashi et al., 2009) also found significant gender differences among SPD (mean age male and female=25 yrs) and HC subjects (mean age male=25 yrs, female=24 yrs). In 1990, Lurie et al. (Lurie et al., 1990) found no PV gender difference in healthy adults, but found a negative correlation between PV and age (Lurie et al., 1990). The later consistent with Doraiswamy (Doraiswamy et al., 1992) and Grams et al. (Grams et al.)
Overall, women had larger PV than men, and within the male sample all SSD subjects had smaller PV than HC, statistically significant only for the SPD group. Discrepancies with previous PV assessments in SSD suggest that the pituitary gland is a dynamic structure that is sensitive to various sociodemographic variables, disease-related factors and biological mechanisms. Additionally, atypical antipsychotics have consistently shown a down- regulating effect on the proposed HPA hyperactivity in schizophrenia.(Bradley and Dinan, 2010) Chronic, untreated HPA axis hyperactivity in SPD subjects could partially explain the smaller PV finding, whereas treatment related acute and chronic HPA and neurotransmitter modulation in FESZ and CHSZ patients might have a normalizing effect on PV in this two groups. PV changes in SSD may be the result of a combined effect of the use of prolactin elevating drugs increasing PV, and chronic elevated cortisol levels as a result of HPA axis hyperactivity leading to decreased PV, as suggested by Pariante (Pariante et al., 2004). Other studies have found that HPA axis dysfunction may be one of many vulnerability factors shared by SSD. It is important to acknowledge that HPA axis activity is also regulated by other systems (e.g., GABA, glutamate, dopamine), which at the same time might exert volumetric changes by more direct pathways. For example, glutamatergic transmission has been implicated as a critical regulator of the stress response (van den Pol et al., 1990). In particular metabotropic glutamate receptors (mGluR) appear to play a crucial role in mediating neuroendocrine responses to stress (Lang and Ajmal, 1995). Group II mGluR agonists have shown to induce apoptosis in lactotropes indicating that the cytotoxic action of glutamate is mainly exerted on this cell type through activation of group II mGluRs (Caruso et al., 2004). Also, group II mGluR stimulation may increase the activity of tubero-infundibular dopaminergic neurons by inhibiting the GABAergic input (Johnson and Chamberlain, 2002). Dopamine exerts a tonic inhibitory effect on PRL release and reduces the proliferation of lactotropes via D2 receptors (Sarkar et al., 2005), with a possible effect on PV. This could help explain why a chronic, untreated HPA axis hyperactivity in neuroleptic naive SPD subjects leads to a decreased pituitary volume, whereas treatment related acute and chronic HPA and neurotransmitter modulation in FESZ and CHSZ patients might have a normalizing effect on PV in these two groups. In other words, PV changes in SSD may be the result of a combined effect of the use of prolactin elevating drugs (leading to increased PV), and chronic elevated cortisol levels as a result of HPA axis hyperactivity (leading to decreased PV), as suggested by Pariante (Pariante et al., 2004). These mechanisms and others yet to be fully understood could contribute to the dynamic volumetric changes and conflicting results that have been reported across studies (MacMaster et al., 2007a; Mondelli et al., 2008; Pariante et al., 2004; Pariante et al., 2005; Takahashi et al., 2009; Tournikioti et al., 2007; Upadhyaya et al., 2007).
Some limitations in this study have to be acknowledged. First, data has been gathered from two different scanners. Although there is evidence supporting that volumetric variations across different scanners is minimal (Jovicich et al., 2009), and we controlled for scanner-site in the analyses, this should be considered as a possible source of bias in results. Second, no endocrine measurements were made, and assumptions about the underlying substrate for pituitary volumetric changes are made based on previous basic and clinical information regarding HPA functioning and modulation by diverse neurotransmitter systems. It is necessary to continue research with direct measurements of HPA axis products or byproducts in order to confirm this hypothesis. Third, the FESZ and CHSZ group only consisted of males. Examination of gender differences was thus not possible for these groups. Lastly, even though we found no correlation between chlorpromazine equivalence and PV, our sample did not include enough medication-free patients to fully determine the potential confounding effects of medication.
Acknowledgments
Role of the funding source
This work was supported by grants from the US Department of Veterans Affairs (Merit Review Awards to MES and RWM; Schizophrenia Center to RWM and MES); US National Institutes of Health (R01 MH50747 and K05 MH070047 to MES; P50 MH080272 to MES and RWM; R01 MH040799 to RWM; MFP was supported by the Cuthbertson and Fischer Chair in Paediatric Mental Health.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Contributors.
All persons designated as authors qualify for authorship as each author substantially contributed to the study by either being involved in the study conception and design (Romo-Nava, Hoogenboom, Shenton, MacMaster, Keshavan, McCarley), acquisition of data (Romo-Nava, Hoogenboom, Pelavin, Alvarado, Bobrow), data analysis (Romo-Nava, Hoogenboom), writing (Romo-Nava, Hoogenboom), or contributed to discussion and revising of the manuscript for intellectual content (all authors). All persons that were involved in the preparation of this manuscript are listed as authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME. An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res. 2002;58(2–3):123–134. doi: 10.1016/s0920-9964(01)00372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. 1989;(Suppl 7):49–58. [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P. Correlational studies of the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms: an overview and update. Psychopathology. 1995;28(1):7–17. doi: 10.1159/000284894. [DOI] [PubMed] [Google Scholar]
- Bouix S, Martin-Fernandez M, Ungar L, Nakamura M, Koo MS, McCarley RW, Shenton ME. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36(4):1207–1224. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24(4 Suppl):91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- Caruso C, Bottino MC, Pampillo M, Pisera D, Jaita G, Duvilanski B, Seilicovich A, Lasaga M. Glutamate induces apoptosis in anterior pituitary cells through group II metabotropic glutamate receptor activation. Endocrinology. 2004;145(10):4677–4684. doi: 10.1210/en.2004-0550. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Boccio AM, Riordan H, Hoff AL, Dorfman A, McClelland J, Kushner M, Van Eyl O, Oden N. Familial thyroid disease and delayed language development in first admission patients with schizophrenia. Psychiatry Res. 1991;38(1):39–50. doi: 10.1016/0165-1781(91)90051-p. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Kushner M, Finer DL, Hoff AL, Crow TJ. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr Bull. 1997;23(2):255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer I, Teh EK, Van Rhoads R, Jakab M, Kikinis R, Jolesz FA, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999;45(11):1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Xu ML, Seidman LJ, Voglmaier MM, Niznikiewicz MA, Connor E, Shenton ME. MRI abnormalities of the hippocampus and cavum septi pellucidi in females with schizotypal personality disorder. Schizophr Res. 2007;89(1–3):49–58. doi: 10.1016/j.schres.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Fraone S, McCarley RW. Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry. 2000;157(1):48–54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy PM, Potts JM, Axelson DA, Husain MM, Lurie SN, Na C, Escalona PR, McDonald WM, Figiel GS, Ellinwood EH, Jr, et al. MR assessment of pituitary gland morphology in healthy volunteers: age- and gender-related differences. AJNR Am J Neuroradiol. 1992;13(5):1295–1299. [PMC free article] [PubMed] [Google Scholar]
- Fujisawa I, Asato R, Nishimura K, Togashi K, Itoh K, Nakano Y, Itoh H, Hashimoto N, Takeuchi J, Torizuka K. Anterior and posterior lobes of the pituitary gland: assessment by 1.5 T MR imaging. J Comput Assist Tomogr. 1987;11(2):214–220. doi: 10.1097/00004728-198703000-00003. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58(5):417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Grams AE, Gempt J, Stahl A, Forschler A. Female Pituitary Size in Relation to Age and Hormonal Factors. Neuroendocrinology. doi: 10.1159/000314196. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000a;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155(10):1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, McCarley RW. Hippocampal and superior temporal gyrus volume in first-episode schizophrenia. Arch Gen Psychiatry. 2000b;57(6):618–619. doi: 10.1001/archpsyc.57.6.618. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11(4):374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. Yale Station; New Haven: 1965. [Google Scholar]
- Johnson MP, Chamberlain M. Modulation of stress-induced and stimulated hyperprolactinemia with the group II metabotropic glutamate receptor selective agonist, LY379268. Neuropharmacology. 2002;43(5):799–808. doi: 10.1016/s0028-3908(02)00142-9. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Yokoyama F, Hoshino Y, Takahagi K, Murata S, Watanabe M, Kumashiro H. Hypothalamic-pituitary-adrenal axis function in chronic schizophrenia: association with clinical features. Neuropsychobiology. 1992;25(1):1–7. doi: 10.1159/000118800. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50(10):781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Lang CH, Ajmal M. Metabolic, hormonal, and hemodynamic changes induced by metabotropic excitatory amino acid agonist (1S,3R)-ACPD. Am J Physiol. 1995;268(4 Pt 2):R1026–1033. doi: 10.1152/ajpregu.1995.268.4.R1026. [DOI] [PubMed] [Google Scholar]
- Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Lurie SN, Doraiswamy PM, Husain MM, Boyko OB, Ellinwood EH, Jr, Figiel GS, Krishnan KR. In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab. 1990;71(2):505–508. doi: 10.1210/jcem-71-2-505. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, El-Sheikh R, Upadhyaya AR, Nutche J, Rosenberg DR, Keshavan M. Effect of antipsychotics on pituitary gland volume in treatment-naive first-episode schizophrenia: a pilot study. Schizophr Res. 2007a;92(1–3):207–210. doi: 10.1016/j.schres.2007.01.022. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Keshavan M, Mirza Y, Carrey N, Upadhyaya AR, El-Sheikh R, Buhagiar CJ, Taormina SP, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR. Development and sexual dimorphism of the pituitary gland. Life Sci. 2007b;80(10):940–944. doi: 10.1016/j.lfs.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulou V, Goodman M, Sevy S, Elman I, New AS, Iskander EG, Silverman JM, Breier A, Siever LJ. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizotypal personality disorder. Schizophr Res. 2004;70(1):27–31. doi: 10.1016/j.schres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Gabilondo A, Tournikioti K, Walshe M, Marshall N, Schulze KK, Murray RM, McDonald C, Pariante CM. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33(7):1004–1012. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Pituitary volume in psychosis: the first review of the evidence. J Psychopharmacol. 2008;22(2 Suppl):76–81. doi: 10.1177/0269881107084020. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30(10):1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. Br J Psychiatry. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Bouix S, Nakamura M, Rohlfing T, McCarley RW, Kikinis R, Grimson WE, Shenton ME, Wells WM. A hierarchical algorithm for MR brain image parcellation. IEEE Trans Med Imaging. 2007;26(9):1201–1212. doi: 10.1109/TMI.2007.901433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291–326. doi: 10.1146/annurev.clinpsy.2.022305.095318. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28(11):1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chaturvedi K, Oomizu S, Boyadjieva NI, Chen CP. Dopamine, dopamine D2 receptor short isoform, transforming growth factor (TGF)-beta1, and TGF-beta type II receptor interact to inhibit the growth of pituitary lactotropes. Endocrinology. 2005;146(10):4179–4188. doi: 10.1210/en.2005-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Harenski K, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Decreased pituitary volume in patients with bipolar disorder. Biol Psychiatry. 2001;50(4):271–280. doi: 10.1016/s0006-3223(01)01086-1. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161(3):398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kido M, Nakamura K, Furuichi A, Zhou SY, Kawasaki Y, Noguchi K, Seto H, Kurachi M, Suzuki M. Longitudinal MRI study of the pituitary volume in chronic schizophrenia: a preliminary report. Psychiatry Res. 2012;202(1):84–87. doi: 10.1016/j.pscychresns.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Velakoulis D, Lorenzetti V, Soulsby B, Zhou SY, Nakamura K, Seto H, Kurachi M, Pantelis C. Increased pituitary volume in schizophrenia spectrum disorders. Schizophr Res. 2009;108(1–3):114–121. doi: 10.1016/j.schres.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Zhou SY, Nakamura K, Tanino R, Furuichi A, Kido M, Kawasaki Y, Noguchi K, Seto H, Kurachi M, Suzuki M. Longitudinal volume changes of the pituitary gland in patients with schizotypal disorder and first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):177–183. doi: 10.1016/j.pnpbp.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Tournikioti K, Tansella M, Perlini C, Rambaldelli G, Cerini R, Versace A, Andreone N, Dusi N, Balestrieri M, Malago R, Gasparini A, Brambilla P. Normal pituitary volumes in chronic schizophrenia. Psychiatry Res. 2007;154(1):41–48. doi: 10.1016/j.pscychresns.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Upadhyaya AR, El-Sheikh R, MacMaster FP, Diwadkar VA, Keshavan MS. Pituitary volume in neuroleptic-naive schizophrenia: a structural MRI study. Schizophr Res. 2007;90(1–3):266–273. doi: 10.1016/j.schres.2006.09.033. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250(4985):1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]