Abstract
The retinoblastoma (Rb) family of proteins are well known for their tumor suppressor properties and for their ability to regulate transcription. The action of Rb-family members correlates with the appearance of repressive chromatin marks at promoter regions of genes encoding key regulators of cell proliferation. Recent studies raise the possibility that Rb family members do not simply act by controlling the activity of individual promoters but that they may also function by promoting the more general organization of chromatin. In several contexts, Rb-family members stimulate the compaction or condensation of chromatin and promote the formation of heterochromatin. In this review, we summarize studies that link pRb family members to the condensation or compaction of DNA.
The structure of chromatin regulates its accessibility
Chromatin is the physical form that allows approximately two meters of DNA to be compacted into the nucleus of a single cell without compromising the genetic information stored in the DNA sequence. The basic building block of chromatin is the nucleosome, containing 147 bp of DNA wrapped around two copies of the core histones H2A, H2B, H3, and H4 (Luger et al. 1997). Post-translational modification of histone proteins changes the accessibility of chromatin to regulators of transcription and affects the binding of transcription factors, the basal transcription machinery, and chromatin remodelers (Strahl and Allis 2000; Jenuwein and Allis 2001). The degree of accessibility in a given region of chromatin determines which genes are transcribed, as well as the order in which they are replicated (Shilatifard 2006; Birney et al. 2007; Groth et al. 2007; Karnani et al. 2007; Kouzarides 2007; Li et al. 2007).
Chromatin is organized into highly accessible domains, or euchromatic regions, and regions which are predominantly inaccessible, described as heterochromatin. In heterochromatic regions, chromatin is highly condensed into a dense, tightly wound structure. In general, chromatin condensation occurs during four physiological situations within a cell: mitosis, apoptosis, senescence, and during the formation and/or redistribution of heterochromatin. While post-translational modifications of histones have been linked to all four of these processes, the complex restructuring of the DNA into condensed chromatin and heterochromatin involves a myriad of proteins and the protein complexes that facilitate the compaction of the chromatin and/or respond to changes in histone modification are still being discovered (Kouzarides 2007; Wang et al. 2007; Cam et al. 2009).
The pRb tumor suppressor
The retinoblastoma gene (RB1) is one of the most intensively studied tumor suppressor genes. Mutation of RB1 is the key rate-limiting event in the development of the childhood cancer, retinoblastoma, and the RB1 gene product (pRb) is thought to be absent or misregulated in over 90% of human cancers (Hanahan and Weinberg 2000; Sherr and McCormick 2002). pRb is a member of a family of proteins (pRb, p107, and p130) whose best-known activity is an ability to repress transcription of E2F-regulated genes (Flemington et al. 1993; Helin and Ed 1993; Frolov et al. 2001). The E2F transcription factor co-ordinates the temporal expression of a large set of genes that are needed for cell proliferation (Farnham et al. 1993; van Ginkel et al. 1997; Bracken et al. 2004; DeGregori and Johnson 2006). In many plants and animals, pRb-family members provide an important brake on E2F activity. The absence of this constraint places cells in a state where they are permissive for cell proliferation and poised to respond to pro-proliferative signals. pRb-family members block transcription that is promoted by “activator” E2Fs, (such as the mammalian E2F1, -2, and –3a proteins) and repress transcription of these targets in complexes with “repressor” E2Fs (such as mammalian E2F3b, -4, and E2F5) (Trimarchi and Lees 2002).
pRb-family members contain distinct binding sites for E2F proteins and chromatin modifying complexes and this architecture enables them to recruit regulatory activities to E2F-regulated promoters (Lee et al. 1998; Rubin et al. 2005). In the past decade, more than 100 different proteins have been reported to associate with pRb-family proteins (Morris and Dyson 2001). Most of the proteins linked to pRb are chromatin-associated proteins with roles in transcriptional regulation, chromatin modification, and/or chromatin remodeling (Brehm et al. 1998a; Ferreira et al. 1998; Luo et al. 1998; Magnaghi-Jaulin et al. 1998a; Nielsen et al. 2001a; Narita et al. 2003; Gunawardena et al. 2007; Litovchick et al. 2007).
Estimates of the number of genes regulated by E2F vary from several hundred to more than a thousand (Kel et al. 2001; Bieda et al. 2006). In addition to their effects on the expression of cell-cycle genes, pRb-family members also promote the expression of several markers of cell differentiation. In muscle cells, where this role has been studied in detail, pRb enables differentiated cells to irreversibly exit the cell cycle (Schneider et al. 1994; Novitch et al. 1996; Zacksenhaus et al. 1996). pRb has also been implicated in bone differentiation and shown to interact with the Runx2 transcription factor, an important regulator of osteoblastic differentiation (Thomas et al. 2001). Through these types of interactions, pRb-family members appear to co-ordinate extensive programs of gene expression that are important for cells to either proliferate or to differentiate
Traditionally the effects of pRb action have been studied at the level of individual promoters. However, as described below, several lines of evidence suggest that the activation or inactivation of pRb-family members can have general consequences. Remarkably, pRb proteins have been shown to be important for at least three of the four processes mentioned above that employ chromatin condensation, including mitosis, senescence, and the formation and/or redistribution of heterochromatin. These findings raise the possibility that pRb-family members do not act simply at the level of individual promoters but that they may function more broadly to control the local organization of chromatin structure. The large number of changes in gene expression that are needed for the transition between cell proliferation and cell differentiation are likely to involve an extensive reorganization of chromosomal domains. The links to chromatin organization may explain why pRb-family member proteins are able to act as master regulators of the transition between proliferation and differentiation. In this review, we summarize the evidence linking pRb to chromatin compaction and condensation.
pRb associates with enzymes that modify histones, remodel nucleosomes, and that promote the formation of heterochromatin
The cessation of cell proliferation and the initiation of differentiation involve widespread changes in the patterns of gene expression. Because repressed genes and actively transcribed genes are concentrated in different sub-compartments in the nucleus these transitions are predicted to involve a substantial reorganization of chromatin. Indeed, several studies have noted general changes in the properties of chromatin during cell differentiation. During tobacco protoplast regeneration, for example, cellular dedifferentiation is accompanied by the reorganization of chromatin reorganized into a less condensed state that is more sensitive to Micrococcal nuclease digestion (Williams et al. 2003). Consistent with the idea that Rb family members control the redistribution of chromatin of E2F-regulated genes as cells enter or exit a differentiation program, the promoters and regions around the E2F regulated RNR2 and PCNA genes were resistant to Micrococcal nuclease digestion in the differentiated cells, but became sensitive in dedifferentiated cells.
The discovery that pRb actively represses transcription when recruited to promoters (Weintraub et al. 1995) prompted searches for pRb-interacting proteins that confer this activity. Several different proteins have been found that contribute to the control of E2F-regulated genes and appear to be involved in pRb-mediated repression (described below and reviewed in (Frolov and Dyson 2004)). Strikingly, several of the proteins recruited to DNA by pRb, or by pRb-family proteins, have activities that promote the formation and or spreading of heterochromatin.
Co-repressor molecules that associate with pRb-family members and have been implicated in the repression of E2F-regulated promoters include histone deacetylases (HDACs), and SWI/SNF chromatin remodelers, histone methyltransferases (HMTases), and histone demethylases (Dunaief et al. 1994; Singh et al. 1995; Brehm et al. 1998b; Luo et al. 1998; Magnaghi-Jaulin et al. 1998b; Nielsen et al. 2001b). These interactions are heavily dependent on a particular binding surface, the LXCXE-binding cleft, in the pocket domain of pRb (reviewed in (Morris and Dyson 2001)). ChIP-on-chip experiments using cultured mammalian cells suggest that p130, E2F4, and components of the hDREAM repressor complex are present at most E2F-regulated promoters in arrested cells (Litovchick et al. 2007). In differentiated myotubes, many of the same promoters are occupied by Sin3 repressor complexes that bind downstream of the transcriptional start site and appear to re-position nucleosomes (van Oevelen et al. 2008).
To date, the full spectrum of complexes recruited to individual E2F regulated promoters by pRb and/or pRb-family members, and the sequence in which they act is not completely clear. Repressor activities vary in importance at individual promoters, and rather than a single type of pRb-recruited repressor acting alone, the most likely scenario is that multiple activities act co-operatively to repress gene expression. For example, Class 1 HDACs are thought to repress transcription, in large part, by deacetylating H3 and H4, and removing modifications that open chromatin and promote gene expression reviewed in (Kuo and Allis 1998). SWI/SNF chromatin remodeling complexes are able to reposition nucleosomes, and may help to promote the specific placing of nucleosomes at E2F regulated promoters (Dunaief et al. 1994; Eisen et al. 1995; Trouche et al. 1997; Havas et al. 2000; Gavin et al. 2001). The Suv39H1 HMTase methylates Histone H3K9 and creates a binding site for the methyl lysine binding protein HP1, providing a chromatin mark that is linked to transcriptional repression (Rea et al. 2000; Bannister et al. 2001; Lachner et al. 2001; Nielsen et al. 2001a). pRb-mediated repression may well involve all of these activities, and perhaps additional types of complexes, such as demethylases, arginine methyltransferases, etc (see (Frolov and Dyson 2004) for review). The artificial targeting of pRb to promoter sequences promotes H3/H4 deacetylation, binding of Suv39H1, the enrichment of trimethylated H3K9, and the recruitment of HP1. Consistent with this, analysis of Rb -/- MEFs, shows less HP1 and H3K9 methylation at the cyclin E promoter (a classic E2F-regulated gene) compared to wild type cells (Nielsen et al. 2001a).
HP1 association with chromatin is influenced by HDAC activity in both yeast and mammalian cells (Vaute et al. 2002; Yamada et al. 2005). Chromatin-bound HP1 acts in conjunction with Suv39H1 to promote the further recruitment of endogenous HP1, allowing both the formation and spreading of heterochromatin (Verschure et al. 2005). The recruitment of HDAC, and the subsequent action of Suv39H1 and HP1, therefore, may not only lead to transcriptional repression of individual promoters but can lead to more extensive changes in chromatin structure. Hence, the activation of pRb at a specific locus may potentially affect the chromatin configuration over a much larger region.
The possibility that pRb recruitment of Suv39H1 may influence the general placement of HP1 is supported by FRAP experiments performed in RbloxP/loxP Mouse Adult Fibroblasts showing that the loss of pRb increases the mobility of HP1 protein (Siddiqui et al. 2007). The idea that pRb-recruited proteins might not simply repress transcription but might promote the formation of heterochromatin is further supported by studies that have examined H4K20 methylation. Trimethylated H4K20 is a feature of pericentric heterochromatin. Suv4-20H1 and Suv4-20H2, the HMTases that generate this modification, are localized to pericentric heterochromatin through an interaction with HP1 isoforms (Schotta et al. 2004). These proteins also associate with pRb family members and, intriguingly, MEFs deficient for pRb, p107 and p130 (Triple Knock-Out or TKO cells) exhibit a decrease in trimethylated H4K20 at pericentric heterochromatin (Gonzalo et al. 2005). A similar decrease is seen in MEFs that express a mutant form of pRb that is mutated in the LXCXE cleft (RbΔLXCXE) (Isaac et al. 2006). However, it is thought that pRb mediates the stabilization of trimethylated H4K20 at pericentric heterochromatin instead of directing Suv420 HMTases to the region, since they still localize to pericentric heterochromatin in TKO cells (Gonzalo et al. 2005). Curiously, the ability of pRb family members to promote H4K20 trimethylation was unaffected by a dominant negative form of E2F, suggesting that this role of pRb is likely to be distinct from its role in E2F regulation (Gonzalo et al. 2005). The compaction of pericentric heterochromatin is thought to aid chromosome segregation, and abnormalities in pericentric heterochromatin are one of the potential causes of the anaphase bridges that appear with elevated frequency in RbΔLXCXE cells (Isaac et al. 2006).
An additional link between Rb and chromatin compaction is provided by studies of L3MBTL1. Studies in Drosophila have shown that L(3)MBT physically associates with the dREAM/myb-Muv complex, a repressor complex that contains RBF proteins (the Drosophila homologs of pRb, p107 and p130), and that L(3)MBT is required for the repression of a subset of E2F-regulated genes (Lewis et al. 2004). Experiments in mammalian cells revealed that L3MBTL1, a mammalian ortholog of L(3)MBT, not only interacts with pRb but also has the ability to compact nucleosomal arrays (Boccuni et al. 2003; Trojer et al. 2007). This compaction is mediated through a bivalent domain that binds to methylated H4K20 and to H1bK26. Interestingly, L3MBTL1 was found to ChIP to E2F regulated promoters, coincident with high levels of methylated H4K20 and H1bK26 (Trojer et al. 2007). Recently, L3MBTL1 was shown to associate with the H4K20 methyltransferase, PR-SET7 (Kalakonda et al. 2008), raising the possibility that L3MBTL1 may function in both the “reading” and “writing” of the methylated H4K20 mark. It is tempting to speculate that the links between pRb and L3MBTL1 could help to promote H4K20 methylation and the compaction of heterochromatin.
pRb may also be able to influence compaction and regulate transcription through an association with the H3K4 tri-demethylase RBP2. pRb and RBP2 complexes have been identified in the chromatin associated fraction of differentiated leukemia cells and in SAOS2 cells that have been induced to senesce by the re-expression of pRb (Benevolenskaya et al. 2005; Iwase et al. 2007; Klose et al. 2007). RBP2 demethylates residues at the transcriptional start sites of specific genes. pRb prevents RBP2 mediated repression of some targets (such as osteocalcin) by displacing RBP2 from the promoter, but it also cooperates with RBP2 to activate other genes (such as BRD2 and BRD8) (Benevolenskaya et al. 2005). The targets of RBP2 include differentiation dependent genes and differentiation-independent genes (Lopez-Bigas et al. 2008). Potentially, pRb may co-operate with RBP2 to induce a change in the chromatin state at specific promoters that promotes the cessation of proliferation and the initiation of differentiation.
pRb and Senescence-Associated changes in heterochromatin
Major changes in the formation and distribution of heterochromatin occur naturally during cellular senescence. Senescence is a physiological process that is characterized by the cessation of cell proliferation, an irreversible cell cycle arrest, and the increased expression of specific markers such as β-galactosidase (Dimri et al. 1995; Narita et al. 2003; Collado et al. 2005). This process provides an important protection against cell immortalization and neoplastic transformation in vitro (Lowe et al. 2004) and suppresses tumorigenesis in vivo (reviewed in (Campisi 2005) and (Schmitt 2003)). Genetic studies show that pRb-family members play a key role in the senescence response. The loss of all three pRb family members is sufficient to bypass senescence in primary MEFs (Harvey et al. 1993; Sage et al. 2000). Conversely, the re-introduction of pRb into tumor cell lines, such as Saos2 cells, that are mutant for RB-1, rapidly induces senescence (Huang et al. 1988; Templeton et al. 1991; Hinds et al. 1992).
Senescent Associated Heterochromatin Foci (SAHFs) are a common feature of senescent cells. SAHFs are formed by regions of highly-condensed chromatin. They contain concentrated areas of trimethylated H3K9 and HP1 and appear to represent heterochromatinized portions of individual chromosomes (Zhang et al. 2005; Funayama et al. 2006; Narita et al. 2006). Biochemical studies of tissue culture cells that have been induced to undergo senescence by the expression of activated Ras or the cdk-inhibitor p16 show that senescence is accompanied by a general decrease of the linker histone H1 and accumulation of HMGA2 (Funayama et al. 2006). H1 is an important mediator of higher order chromatin structure (Bednar et al. 1995; Sato et al. 1999). Among its activities, H1 competes with HMGA proteins for binding to chromatin. The purification of SAHFs revealed that these structures contain high levels of HMGA proteins, and subsequent experiments confirmed that they are essential components of SAHFs (Catez et al. 2004; Funayama et al. 2006; Narita et al. 2006). There is a great deal of evidence to suggest that HMG proteins decompact chromatin structure and allow chromatin associated factors access to DNA (Bustin 1999; Reeves 2001; Thomas and Travers 2001; Agresti and Bianchi 2003). The knock down of HMGA in normal fibroblasts downregulated the expression of many genes. However, HMGA cooperates with p16 expression to induce senescence in primary IMR90 fibroblasts and, in the context of senescent cells, HMGA proteins acts to repress the transcription of genes necessary for proliferation (Narita et al. 2006).
Not only are pRb-family members needed for senescence, but the appearance of SAHFs correlates with the repression of E2F-regulated genes as well as the detection of pRb and molecular marks of heterochromatin at E2F regulated promoters. FISH experiments show that repressed promoters localize to SAHF’s in senescent cells (Narita et al. 2003). pRb may to contribute to this process in several different ways. Through its interactions with HDAC’s, Suv39H1 and HP1, pRb is thought to mediate the repression of E2F-regulated genes in senescent cells and the spreading of heterochromatin (Narita et al. 2003). In addition, pRb has been shown to bind to HMGA1, and may even possess the ability to direct HMGA to E2F regulated promoters (Ueda et al. 2007).
Intriguingly, G1 arrested Rb −/− MEFs are more susceptible to Micrococcal nuclease digestion than wild type MEFs, suggesting that these cells have a more open chromatin configuration (Herrera et al. 1996). Rb −/− MEFs also exhibit an increase in Histone H1 phosphorylation. Studies in Tetrahymena have shown that H1 phosphorylation regulates the expression of some genes, and chromatin assembled with phosphorylated H1 in vitro exhibits a more open structure (Dou et al. 1999). H1 phosphorylation has also been demonstrated to allow for chromatin remodeling and accessibility of the MMTV promoter (Kaplan et al. 1984; Halmer and Gruss 1996; Bhattacharjee et al. 2001). Hence, the inactivation of pRb and pRb-family members may not only affect the recruitment of repressor complexes and proteins needed for SAHF formation but the chromatin present in these cells may be more open and more difficult to compress.
The inactivation of Rb family members leads to chromosomal defects
The compression of chromatin becomes extremely important during cell division. Staining of mitotic chromosomes from TKO MEFs revealed the presence of butterfly chromosomes, abnormal structures that appeared to be caused by a defect in chromosome cohesion (Gonzalo et al. 2005). These chromosomes appear to contain hypocondensed chromatin, suggesting that the activities of pRb-family members have an impact on processes affecting chromosome condensation and/or separation.
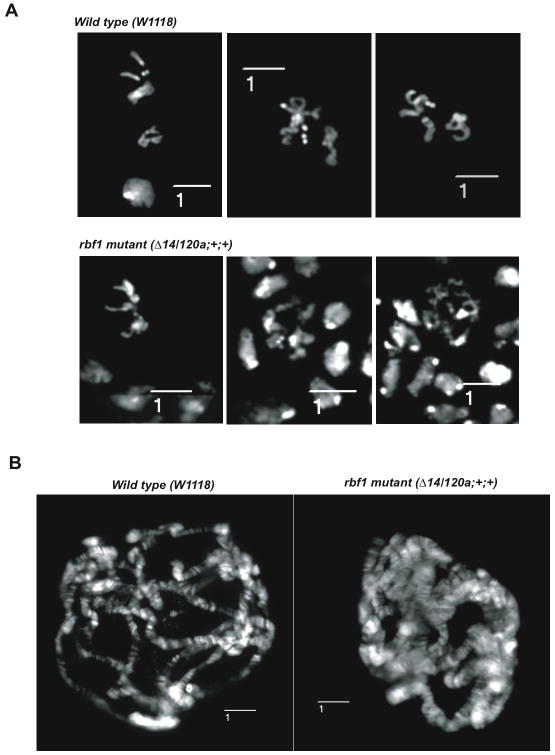
pRb family members are traditionally thought to function during G0 or G1 phases of the cell cycle. The idea that these proteins might also influence the normal progression through mitosis has received strong support from studies of the Drosophila homolog of pRb, RBF1. Recent work shows that mitotic chromosomes of rbf1 mutant animals, visualized in larval neuroblast squashes, often have an abnormal appearance during the early stages of mitosis (Figure 1A and (Longworth et al. 2008). These chromosomes frequently contain regions of normally condensed chromatin interspersed with hypocondensed regions that appear spindly and unstable. These defects do not prevent rbf1 mutant cells from dividing. Fully condensed chromosomes are seen in metaphase cells, illustrating that condensation can occur if given time. However, the appearance of fused or broken chromosomes and anaphase bridges indicates that chromosome segregation is error-prone.. In converse experiments, the overexpression of RBF1 in imaginal discs leads to the appearance of hypercondensed PH3 positive chromosomes that are distinct from the fragmented chromosome that can arise during apoptosis (Longworth et al. 2008). Polytene chromosomes prepared from rbf1 mutant larvae are unusually broad and hypocondensed compared to wild type chromosomes (Figure 1B), strongly supporting the idea that RBF1 is needed for normal chromosome morphologies.
Figure 1. rbf1 mutant Drosophila larvae exhibit abnormal chromatin condensation.
A. Prometaphase spreads of neuroblast squashes from wild type, and rbf1 mutant (rbf1120/Δ14) Drosophila third instar larvae. In contrast to the uniformly condensed, wild-type chromosomes, the chromosomes of rbf1 mutant neurblasts are irregular in appearance and contain hypocondensed regions. B. Polytene chromatin spreads of salivary gland squashes from third instar larvae of the same genotypes as in A illustrating hypocondensed chromatin in the rbf1 mutants. Measurement bars labeled with (1) measure 10 μM. For Methods, see [82].
RBF1 complexes control the expression of genes needed during mitosis
Why do these changes in chromosome morphology occur? The E2F-transcriptional program is not limited to genes needed for the G1/S transition but affects a diverse array of cellular processes. E2F targets include, for example, genes such as SMC2, SMC4, BRD1, BRD2 ial, Cap-G, and barren (Ren et al. 2002; Georlette et al. 2007; Litovchick et al. 2007) that are important for chromatin condensation and changes in expression the expression of these types of genes may affect the chromatin condensation or Mitotic progression in cells lacking Rb-family members. In addition, changes in gene expression may also affect the ways in which cells respond to abnormal chromosome structures. Indeed, studies in mammalian cells have shown that Mad2 expression is deregulated in cells lacking functional pRb and that this change compromises the spindle assembly checkpoint, promoting the mis-segregation of chromosomes (Hernando et al. 2004). Studies of the dREAM/Myb-Muv repressor complex in Drosophila (a complex containing Mip130/TWIT, Mip120, dMyb, CAF1p55, dE2F2, dDP, Mip40, and either RBF1 or RBF2 that is present at most E2F target genes in Drosophila) indicates that changes in dREAM activity affect the expression of the polo kinase, an important mitotic regulator (Wen et al. 2008).
dMyb, a subunit of the dREAM complex that is important for both the activation and repression of transcription, is also required for the timely condensation of euchromatin in early mitosis (Manak et al. 2007). In contrast to the hypocondensation defects seen in rbf1 mutants, imaginal discs homozygous for two separate null alleles of dMyb exhibited hypercondensed chromosomes and increased Phosphorylated Histone 3 staining (Manak et al. 2002). Furthermore, the long-term depeletion of dREAM subunits Myb, Mip120 and Mip130 in Drosophila Kc cells resulted in abnormal chromosomes and chromosome condensation defects (Georlette et al. 2007). The different effects of RBF1 and dMyb on chromatin condensation may be due to antagonistic effects on dREAM complexes, or potentially these may be distinct effects that are mediated through different sets of targets. Nevertheless, dREAM complexes are clearly important for normal chromosome condensation and genome stability.
Links between RBF and Condensin II
A more direct explanation for the link between rbf1 and chromatin condensation has been provided by the discovery of genetic and physical interactions between RBF1 and components of the Condensin II complex (Longworth et al. 2008).
Condensin complexes promote the uniform, stable condensation of chromatin during prophase of mitosis (Steffensen et al. 2001; Ono et al. 2003; Hirota et al. 2004; Nasmyth and Haering 2005; Gerlich et al. 2006; Hudson et al. 2008). Although yeast contain a single form of Condensin, two distinct types complexes (Condensin I and II) are conserved from worms to humans. Both complexes contain a SMC2/SMC4 heterodimer, as well as 3 unique, non-SMC subunits (Hirano and Mitchison 1994; Nasmyth and Haering 2005; Onn et al. 2007). In Condensin I, the non-SMC kleisin protein, Cap-H connects the SMC heterodimer to the remaining non-SMC HEAT repeat proteins, Cap-D2 and Cap-G (Onn et al. 2007). Similar associations occur in Condensin II, with Cap-H2 replacing Cap-H as the kleisin, and Cap-D3 and Cap-G2 comprising the HEAT repeat subunits. The SMC proteins heterodimerize to form an active ATPase which acts to constrain positive supercoils, and which has been shown to condense DNA in vitro (Strick et al. 2004). This ATP dependent supercoiling requires the presence of the non-SMC subunits, which can also form a complex on their own (Kimura et al. 2001).
Immunostaining experiments suggest that Condensin I and Condensin II complexes localize to different domains of mammalian chromosomes (Ono et al. 2004) but precisely how Condensins function is still not well understood. It has been proposed that a single Condensin complex traps one or more double helixes within a protein ring. In Bacillus subtilis, this is accomplished in a two step cycle in which less stable DNA binding facilitated by opening of the SMC coiled coil ring is followed by more stable DNA binding after the ring closes and the SMC heads reengage(Hirano and Hirano 2006). This model is similar to that described for eukaryotic cohesion complexes, which are very similar in structure to condensins (Haering et al. 2008). However, to date, there is no definitive model for eukaryotic Condensin complexes with DNA. Condensin subunits are phosphorylated in early mitosis, and it is the phosphorylated mitotic form of each holocomplex which promotes the compaction of DNA (Kimura et al. 1998; Strick et al. 2004).
In mammalian cells, Condensin II localizes to the nucleus throughout the cell cycle, whereas Condensin I only enters the nucleus after Nuclear Envelope Breakdown (NEB) and is mostly unassociated with the DNA after anaphase (Hirota et al. 2004; Gerlich et al. 2006). Condensins are important for complete removal of cohesins and subsequent separation of sister chromatids during mitosis (Steffensen et al. 2001; Coelho et al. 2003; Dej et al. 2004; Yu and Koshland 2005; Lam et al. 2006). In vivo studies using mutants, or RNAi-mediated depletion, of Condensin I and II subunits show that chomosomes still undergo condensation, albeit abnormally, in the absence each type of Condensin complex. These studies suggest that Condensins may be more essential for chromosome rigidity than for condensation per se (Steffensen et al. 2001; Dej et al. 2004; Hirota et al. 2004; Gerlich et al. 2006).
pRb-family members physically associate with the Condensin II subunit CAP-D3 in both human cells and in Drosophila, but not with CAP-D2/dCAP-D2, the equivalent component of the Condensin I complex(Longworth et al. 2008). pRb/RBF1 proteins also associate with SMC4, suggesting they can interact with the entire Condensin II holocomplex. RBF1 and dCAP-D3 show a striking pattern of colocalization on polytene chromosomes. The association of dCAP-D3 with chromatin is reduced in rbf1 mutants, and the re-expression of pRb in Saos2 tumor cells promotes the association of CAP-D3 with DNA, indicating that RBF1/pRb help to maintain normal levels of CAP-D3/dCAP-D3 complexes on chromatin. Genetic studies show that mutant alleles of dCAP-D3 suppress the ability of ectopic RBF1 to reduce cell proliferation and to induce hypercondensed chromosomes (Longworth et al. 2008). dCAP-D3 mutant larvae possess partially hypocondensed chromosomes and mitotic defects that are very similar to the defects of rbf1 mutants, raising the possibility that the rbf1 mutant phenotype may be due, at least in part to reduced dCAP-D3 activity. All three of the mammalian pRb-family members are able to interact with CAP-D3 suggesting that functional inactivation of all three proteins may be necessary to generate condensation defects that are similar to rbf1 mutants.
These physical and genetic interactions reveal an important connection between pRb-family members and Condensin II complexes but the purpose of this link is unclear. It is possible that this interaction reflects a previously unappreciated role for pRb-family members in chromatin condensation during Mitosis. Alternatively, Condensin II complexes may have roles that extend beyond M-phase.
CAP-D3 proteins contain HEAT repeats (Neuwald and Hirano 2000), motifs that are present in several chromatin associated proteins that act as molecular scaffolds (eg the budding yeast SWI2/SNF2 family member, Mot1p (Neuwald and Hirano 2000). It is likely therefore that the RBF1/dCAP-D3 interaction is part of a larger structure. Given that pRb-family members are important regulators of gene expression and Condensin complexes are important for chromosome structure, one appealing possibility is that Condensin II components may help to organize chromosomal domains containing pRb-regulated genes. Indeed, Condensin subunits have been shown to affect transcription in multiple organisms (Dej et al. 2004; Cobbe et al. 2006; Xu et al. 2006; Gosling et al. 2007) and recent studies in Drosophila show that dCAP-D3 is required for transvection, a process in which the structure of an allele on one chromosome can affect the expression of another allele (Hartl et al. 2008). Recent studies in budding yeast show that Condensin I complexes associate preferentially with intergenic regions, but that high concentrations of Condensin I were also found at tRNA genes, pol III genes, and Pol II genes which encoded small nuclear and nucleolar RNAs (D’Ambrosio et al. 2008). Condensin I was also shown to bind the TFIIIC B box and to physically interact with TFIIIC/TFIIIB complexes (D’Ambrosio et al. 2008; Haeusler et al. 2008). Whether these associations are conserved among higher eukaryotes, whether they are Condensin I specific, and whether pRb could direct Condensin II components to similar DNA regions remains to be determined.
pRb, a master-regulator of chromatin structure?
pRb-family members interact with many different proteins, affect the expression of numerous genes, and have been implicated in diverse biological processes. Because of this complexity, identifying themes that run through multiple studies helps to provide a framework for thinking about the overall role played by this important family of proteins.
As highlighted here, one common theme is the idea that pRb-family members recruit chromatin-modifying enzymes to specific places in the genome. In a few specific examples, the recruited activities promote gene expression (Gu et al. 1993; Thomas et al. 2001; Ianari et al. 2009). However, in most studies, pRb-family members mediate transcriptional repression, and this activity is typically associated with a change in chromatin state that makes it less accessible - the closing, compression, compaction, or condensation of chromatin. Many of the properties of Rb-mutant cells may simply reflect a failure to properly compress regions of chromatin.
Why should proteins that are important regulators of cell proliferation possess this activity? One could argue that pRb-family members simply provide a mechanism to prevent the expression of proliferation genes. However, there are many ways to suppress transcription and it is striking that pRb-family members do not recruit a single type of chromatin modulating activity; instead this family of proteins provides a bridge to many different activities that are important in different cellular contexts or different parts of the genome. This versatility may be a clue as to why pRb family members are such key suppressors of cell proliferation – the inactivation of pRb-family proteins does not remove one brake, but several different brakes.
The global changes in gene expression that accompany the transition from proliferation to cell differentiation in normal cells require an extensive re-organization of chromatin domains and the proteins that co-ordinate this process are largely mysterious. As master-regulators of proliferation/differentiation, pRb-family members need to act locally at individual targets but their activities also need to be integrated with larger changes in nuclear organization. Characterization of the altered chromatin states and chromosomal abnormalities in cells lacking Rb-family members provides insights into the regulation of individual promoters. Perhaps even more importantly, these studies also provide insight into the processes that control the more general re-organization of chromatin that occurs during the transitions between cell proliferation, differentiation and senescence.
Acknowledgments
We thank members of the Dyson laboratory for helpful discussions. ML was supported by fellowships from the Leukemia and Lymphoma Society and the Charles A. King Trust. This work was supported by a grant from the NIH to ND (CA064402).
References
- Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr Opin Genet Dev. 2003;13:170–8. doi: 10.1016/s0959-437x(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bednar J, Horowitz RA, Dubochet J, Woodcock CL. Chromatin conformation and salt-induced compaction: three-dimensional structural information from cryoelectron microscopy. J Cell Biol. 1995;131:1365–76. doi: 10.1083/jcb.131.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG., Jr Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18:623–35. doi: 10.1016/j.molcel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee RN, Banks GC, Trotter KW, Lee HL, Archer TK. Histone H1 Phosphorylation by Cdk2 Selectively Modulates Mouse Mammary Tumor Virus Transcription through Chromatin Remodeling. Mol Cell Biol. 2001;21:5417–5425. doi: 10.1128/MCB.21.16.5417-5425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieda M, Xu X, Singer MA, Green R, Farnham PJ. Unbiased location analysis of E2F1-binding sites suggests a widespread role for E2F1 in the human genome. Genome Res. 2006;16:595–605. doi: 10.1101/gr.4887606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuni P, MacGrogan D, Scandura JM, Nimer SD. The human L(3)MBT polycomb group protein is a transcriptional repressor and interacts physically and functionally with TEL (ETV6) J Biol Chem. 2003;278:15412–20. doi: 10.1074/jbc.M300592200. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–17. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998a;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Bustin M. Regulation of DNA-Dependent Activities by the Functional Motifs of the High-Mobility-Group Chromosomal Proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam HP, Chen ES, Grewal SI. Transcriptional scaffolds for heterochromatin assembly. Cell. 2009;136:610–4. doi: 10.1016/j.cell.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, Bustin M. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol Cell Biol. 2004;24:4321–8. doi: 10.1128/MCB.24.10.4321-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbe N, Savvidou E, Heck MM. Diverse mitotic and interphase functions of condensins in Drosophila. Genetics. 2006;172:991–1008. doi: 10.1534/genetics.105.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho PA, Queiroz-Machado J, Sunkel CE. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J Cell Sci. 2003;116:4763–76. doi: 10.1242/jcs.00799. [DOI] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio C, Schmidt CK, Katou Y, Kelly G, Itoh T, Shirahige K, Uhlmann F. Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev. 2008;22:2215–27. doi: 10.1101/gad.1675708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–48. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- Dej KJ, Ahn C, Orr-Weaver TL. Mutations in the Drosophila condensin subunit dCAP-G: defining the role of condensin for chromosome condensation in mitosis and gene expression in interphase. Genetics. 2004;168:895–906. doi: 10.1534/genetics.104.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Mizzen CA, Abrams M, Allis CD, Gorovsky MA. Phosphorylation of linker histone H1 regulates gene expression in vivo by mimicking H1 removal. Mol Cell. 1999;4:641–7. doi: 10.1016/s1097-2765(00)80215-4. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Strober BE, Guha S, Khavari PA, Alin K, Luban J, Begemann M, Crabtree GR, Goff SP. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell. 1994;79:119–30. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Eisen JA, Sweder KS, Hanawalt PC. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucl Acids Res. 1995;23:2715–2723. doi: 10.1093/nar/23.14.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham PJ, Slansky JE, Kollmar R. The role of E2F in the mammalian cell cycle. Biochim Biophys Acta. 1993;1155:125–31. doi: 10.1016/0304-419x(93)90001-s. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc Natl Acad Sci U S A. 1998;95:10493–8. doi: 10.1073/pnas.95.18.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington EK, Speck SH, Kaelin WG., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1993;90:6914–8. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173–81. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Huen DS, Stevaux O, Dimova D, Balczarek-Strang K, Elsdon M, Dyson NJ. Functional antagonism between E2F family members. Genes Dev. 2001;15:2146–60. doi: 10.1101/gad.903901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama R, Saito M, Tanobe H, Ishikawa F. Loss of linker histone H1 in cellular senescence. J Cell Biol. 2006;175:869–80. doi: 10.1083/jcb.200604005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin I, Horn PJ, Peterson CL. SWI/SNF chromatin remodeling requires changes in DNA topology. Mol Cell. 2001;7:97–104. doi: 10.1016/s1097-2765(01)00158-7. [DOI] [PubMed] [Google Scholar]
- Georlette D, Ahn S, MacAlpine DM, Cheung E, Lewis PW, Beall EL, Bell SP, Speed T, Manak JR, Botchan MR. Genomic profiling and expression studies reveal both positive and negative activities for the Drosophila MybMuvB/dREAM complex in proliferating cells. Genes & Development. 2007;21:2880–2896. doi: 10.1101/gad.1600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich D, Hirota T, Koch B, Peters JM, Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr Biol. 2006;16:333–44. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Garcia-Cao M, Fraga MF, Schotta G, Peters AH, Cotter SE, Eguia R, Dean DC, Esteller M, Jenuwein T, Blasco MA. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nat Cell Biol. 2005;7:420–8. doi: 10.1038/ncb1235. [DOI] [PubMed] [Google Scholar]
- Gosling KM, Makaroff LE, Theodoratos A, Kim YH, Whittle B, Rui L, Wu H, Hong NA, Kennedy GC, Fritz JA, Yates AL, Goodnow CC, Fahrer AM. A mutation in a chromosome condensin II subunit, kleisin beta, specifically disrupts T cell development. Proc Natl Acad Sci U S A. 2007;104:12445–50. doi: 10.1073/pnas.0704870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–33. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–24. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Gunawardena RW, Fox SR, Siddiqui H, Knudsen ES. SWI/SNF activity is required for the repression of deoxyribonucleotide triphosphate metabolic enzymes via the recruitment of mSin3B. J Biol Chem. 2007;282:20116–23. doi: 10.1074/jbc.M701406200. [DOI] [PubMed] [Google Scholar]
- Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454:297–301. doi: 10.1038/nature07098. [DOI] [PubMed] [Google Scholar]
- Haeusler RA, Pratt-Hyatt M, Good PD, Gipson TA, Engelke DR. Clustering of yeast tRNA genes is mediated by specific association of condensin with tRNA gene transcription complexes. Genes Dev. 2008;22:2204–14. doi: 10.1101/gad.1675908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmer L, Gruss C. Effects of cell cycle dependent histone H1 phosphorylation on chromatin structure and chromatin replication. Nucl Acids Res. 1996;24:1420–1427. doi: 10.1093/nar/24.8.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hartl TA, Smith HF, Bosco G. Chromosome alignment and transvection are antagonized by condensin II. Science. 2008;322:1384–7. doi: 10.1126/science.1164216. [DOI] [PubMed] [Google Scholar]
- Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P, Giovanella BC, Tainsky MA, Bradley A, Donehower LA. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993;8:2457–67. [PubMed] [Google Scholar]
- Havas K, Flaus A, Phelan M, Kingston R, Wade PA, Lilley DM, Owen-Hughes T. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell. 2000;103:1133–42. doi: 10.1016/s0092-8674(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Helin K, Ed H. The retinoblastoma protein as a transcriptional repressor. Trends Cell Biol. 1993;3:43–6. doi: 10.1016/0962-8924(93)90150-y. [DOI] [PubMed] [Google Scholar]
- Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, Lowe SW, Cordon-Cardo C. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- Herrera RE, Chen F, Weinberg RA. Increased histone H1 phosphorylation and relaxed chromatin structure in Rb-deficient fibroblasts. Proc Natl Acad Sci U S A. 1996;93:11510–5. doi: 10.1073/pnas.93.21.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hirano M, Hirano T. Opening closed arms: long-distance activation of SMC ATPase by hinge-DNA interactions. Mol Cell. 2006;21:175–86. doi: 10.1016/j.molcel.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–58. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Hirota T, Gerlich D, Koch B, Ellenberg J, Peters JM. Distinct functions of condensin I and II in mitotic chromosome assembly. J Cell Sci. 2004;117:6435–45. doi: 10.1242/jcs.01604. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Yee JK, Shew JY, Chen PL, Bookstein R, Friedmann T, Lee EY, Lee WH. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988;242:1563–6. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Hudson DF, Ohta S, Freisinger T, Macisaac F, Sennels L, Alves F, Lai F, Kerr A, Rappsilber J, Earnshaw WC. Molecular and genetic analysis of condensin function in vertebrate cells. Mol Biol Cell. 2008;19:3070–9. doi: 10.1091/mbc.E08-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I, Haigis K, Gulino A, Lees JA. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell. 2009;15:184–94. doi: 10.1016/j.ccr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac CE, Francis SM, Martens AL, Julian LM, Seifried LA, Erdmann N, Binne UK, Harrington L, Sicinski P, Berube NG, Dyson NJ, Dick FA. The retinoblastoma protein regulates pericentric heterochromatin. Mol Cell Biol. 2006;26:3659–71. doi: 10.1128/MCB.26.9.3659-3671.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kalakonda N, Fischle W, Boccuni P, Gurvich N, Hoya-Arias R, Zhao X, Miyata Y, MacGrogan D, Zhang J, Sims JK, Rice JC, Nimer SD. Histone H4 lysine 20 monomethylation promotes transcriptional repression by L3MBTL1. Oncogene. 2008;27:4293–4304. doi: 10.1038/onc.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L, Bauer R, Morrison E, Langan T, Fasman G. The structure of chromatin reconstituted with phosphorylated H1. Circular dichroism and thermal denaturation studies. J Biol Chem. 1984;259:8777–8785. [PubMed] [Google Scholar]
- Karnani N, Taylor C, Malhotra A, Dutta A. Pan-S replication patterns and chromosomal domains defined by genome-tiling arrays of ENCODE genomic areas. Genome Res. 2007;17:865–76. doi: 10.1101/gr.5427007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kel AE, Kel-Margoulis OV, Farnham PJ, Bartley SM, Wingender E, Zhang MQ. Computer-assisted identification of cell cycle-related genes: new targets for E2F transcription factors. J Mol Biol. 2001;309:99–120. doi: 10.1006/jmbi.2001.4650. [DOI] [PubMed] [Google Scholar]
- Kimura K, Cuvier O, Hirano T. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J Biol Chem. 2001;276:5417–20. doi: 10.1074/jbc.C000873200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282:487–90. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG., Jr The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–26. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–20. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Lam WW, Peterson EA, Yeung M, Lavoie BD. Condensin is required for chromosome arm cohesion during mitosis. Genes Dev. 2006;20:2973–84. doi: 10.1101/gad.1468806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–65. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- Lewis PW, Beall EL, Fleischer TC, Georlette D, Link AJ, Botchan MR. Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev. 2004;18:2929–40. doi: 10.1101/gad.1255204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–19. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–51. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Herr A, Ji JY, Dyson NJ. RBF1 promotes chromatin condensation through a conserved interaction with the Condensin II protein dCAP-D3. Genes Dev. 2008;22:1011–24. doi: 10.1101/gad.1631508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bigas N, Kisiel TA, Dewaal DC, Holmes KB, Volkert TL, Gupta S, Love J, Murray HL, Young RA, Benevolenskaya EV. Genome-wide analysis of the H3K4 histone demethylase RBP2 reveals a transcriptional program controlling differentiation. Mol Cell. 2008;31:520–30. doi: 10.1016/j.molcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–73. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998a;391:601–5. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- Manak JR, Mitiku N, Lipsick JS. Mutation of the Drosophila homologue of the Myb protooncogene causes genomic instability. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7438–7443. doi: 10.1073/pnas.122231599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak JR, Wen H, Van T, Andrejka L, Lipsick JS. Loss of Drosophila Myb interrupts the progression of chromosome condensation. Nature Cell Biology. 2007;9:581–587. doi: 10.1038/ncb1580. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–14. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Hirano T. HEAT repeats associated with condensins, cohesins, and other complexes involved in chromosome-related functions. Genome Res. 2000;10:1445–52. doi: 10.1101/gr.147400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, O’Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera RE, Kouzarides T. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001a;412:561–5. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- Novitch B, Mulligan G, Jacks T, Lassar A. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn I, Aono N, Hirano M, Hirano T. Reconstitution and subunit geometry of human condensin complexes. Embo J. 2007;26:1024–34. doi: 10.1038/sj.emboj.7601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Fang Y, Spector DL, Hirano T. Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol Biol Cell. 2004;15:3296–308. doi: 10.1091/mbc.E04-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Losada A, Hirano M, Myers MP, Neuwald AF, Hirano T. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109–21. doi: 10.1016/s0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–9. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes & Development. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SM, Gall AL, Zheng N, Pavletich NP. Structure of the Rb C-terminal domain bound to E2F1-DP1: a mechanism for phosphorylation-induced E2F release. Cell. 2005;123:1093–106. doi: 10.1016/j.cell.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Sage J, Mulligan GJ, Attardi LD, Miller A, Chen S, Williams B, Theodorou E, Jacks T. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–50. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato MH, Ura K, Hohmura KI, Tokumasu F, Yoshimura SH, Hanaoka F, Takeyasu K. Atomic force microscopy sees nucleosome positioning and histone H1-induced compaction in reconstituted chromatin. FEBS Lett. 1999;452:267–71. doi: 10.1016/s0014-5793(99)00644-4. [DOI] [PubMed] [Google Scholar]
- Schmitt CA. Senescence, apoptosis and therapy--cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286–95. doi: 10.1038/nrc1044. [DOI] [PubMed] [Google Scholar]
- Schneider J, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science. 1994;264:1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–62. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–69. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Siddiqui H, Fox SR, Gunawardena RW, Knudsen ES. Loss of RB compromises specific heterochromatin modifications and modulates HP1alpha dynamics. J Cell Physiol. 2007;211:131–7. doi: 10.1002/jcp.20913. [DOI] [PubMed] [Google Scholar]
- Singh P, Coe J, Hong W. A role for retinoblastoma protein in potentiating transcriptional activation by the glucocorticoid receptor. Nature. 1995;374:562–565. doi: 10.1038/374562a0. [DOI] [PubMed] [Google Scholar]
- Steffensen S, Coelho PA, Cobbe N, Vass S, Costa M, Hassan B, Prokopenko SN, Bellen H, Heck MM, Sunkel CE. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr Biol. 2001;11:295–307. doi: 10.1016/s0960-9822(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Strick TR, Kawaguchi T, Hirano T. Real-time detection of single-molecule DNA compaction by condensin I. Curr Biol. 2004;14:874–80. doi: 10.1016/j.cub.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Templeton DJ, Park SH, Lanier L, Weinberg RA. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci U S A. 1991;88:3033–7. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–16. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci. 2001;26:167–74. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Trojer P, Li G, Sims RJ, 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, Wang YH, Reinberg D. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell. 2007;129:915–28. doi: 10.1016/j.cell.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Trouche D, Le Chalony C, Muchardt C, Yaniv M, Kouzarides T. RB and hbrm cooperate to repress the activation functions of E2F1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11268–11273. doi: 10.1073/pnas.94.21.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Watanabe S, Tei S, Saitoh N, Kuratsu J, Nakao M. High mobility group protein HMGA1 inhibits retinoblastoma protein-mediated cellular G0 arrest. Cancer Sci. 2007;98:1893–901. doi: 10.1111/j.1349-7006.2007.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel PR, Hsiao KM, Schjerven H, Farnham PJ. E2F-mediated growth regulation requires transcription factor cooperation. J Biol Chem. 1997;272:18367–74. doi: 10.1074/jbc.272.29.18367. [DOI] [PubMed] [Google Scholar]
- van Oevelen C, Wang J, Asp P, Yan Q, Kaelin WG, Jr, Kluger Y, Dynlacht BD. A role for mammalian Sin3 in permanent gene silencing. Mol Cell. 2008;32:359–70. doi: 10.1016/j.molcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaute O, Nicolas E, Vandel L, Trouche D. Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucl Acids Res. 2002;30:475–481. doi: 10.1093/nar/30.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure PJ, van der Kraan I, de Leeuw W, van der Vlag J, Carpenter AE, Belmont AS, van Driel R. In Vivo HP1 Targeting Causes Large-Scale Chromatin Condensation and Enhanced Histone Lysine Methylation. Mol Cell Biol. 2005;25:4552–4564. doi: 10.1128/MCB.25.11.4552-4564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, Part I: Covalent histone modifications. Trends Mol Med. 2007;13:363–72. doi: 10.1016/j.molmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Weintraub SJ, Chow KN, Luo RX, Zhang SH, He S, Dean DC. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–5. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- Wen H, Andrejka L, Ashton J, Karess R, Lipsick JS. Epigenetic regulation of gene expression by Drosophila Myb and E2F2/RBF via the MybMuvB/dREAM complex. Genes & Development. 2008;22:601–614. doi: 10.1101/gad.1626308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Zhao J, Morozova N, Li Y, Avivi Y, Grafi G. Chromatin reorganization accompanying cellular dedifferentiation is associated with modifications of histone H3, redistribution of HP1, and activation of E2F-target genes. Dev Dyn. 2003;228:113–20. doi: 10.1002/dvdy.10348. [DOI] [PubMed] [Google Scholar]
- Xu Y, Leung CG, Lee DC, Kennedy BK, Crispino JD. MTB, the murine homolog of condensin II subunit CAP-G2, represses transcription and promotes erythroid cell differentiation. Leukemia. 2006;20:1261–9. doi: 10.1038/sj.leu.2404252. [DOI] [PubMed] [Google Scholar]
- Yamada T, Fischle W, Sugiyama T, Allis CD, Grewal SI. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell. 2005;20:173–85. doi: 10.1016/j.molcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yu HG, Koshland D. Chromosome morphogenesis: condensin-dependent cohesin removal during meiosis. Cell. 2005;123:397–407. doi: 10.1016/j.cell.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E, Jiang Z, Chung D, Marth JD, Phillips RA, Gallie BL. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 1996;10:3051–64. doi: 10.1101/gad.10.23.3051. [DOI] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]