Abstract
The Dmbt1 gene encodes alternatively spliced glycoproteins that are either membrane associated or secreted epithelial products. Functions proposed for Dmbt1 include it being a tumor-suppressor, having roles in innate immune defense and inflammation, and being a Golgi sorting receptor in the exocrine pancreas. The heavily sulfated membrane glycoprotein Muclin (mucin-like glycoprotein) is a Dmbt1 product that is strongly expressed in organs of the gastrointestinal (GI) system. To explore Muclin’s functions in the GI system the Dmbt1 gene was targeted to produce Muclin-deficient mice. Muclin-deficient mice have normal body weight gain and are fertile. The Muclin-deficient mice did not develop GI tumors, even when crossed with mice lacking the known tumor suppressor p53. When colitis was induced by dextran sulfate sodium, there was not a significant difference in disease severity in Muclin-deficient mice. Also, when acute pancreatitis was induced with supraphysiological caerulein there was no difference in disease severity in the Muclin-deficient mice. Exocrine pancreatic function was impaired as measured by attenuated neuro-hormonal stimulated amylase release from Muclin-deficient acinar cells. Also, by [35S]met/cys pulse-chase analysis, traffic of newly synthesized protein to the stimulus-releasable pool was significantly retarded in Muclin-deficient cells as compared to wild type. Thus, Muclin-deficiency impairs trafficking of regulated proteins to a stimulus-releasable pool in the exocrine pancreas.
INTRODUCTION
Dmbt1 cDNA and its glycoprotein products have been independently discovered in various animal species from human to fish. Each group of investigators has given the gene/protein different names and there are many proposed functions. The different names include Muclin (12); gp-340 (31); salivary agglutinin (SAG) (16; 42); crp-ductin (6); ebnerin (29); hensin (54); vomeroglandin (33); and DMBT1 (37). We coined the name Muclin (mucin-like glycoprotein) which is based on the glycoprotein’s biochemical characteristics (8). Muclin is a 300 kDa glycoprotein with abundant N- and O-linked oligosaccharides that comprise about one half the mass of the mature glycoprotein, and the O-linked sugars are heavily sulfated. We reported on cloning of Muclin cDNA from a mouse pancreas cDNA library (12) at about the same time it was cloned from a mouse intestinal crypt library and called crp-ductin (6).
The Dmbt1 gene exhibits species differences in expression levels in different organs. In mice, Muclin is most strongly expressed in the gastrointestinal system including pancreas [weaker in human pancreas (21)]; gallbladder; salivary glands; crypts throughout the small and large intestines (12); and nasal/olfactory/vomeronasal epithelium (29; 33). It has also been detected weakly in the kidney and liver (53). DMBT1 gene expression in humans is strong in lung [weaker in mouse (12)]; at weak levels in brain (21; 37); strong in salivary glands (16; 29; 42); and at low levels in mammary gland, uterus, testis, and prostate (21). In addition to these tissues, the protein has been detected in human tear fluid (48), sweat glands, hair follicles and epidermis (35), liver (47), alveolar macrophages (21), pancreatic juice (18), bile (28), and airway submucosal gland secretions (49). In general, the gene is expressed most strongly in epithelia and is usually observed on the apical cell surface or in luminal exocrine secretions.
Among the many proposed functions for the products of the gene, those that have been most investigated are as a tumor suppressor (37) and/or a regulator of epithelial functional differentiation (53), as a component of the innate immune defenses (46), and as a Golgi sorting receptor in the regulated secretory pathway (11).
Evidence for a role of the DMBT1 gene in cancer includes reports of chromosomal deletions of the gene (37), decreased expression (2; 36), and increased expression (7; 25) in tumors and cell lines. Also, differentiation of kidney intercalated tubule epithelial cells is affected when they are grown on culture dishes coated with the purified DMBT1 gene product called hensin (52). Hensin causes a switch in the apical/basolateral polarity of the acid/base transporters these cells express (53). It has been suggested that hensin’s ability to affect epithelial cell differentiation may underlie its role in cancer (57).
A role for the DMBT1 gene products in innate defenses is supported by various studies. The gp-340 glycoprotein, expressed in salivary glands, airways, and genital tract, binds various bacterial pathogens and viruses (42; 50; 60; 62). Gp-340 also binds to IgA (30) and the collectin surfactant protein D (32), both of which are known components of the innate immune defense system. Furthermore, expression of Muclin in the gastrointestinal tract is regulated by bacteria (22; 43; 51) and its expression is increased during infection (39). During sepsis, Dmbt1 gene expression is upregulated in the liver and this relies on the MyD88 intracellular toll-like receptor adaptor system (59). A recent study showed that DMBT1 expression is upregulated in Crohn’s (inflammatory bowel) disease via the NOD2 signaling pathway, and that siRNA inhibition of DMBT1 expression in a cell culture model enhances bacterial invasion (46). Another recent study identified an association of a DMBT1 variant allele with Crohn’s disease, and also correlation of expression levels of DMBT1 with inflammatory bowel disease severity (44). Additionally, the pathogen Escherichia coli O157:H7 expresses a protease (StcE) that selectively degrades gp-340 (19). Degradation of gp-340 enhances the attachment of bacteria to epithelial cells, which indicates the importance of this glycoprotein as an innate defense factor.
A role for Muclin as a Golgi sorting receptor in the regulated secretory pathway was suggested by the finding that Muclin is most strongly expressed in the mouse pancreas, and that this 300 kDa sulfated glycoprotein is localized to the regulated secretory granules (8). In vitro, at the mildly acidic pH that mimics the trans Golgi network (TGN) environment, purified Muclin interacts with zymogen granule secretory proteins, but not constitutively secreted protein (4). The most direct evidence for the role of Muclin in granule formation came from experiments where Muclin was ectopically expressed in the poorly differentiated rat pancreatic exocrine cell line AR42J which lacks the regulated secretory pathway. When Muclin was expressed in AR42J cells they acquired regulated secretion: they developed functional granules whose exocytosis could be stimulated by the secretagogue caerulein (a cholecystokinin analog) (11).
Our goal in this work was to investigate in gastrointestinal organs the various proposed functions for Muclin. We established a Muclin-deficient mouse line by targeting the Dmbt1 gene. We looked for GI tumor development in Muclin-deficient mice, as well as in Muclin-deficient mice crossed with mice lacking the known tumor suppressor p53. We also investigated whether Muclin-deficiency would affect the severity of experimentally-induced models of inflammatory diseases, dextran sulfate sodium-induced colitis and supramaximal caerulein-induced acute pancreatitis. Finally, we studied the regulated secretory pathway in pancreatic acinar cells from Muclin-deficient mice.
MATERIALS AND METHODS
Materials
Anti-Muclin rabbit antibodies were generated as described (8). [35S]met/cys (TranSLabel) and carrier-free [35S]sulfate were from MP Biomedicals (Irvine, CA). Collagenase (CLSPA grade) was obtained from Worthington Biochemical (Freehold, NJ). All other chemicals were from Sigma (St. Louis, MO) or as indicated.
Targeting Strategy
A bacterial artificial chromosome (BAC) was obtained by screening a mouse 129/SvJ BAC library (Genome Systems) with a Muclin-specific probe. The BAC was sequenced using procedures standard for cloned, large insert genomic DNA isolation, random shot-gun cloning, and fluorescent-based DNA sequencing as described previously (3; 45). Subsequent analysis showed that it contains the entire Dmbt1 coding region flanked by 124kb on the 5’ side and 20kb on the 3’ side (GenBank accession AC087063). A HindIII fragment of 10kb that contains 6kb 5’ of the start codon and 4kb 3’ of the start codon, including the first two exons, was cloned. The dual-selection pPNT vector (56) was modified by placing a green fluorescent protein (GFP) reporter cassette 5’ of the neomycin (Neo) selection cassette. Then, a 4kb NotI/BsaWI 3’ fragment of the HindIII clone was place downstream of the Neo cassette; and a 6kb BamHI 5’ fragment was placed between the negative selection marker for Herpes simplex thymidine kinase (Hsv-tk) and the GFP cassette. All but the first 9 bp in the first exon and part of the first intron are replaced in the targeting construct with the GFP and Neo cassettes (Fig.1A). The reporter cassette does not interrupt the gene’s TATA box but replaces the endogenous start codon with that of GFP.
Fig. 1. Targeting strategy and production of Muclin-deficient mice.
(A) Schematic diagram of WT gene in the target region, the targeting construct, and the targeted gene. (B) Southern blots of Muclin WT (+/+), heterozygous (+/−), and knockout (−/−) genomic DNA: digested with XbaI and hybridized with the 5’-outside probe to verify correct targeting; digested with BamHI or EcoRI and hybridized with a GFP probe to demonstrate there was a single integration site and there were no nonspecific transgene insertions.
The construct was used for homologous recombination in 129/SvJ-derived RW-4 embryonic stem (ES) cells and positive/negative selection. ES clones were screened by Southern blot of XbaI-digested genomic DNA using probes 5’ and 3’ of the targeted region. To verify a single integration site and that no ectopic transgenes were inserted, Southern blots of BamHI- and EcoRI-digested genomic DNA were hybridized to a GFP cassette probe. Correctly targeted cells were injected into C57BL/6J blastocysts to produce chimeras, which were then bred to establish the Muclin-deficient mice.
Some experiments were performed with mice on a mixed 129/SvJ, C57BL/6J background. Mice were also backcrossed 8 generations onto the C57BL/6J background. There was no apparent change in the mice comparing mixed and inbred backgrounds. The background strains used for different experiments are as indicated in the appropriate Materials and Methods sections. All mice were kept in a specific pathogen free facility in barrier-top cages. SDS-PAGE and Western blot. Pancreatic and small intestinal tissues were lysed in 10 mM Tris-HCl, pH 7.0 plus protease inhibitors by sonication on ice. Pancreatic zymogen granules were isolated from pancreas on Percoll density gradients as described (4). Proteins were separated by SDS-PAGE. The gels were then Coomassie blue stained and dried for total protein imaging, or exposed to a phosphor storage screen to image radio-labeled proteins (below). Gels were also transferred to PVDF membranes and probed with an rabbit anti-Muclin antiserum (diluted 5,000-fold). The blots were subsequently incubated with goat anti-rabbit alkaline phosphatase and color was developed with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium.
Histology and immunohistochemistry
Tissues were immersion fixed overnight in 4% paraformaldehyde. Paraffin sections were prepared and stained with hematoxylin and eosin by a commercial service (Mass Histology, Worcester, MA). For immunohistochemistry, slides were deparaffinized and rehydrated, followed by incubation with rabbit anti-Muclin antiserum as primary antibody at a dilution of 500-fold. This was followed by processing using an avidin-biotin peroxidase kit according to the supplier’s instructions (Vector Labs, Burlingame, CA, CA). Color was developed using the VIP peroxidase substrate (Vector Labs).
Crossing Muclin-deficient mice with p53 mice
Heterozygous Trp53tm1Tyj mice (15) on the C57BL/6J background were obtained from Jackson Labs (Bar Harbor, ME). Muclin/p53 double heterozygous mice were generated and interbred. Offspring from this breeding were killed at 9 months of age or were sacrificed when apparent abnormal growths (mostly subcutaneous) occurred. Mice were necropsied and special attention was paid to the pancreas and the intestinal tract, both tissues that express high levels of Muclin. Some p53 null mice died spontaneously as has been described for this mouse line.
Dextran sulfate sodium-induced colitis
Colitis was induced in 10 week old wild type WT and Muclin-deficient mice on the C57BL/6J background using oral administration of 4% dextran sulfate sodium (DSS, molecular weight = 36,000 − 50,000; MP Biomedicals) in drinking water for 7 days (34). Daily, mice were weighed and fecal pellets were collected for analysis of blood in the stool (Hemoccult SENSA assay; Fisher Scientific, Chicago, IL). After the 7 day treatment mice were killed, the colon was removed, and the length from cecum to anus was measured. The tissue was prepared for histological analysis and sections encompassing the entire length of the colon were scored by a qualified veterinary pathologist unaware of the sample identities (L.D.McGill, DVM, PhD, Diplomate, ACVP; Mass Histology). The following parameters were used for severity of colitis. Inflammatory cell infiltration: “0”= Normal , “1”= Mild, “2”= Moderate, “3”= Marked; Ulceration: “0”= Normal , “1”= Mild, “2”= Moderate, “3”= Marked; and Crypt damage: “0”= Normal , “1”= loss of base, “2”= loss of middle, “3”= loss of entire crypt.
Caerulein-induced acute pancreatitis
Pancreatitis was induced in WT and Muclin-deficient mice on the mixed strain background by 7 hourly i.p. injections of supraphysiological caerulein (50 µg/kg). Mice were sacrificed with CO2 and by exsanguination. The groups of WT and Muclin-deficient mice were controls (without caerulein treatment), and after caerulein injections, at 7 hr, 12 hr, 24 hr, 3 days, and 7 days after beginning the injections. Trunk blood was collected for measurement of serum amylase which was determined using 4,6-ethyldiene(glucose)7-p-nitrophenyl-glucose-α, D-maltoheptaside (Raichem, San Diego, CA). The pancreas was removed and a portion was taken for determination of tissue water content and myeloperoxidase activity; the remainder was fixed overnight in 4% paraformaldehyde and processed for hematoxylin and eosin histology. Tissue water content was calculated from the blotted wet weight of tissue followed by lyophilization to dryness (72 hr) and recording of the dry weight. The dried pancreas samples were homogenized and extracted with hexadecyltrimethylammonium-bromide followed by determination of myeloperoxidase activity as an estimate of neutrophil infiltration as described (38).
Stimulated amylase release
Pancreata from mice on the mixed background were digested with collagenase followed by mechanical dispersion and purification by 150 µm filtration and centrifugation through 4% bovine serum albumin, as previously described (10). Acini were suspended at about 1 mg protein per ml in Hepes buffered Ringer’s solution supplemented with amino acids, bovine serum albumin, soybean trypsin inhibitor, and glucose. Acini from WT and Muclin-deficient mice were incubated with the indicated concentrations of the cholinergic agonist carbachol (carbamylcholine chloride) or the cholecystokinin analog caerulein for 30 min at 37°C followed by separation of cells and media. Cells were lysed by sonication. Media and cell samples were assayed for amylase activity as described above. Release data are expressed relative to the initial cell content of amylase and are corrected by subtracting amylase activity in the media at the beginning of the incubation period.
Metabolic radio-labeling and pulse-chase analysis of the secretory pathway
Acini from WT and Muclin-deficient mice on the mixed strain or C57BL/6J backgrounds were prepared for radio-labeling. Sulfated proteins in acini were metabolically labeled by incubation for 1 hr at 37°C in medium containing 0.1 mCi/ml carrier free [35S]sulfate followed by SDS-PAGE. For pulse-chase analysis with [35S]met/cys, acini were prepared in methionine and cysteine deficient buffer to deplete cellular pools of these amino acids. Cells were then pulse-labeled by incubation in 0.5 mCi/ml [35S]met/cys for 30 min. The cells were washed in Hepes buffered Ringer’s solution containing 3x unlabeled amino acids (chase medium), resuspended in chase medium at about 1 mg protein/ml, and incubated for the indicated times. Where indicated, cells were stimulated by adding carbachol (1 µM final) and 8-Br-cAMP (1 mM final). Cells were pelleted at 2,000 g × 30 sec and media and cells were saved for analysis. Samples were separated on 7.5% acrylamide SDS-PAGE followed by exposure to a Packard Cyclone phosphor storage screen for imaging and quantification of incorporated radioactivity.
Statistics
Data are presented as means ± SE. Statistical analysis was performed using Systat software (San Jose, CA). When the number of groups was two, a t-test was used; for data with more than two groups an ANOVA with a post-hoc Fisher’s least-significant-difference test was used. Significance was set to p-values of less than 0.05.
RESULTS
Targeting the Dmbt1 gene to produce Muclin-deficient mice
A standard targeting approach was used to generate Muclin knockout mice with a disrupted Dmbt1 gene (see Materials and Methods). The targeting construct and the targeted gene are shown schematically in Fig.1A. Southern blotting with a probe 5’ outside the targeted region shows the expected bands in Muclin WT (+/+), heterozygous (+/−), and knockout (−/−) genomic DNA (Fig.1B). A probe 3’ of the targeted region was also used to confirm the expected targeting (data not shown). A probe to the green fluorescent protein (GFP) cassette, which was inserted into the targeted gene, shows that there is a single integration site and no ectopic insertions elsewhere in the genome (Fig.1B).
Two different targeted ES cell lines were used to generate chimeras and obtain Muclin-deficient mice. Initial characterization showed that both targeted strains were identical and the results presented here are from the D5 line. Muclin-deficient mice were fertile, appeared healthy, and had normal body weight gain as compared to WT mice. A GFP cassette that is part of the targeted allele was intended to serve as a reporter for Muclin gene expression, but it failed to function. There was no expression of GFP protein (no fluorescence in fresh tissues or signal by anti-GFP Western blot of tissue homogenates) in any of the tissues that normally express Muclin (data not shown). In knockout mice Muclin mRNA was undetectable in the pancreas by realtime RT-PCR (using Muclin primers outside of the targeted region, the CT using 1 ng total RNA as template was about 24 cycles in WT samples; the CT in knockout mice was undetectable at 34 cycles, at least a 1000-fold decrease in signal). There was also no detectable signal using GFP primers for RT-PCR (data not shown). Thus, it appears that the targeted allele is either not transcribed or that its message is very unstable. These mice will be made available under the strain name Dmbt1tm1KUMC on the C57BL/6J background when deposited in the Mouse Genome Informatics database (http://www.informatics.jax.org/).
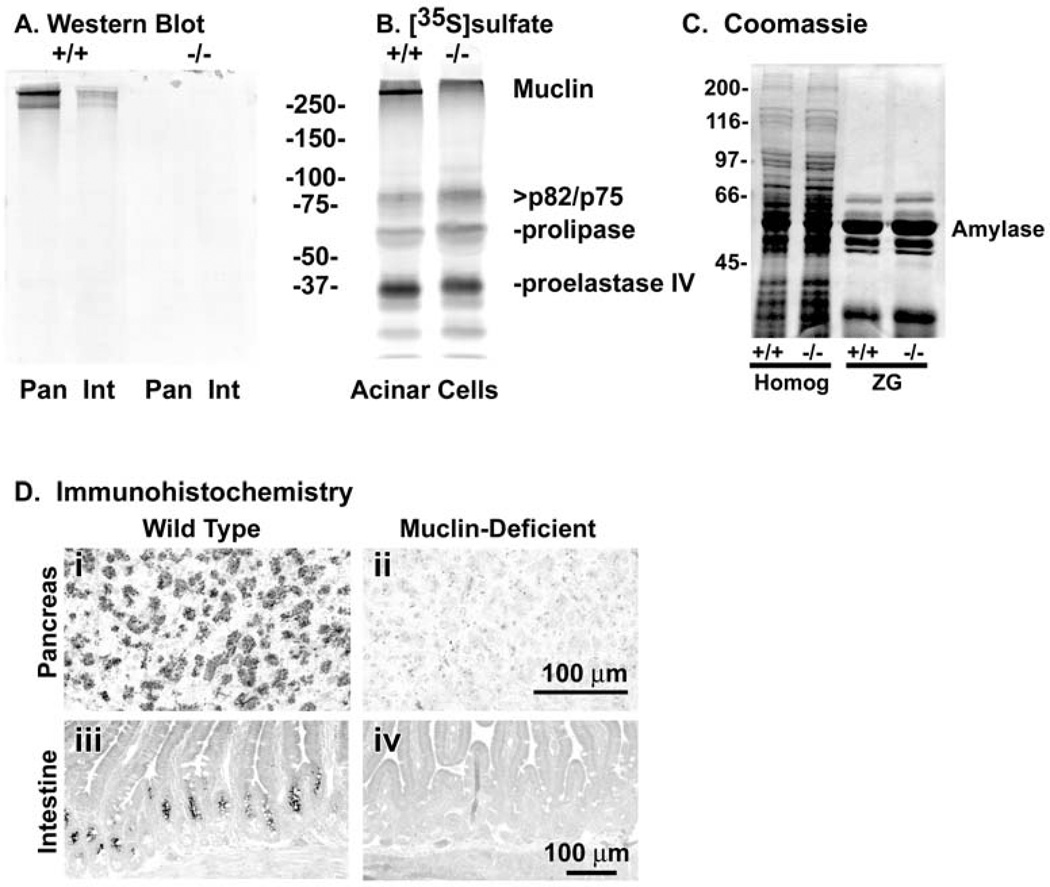
To verify loss of Muclin expression, Western blots and immunohistochemistry were performed on samples from the exocrine pancreas and small intestine where Muclin expression is high in WT mice. There was a strong signal by Western blot in the Muclin (+/+) tissues while there was no detectable Muclin in either pancreas or small intestine from the Muclin (−/−) mice (Fig.2A). In the pancreas, Muclin is a major sulfated protein (sulfation of O-linked oligosaccharides) (9). In acinar cells from Muclin (−/−) mice there was no metabolic incorporation of [35S]sulfate into the high molecular mass band corresponding to Muclin (Fig.2B). The other sulfated proteins of the acinar cell appear to have similar degrees of incorporation in the knockout cells as compared to WT. By Coomassie blue staining of SDS-PAGE gels, the protein composition of the Muclin-deficient mouse pancreas was not noticeably different as compared to WT (Fig.2C). Also, the relative composition of digestive enzymes was the same in zymogen granules (ZG) isolated from the Muclin-deficient pancreas as compared to WT (Fig.2C). There was no difference in the specific activity of amylase, the major digestive enzyme, in pancreatic tissue comparing Muclin-deficient to WT (not shown).
Fig. 2. Analysis of Muclin protein expression in WT (+/+) and Muclin-deficient (−/−) mice.
(A) Western blot for Muclin in pancreas (Pan) and small intestine (Int). There is no signal in samples from the knockout mice. (B) Metabolic [35S]sulfate incorporation into pancreatic acinar cell proteins. The high molecular mass Muclin band shows [35S] incorporation in WT cells but not in the knockout cells. Other sulfated species are unchanged in the knockout. (C) Coomassie blue stained gels showing identical pancreas and isolated zymogen granule protein compositions in WT (+/+) and Muclin-deficient (−/−) mice. Homog = homogenate of total pancreas; ZG = isolated zymogen granules. (D) Muclin immunohistochemistry in WT and Muclin-deficient pancreas and small intestine. (i) In the WT pancreas the zymogen granules are strongly labeled and (ii) there is no labeling in the knockout tissue. (iii) In the WT small intestine labeling is mostly confined to the crypt epithelium above the Paneth cells and (iv) there is no labeling in the knockout tissue.
By immunohistochemistry zymogen granules were strongly labeled in the WT pancreas whereas there was no specific labeling in the Muclin-deficient tissue (Fig.2D i and ii, respectively). In the small intestine in WT tissue labeling was primarily in the intestinal crypts above the Paneth cells while there was no labeling in the Muclin-deficient intestine (Fig.2D iii and iv, respectively).
Muclin-deficient mice do not develop GI tumors
Since Dmbt1 has been suggested to be a tumor suppressor, tumor formation was looked for, paying special attention to the pancreas and intestinal tract. No tumors were observed in Muclin-deficient mice as old as one year. To further test this idea we used mice deficient in p53, a known tumor suppressor gene, which when crossed with other cancer-related genes can enhance tumor formation in specific organs such as the pancreas (58). Offspring of Muclin/p53 double heterozygous mice were sacrificed at 9 months of age or when an obvious abnormal growth occurred. As is common with p53 null mice, some mice died spontaneously before the end point. The majority (82%) of Muclin (+/+)/p53 (−/−) mice died early or were sacrificed due to development of obvious growths (Table). A similar number (86%) of Muclin (−/−)/p53 (−/−) mice also died early or were sacrificed because of an obvious tumor mass (Table). None of the Muclin knockout mice exhibited any tumors in the pancreas or intestinal tract, regardless of their p53 status.
Table 1.
Table Effect of Muclin-deficiency on tumor development alone and in combination with p53-deficiency
| Genotype (Muclin/p53) |
Normal | Died/Abnormal Mass |
|---|---|---|
| WT/WT | 100% | 0% |
| Muclin-deficient/WT | 100% | 0% |
| WT/p53-deficient | 18% | 82% |
| Muclin-deficient/p53-deficient | 14% | 86% |
Double heterozygous mice were interbred and 25 litters (173 offspring) were born. The distribution of possible genotypes was not significantly different from that expected by Mendelian genetics (χ-square p-value = 0.81). Some mice died spontaneously and the remainder were sacrificed at 9 months of age or when there was an obvious tumor (abnormal mass). None of the mice had pancreatic or intestinal tumors or other morphological abnormalities in these organs. There were no statistical differences between Muclin-deficient/p53-deficient and WT/p53-deficient with respect to death and sacrifice due to an abnormal mass (χ-square p-value = 0.92).
Muclin-deficient mice have a similar severity of dextran sulfate sodium-induced colitis
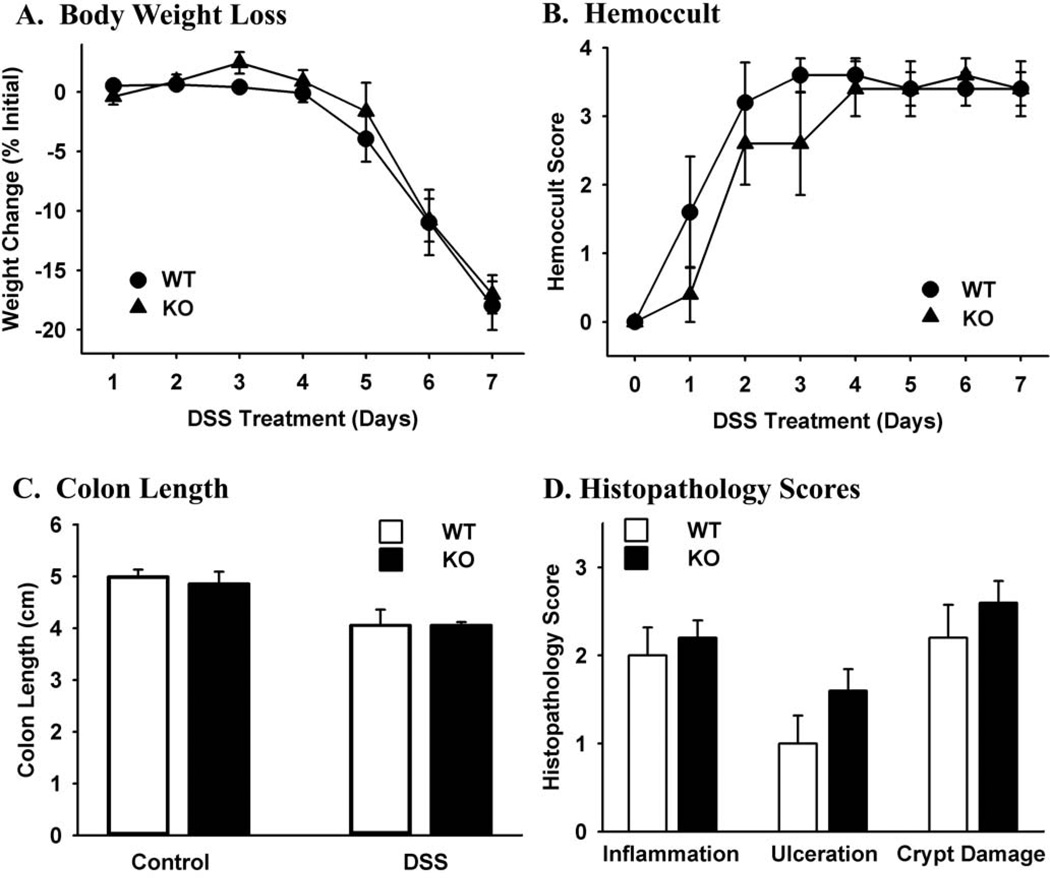
DMBT1 has been reported to be regulated by NOD2 signaling component of the innate defense system, and it is upregulated in inflamed tissue in Crohn’s patients (46). Knockout of other innate defense genes has been shown to either increase (17) or decrease (34) the severity of experimental colitis. Since the DMBT1 product gp-340 is involved in innate defenses, we explored whether Muclin-deficiency would affect DSS-induced colitis. Loss of body weight began at 5 days of DSS treatment and it was the same in WT and Muclin-deficient mice (Fig.3A). Blood in the stool was evident at 1–2 days of DSS treatment and increased until all animals were maximally positive by day 3–4 (Fig.3B). There was not a significant difference in blood in the stool comparing DSS-treated Muclin-deficient to WT mice. The length of the colon from cecum to anus was significantly decreased after 7 day DSS treatment compared to control mice, and the decrease was the same in Muclin-deficient mice as in WT (Fig.3C). In untreated mice, there were no observable histological differences comparing WT and Muclin-deficient colons, and all control tissues were scored as normal for all histopathology parameters (not shown). After 7 days DSS treatment, histopathology scores (inflammation, ulceration, crypt damage) were increased in mice of both genotypes but the differences were not statistically significant comparing Muclin-deficient to WT (Fig.3D).
Fig. 3. Dextran sulfate sodium-induced colitis in WT and Muclin-deficient mice.
(A) Body weight loss during DSS administration. Mice began to lose body weight by 5 days of treatment, and there were no significant differences comparing Muclin-deficient (KO) to WT mice. (B) Hemoccult scores during DSS administration. There were no significant differences comparing Muclin-deficient to WT mice. (C) Colon length in controls and after 7 days DSS administration. There were no significant differences in controls comparing Muclin-deficient to WT mice. After 7 days DSS administration both WT and Muclin-deficient mice had significantly shorter colons as compared to untreated mice (p=0.0002) and there was not a significant difference comparing Muclin-deficient to WT mice. (D) Histopathology scores of WT and Muclin-deficient colon after 7 days DSS treatment. All mice showed pathological changes but there were no significant differences comparing DSS-treated Muclin-deficient and WT mice. Data are means ± SE from 5 WT and 5 Muclin-deficient mice.
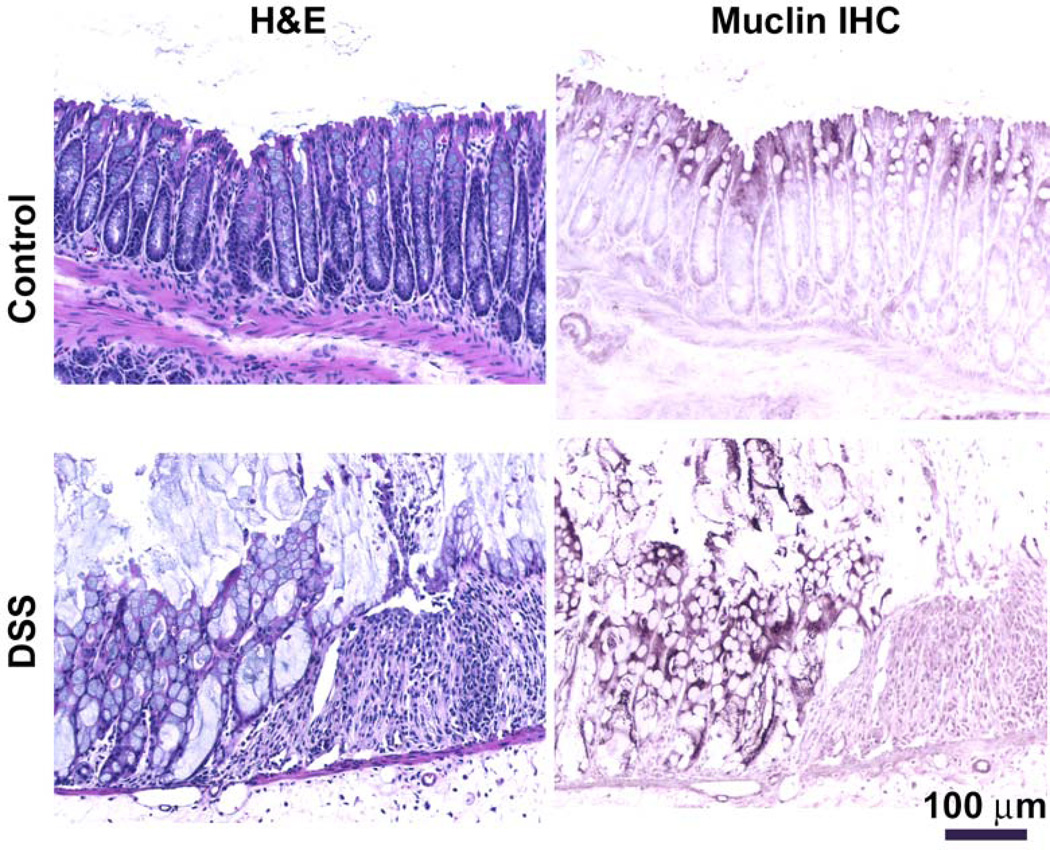
By Muclin immunohistochemistry, the colon from control WT mice exhibits expression in the epithelial cells near the intestinal lumen (Fig.4B) and there is a normal histological appearance (Fig.4A). After DSS administration for 7 days, the colonic epithelium is focally damaged, ulcers occur, and there are large inflammatory infiltrates (Fig.4C). In areas where the epithelium is still present, the intensity of Muclin immunoreactivity is noticeably greater in tissue from DSS-treated mice (Fig.4D) as compared to control tissue (Fig.4B). Also, the immunolabeling goes deeper into the epithelium and cells near the base of the crypts are now immunoreactive (Fig.4D). The histological appearance in Muclin-deficient mice after DSS (not shown) was not distinguishable from that of DSS-treated WT mice, as evidenced by the similar histopathology scores (Fig.3D).
Fig. 4. Histology and Muclin immunohistochemistry in dextran sulfate sodium-induced colitis in WT mice.
(A and C) Routine hematoxylin and eosin (H&E) histology. (B and D) Immunohistochemistry for Muclin (Muclin IHC). The control colon shows (A) normal histology and (B) Muclin immunoreactivity is mostly in the surface epithelium with little labeling deeper in the crypts. After 7 days DSS treatment, (C) the epithelium is focally destroyed and there are inflammatory infiltrates; (D) Muclin immunoreactivity is more intense and cells deep in the crypts now express immunoreactive Muclin. Representative images from 5 each Control and DSS-treated WT mice.
Caerulein-induced pancreatitis is similar in Muclin-deficient and WT mice
Since the highest site of Muclin expression is in the secretory pathway in the exocrine pancreas, the rest of our experiments focused on pancreatic function. We have previously shown that experimental pancreatitis disrupts posttranslational processing of glycoproteins such as Muclin, and we suggested that these incompletely processed glycoproteins might contribute to the cellular events in pancreatitis (10). We tested whether Muclin serves a protective role in the pancreas and if its absence would result in a more severe acute pancreatitis. The widely-used supraphysiological caerulein stimulation model was employed.
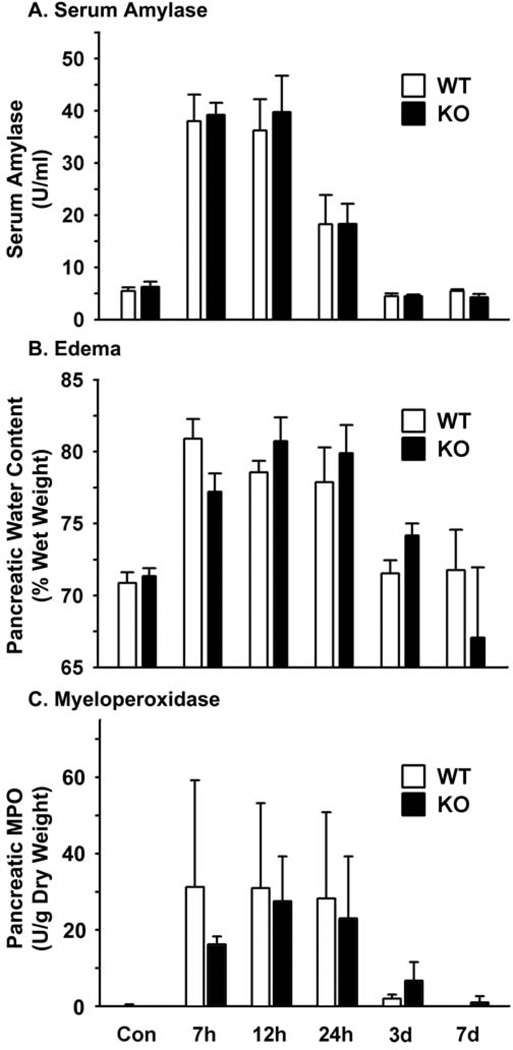
Without induction of pancreatitis, Muclin-deficient mice were the same as WT with respect to low serum amylase activity (Fig.5A), pancreatic water content (a measure of edema; Fig.5B), and unmeasurable pancreatic myeloperoxidase activity (a measure of neutrophil influx during inflammation; Fig.5C). The histological appearance of the pancreas from untreated Muclin-deficient mice was indistinguishable from WT (Fig.6A and B).
Fig. 5. Biochemical analysis of caerulein-induced pancreatitis in Muclin-deficient mice as compared to WT.
Mice were injected 7 times at hourly intervals with 50 µg/kg caerulein i.p. (A) At sacrifice trunk blood was collected for measurement of serum amylase. (B) The pancreas was harvested and wet weight was recorded followed by lyophilization to dryness to determine tissue water content. (C) The tissue was used to measure myeloperoxidase (MPO) activity as a measure of neutrophil infiltration. Data are means ± SE from 4 individual animals per time point and genotype. There were no significant differences in the severity or resolution of pancreatitis comparing Muclin-deficient (KO) to WT mice.
Fig. 6. Histology of caerulein-induced pancreatitis in Muclin-deficient mice as compared to WT.
(A) WT and (B) Muclin-deficient untreated (Control) pancreas. There were no apparent differences comparing Muclin-deficient to WT pancreas. (C) WT and (D) Muclin-deficient pancreas 7 hr after starting supraphysiological caerulein injections. Both genotypes exhibited similar degrees of edema, inflammatory cell infiltrates, and acinar cell apoptosis. (E) WT and (F) Muclin-deficient pancreas 7 days after caerulein treatment. There were no histological differences comparing Muclin-deficient to WT mice. (I) = islet of Langerhans. Representative images from 4 each WT and Muclin-deficient mice for each time point.
Induction of acute pancreatitis with supraphysiological caerulein produced elevations in serum amylase in Muclin-deficient mice similar to WT, and the time course of resolution was also the same and occurred by 3 days (Fig.5A). Pancreatic tissue edema followed the same time course in Muclin-deficient mice as in WT mice (Fig.5B). Similarly, the increase in tissue myeloperoxidase upon induction of pancreatitis and the subsequent resolution of inflammation were the same in Muclin-deficient and WT mice (Fig.5C). Histologically, both WT and Muclin-deficient mice showed similar changes after caerulein injection. There was edema, infiltration of leukocytes, and acinar cell apoptosis at 7 hr after starting the caerulein injections (Fig.6C and D). Recovery from pancreatitis was complete by 7 days and the histological appearance was not noticeably different comparing WT and Muclin-deficient mice (Fig.6E and F).
Protein traffic in the regulated secretory pathway is altered in Muclin-deficient mice
The major function of the pancreatic acinar cell is the synthesis, storage, and neuro-hormonal stimulated release of digestive enzymes (61). To measure if enzyme secretion is affected in the Muclin-deficient pancreas, release was measured from freshly isolated pancreatic acini stimulated with the cholinergic agent carbachol or with the cholecystokinin analog caerulein. Basal (unstimulated) release was not statistically different from Muclin-deficient (KO) as compared to WT cells (Fig.7A). Maximal stimulation of secretion from WT acini was about 5-fold over basal and occurred at 1 µM carbachol (Fig.7A). At higher carbachol concentrations there was reduced release. Muclin-deficient cells exhibited maximal release at 0.3 µM carbachol and there was a small but not statistically significant difference as compared to WT cells (Fig.7A).
Fig. 7. Amylase release from Muclin-deficient and WT pancreatic acini in response to cholinergic (carbachol) or caerulein stimulation.
Acini were isolated and incubated with the indicated concentrations of stimulus for 30 min at 37°C. Amylase released into the media is expressed as percent of the initial cellular content corrected for activity in the media at the start of the release period. (A) Carbachol stimulated amylase release. There were no significant differences in amylase release comparing Muclin-deficient (KO) to WT acini. (B) Caerulein stimulated amylase release. (*) p<0.05 comparing WT to Muclin-deficient (KO) cells. Data are means ± SE from acinar preparations from 5 mice each per genotype.
When caerulein was used as the stimulus, maximal amylase release from WT cells was about 5-fold over basal and occurred at 10 pM caerulein (Fig.7B). In contrast, maximal amylase release from Muclin-deficient cells was at 3 − 10 pM caerulein (Fig.7B). In addition, at 30 − 100 pM caerulein there was about 40% less stimulated release from Muclin-deficient cells as compared to WT, which was statistically significant (Fig.7B).
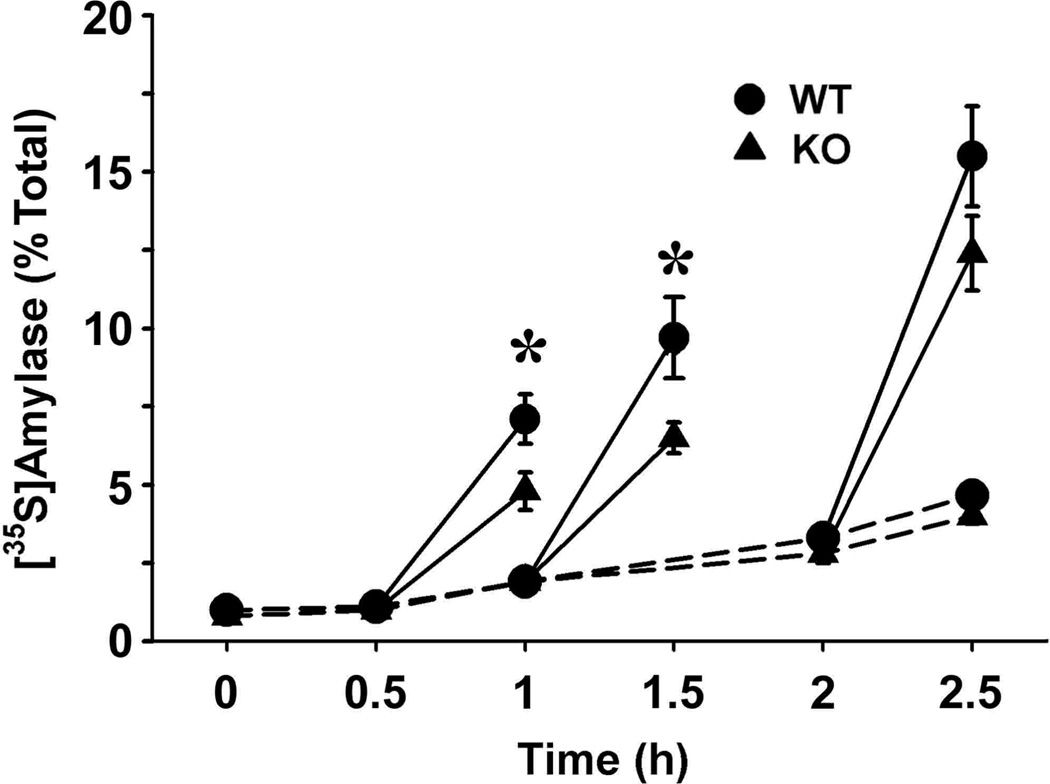
We have previously shown that ectopic expression of Muclin in the rat pancreatic exocrine cell line AR42J induces functional regulated secretory granules (11). Along with the decrease in stimulated amylase release from Muclin-deficient acini, this indicates an important role of Muclin in the formation of zymogen granules. We investigated the secretory pathway in greater detail using a pulse-chase approach. Freshly isolated mouse pancreatic acini were pulse-labeled with [35S]met/cys and then trafficking of the newly synthesized zymogen granule proteins to a stimulus releasable pool was followed. Basal release of newly made amylase was low over a 2.5 hr chase period, and there were no differences between WT and Muclin-deficient (KO) cells (Fig.8B). When the chase period was extended to 4 hr the basal release was 8.70 ± 0.80% of total from WT cells and was 7.50 ± 0.50% from Muclin-deficient cells (not statistically significant). With increasing times of chase, in both WT and Muclin-deficient cells, there was an increase in newly made protein which could be released upon stimulation with the combination of 1 µM carbachol plus 1 mM 8-Br-cAMP (Fig.8B). However, there was significantly less stimulated release of newly synthesized protein from Muclin-deficient (KO) cells as compared to WT cells, stimulated between 0.5−1 hr and between 1−1.5 hr of chase (Fig.8B). From the Muclin-deficient cells, stimulated release was reduced about 40% as compared to WT. This effect was transient, and with stimulation between 2−2.5 hr of chase, release from the Muclin-deficient cells was about 80% that of WT; the difference was not statistically significant (Fig.8).
Fig. 8. [35S]met/cys pulse-chase analysis of protein trafficking through the secretory pathway in WT and Muclin-deficient pancreatic acini.
Pancreatic acini were prepared and pulse-labeled with [35S]met/cys, washed, and then chased for the indicated times. Where indicated, cells were stimulated with 1 µM carbachol and 1 mM 8-Br-cAMP for 0.5 hr. [35S]amylase in the media was quantified from the phosphor storage data and are expressed as percent of labeled amylase in the media relative to the total in the cell pellets at the end of the labeling period. The data are from 6 each WT and Muclin-deficient (KO) acinar preparations. Basal secretion (unstimulated) is indicated by the dashed lines. Stimulated secretion is shown in solid lines. (*) Stimulated secretion between 0.5−1 hr and between 1−1.5 hr of chase was significantly less in Muclin-deficient acini (p=0.029, 0.0031, respectively).
DISCUSSION
In this study we explored the roles of the glycoprotein Muclin in the gastrointestinal system. Muclin is encoded by the Dmbt1 gene on mouse chromosome 7F4 and the human ortholog is on chromosome 10q25.3-q26.1. The Muclin protein is modular and has repeats of SRCR (scavenger receptor cysteine rich) domains with short interspersed Ser/Thr-rich domains that are O-glycosylated; repeated CUB (complement C1r/C1s–sea urchin-EGF-bone morphogenic factor 1) domains; and a single ZP (zona pellucida) domain. There are alternatively spliced transcripts of the gene in different tissues that vary the number of repeated domains and the presence or absence of a membrane spanning domain and a short cytosolic tail. The mouse GI system expresses two alternatively spliced transcripts and the major form includes a C-terminal transmembrane domain and cytosolic tail (6). Although there is an exon for a C-terminal transmembrane domain in the human genomic sequence (http://atlasgeneticsoncology.org/Genes/DMBT1ID309ch10q26.html), the human mRNA transcripts so far discovered do not include this exon.
Two other Dmbt1-targeted mouse lines have been previously reported. One line (hensin knockout) was found to be lethal by embryonic day 4.5 (52). The other line (Dmbt1 knockout) had no spontaneous phenotype (44). It is unclear why the hensin knockout is embryonic lethal while our strain and the other Dmbt1 knockout are healthy. The targeting strategies and background mouse strains used were very similar in all cases. In any case, our targeted mice are Muclin-deficient from at least 2 weeks of age and they are fertile. These mice can serve as a model to explore the functions of the Dmbt1 gene.
The official gene name DMBT1, ‘deleted in malignant brain tumor 1’, is based on the fact that this was the first full-length human transcript reported and the authors suggested that it is a tumor suppressor based on its deletion in a significant number of glioblastomas (37). There is a large body of literature supporting an association of DMBT1 to cancer (2; 5; 23; 26; 35; 37). However, other studies have failed to provide a link between DMBT1 and cancer (20; 40; 41). We did not find evidence that a lack of Muclin caused GI tumors. Even when crossed with mice deficient in the known tumor suppressor gene p53, Muclin-deficient mice did not exhibit any GI tumors. It is possible that on a different genetic background we may have detected an effect of Muclin-deficiency on tumor formation. For example, Dmbt1 was recently identified as a potential mammary tumor suppressor gene in the SuprMam1 locus of mice on a C57BL/6 × BALB/c mixed genetic background (2).
There is strong evidence that the various Dmbt1 products are components of the epithelial innate defenses and that their expression is regulated by inflammation. DMBT1 is dramatically upregulated in inflamed regions of colon from patients with Crohn’s, but not in uninvolved tissue (46). Furthermore, in that studyDMBT1 expression levels were found to be significantly less in patients with the NOD2 L1007fs (SNP13) mutation, which is part of an innate defense pathway that is activated by bacterial infection.
We attempted to demonstrate a role for Muclin in innate defenses or inflammation by exploring the effects of Muclin-deficiency on DSS-induced colitis and caerulein-induced acute pancreatitis. In DSS-induced colitis there was not significant difference in disease severity in the Muclin-deficient colon. However, Muclin protein expression was increased in the inflamed colonic epithelium of WT mice (Fig.4D), consistent with published data on human inflammatory bowel disease (44; 46). Our data differ as compared to those using the Dmbt1 knockout mouse, in which there is a significantly increased severity of DSS-colitis (44). There were some technical differences between our study and that study. Our mice for the colitis experiments were on the C57BL/6J background while theirs were on a C57BL/6, 129/ola mixed background. Also, our targeted allele still includes the Neo selection cassette while they used Cre-recombinase to remove the Neo. For inducing colitis, we used 4% DSS for 7 days, whereas they used 2.5% DSS for 10 days. After DSS treatment, they found a small increase in weight loss and slightly higher histopathology scores in Dmbt1−/− as compared to wild type mice. It appears that Muclin/Dmbt1 may have a protective role in inflammatory bowel disease but loss of this gene does not have a profound effect on the severity of experimental colitis in mice.
In experimentally-induced pancreatitis, posttranslational processing and progression along the secretory pathway of Muclin is impaired (10). We suggested that glycoproteins such as Muclin may have protective roles in the secretory pathway, and that deficient posttranslational processing of glycoproteins could be involved in cellular damage in pancreatitis. The current work shows that Muclin-deficiency does not increase the severity of experimental pancreatitis. Thus, it appears that these changes in glycoproteins are likely a consequence of perturbed acinar cell function during pancreatitis rather than having a causative relationship.
There are two other mouse knockouts of proteins structurally related to Muclin. The first is GP2, a ZP domain containing protein that is membrane associated through a glycosylphosphatidylinositol linkage; Muclin also has a juxtamembrane ZP domain. GP2 knockout mice have no spontaneous phenotype. During caerulein-induced pancreatitis there was a transient increase in the number of apoptotic acinar cells in GP2 knockout cells, but otherwise the severity of pancreatitis was identical to WT mice (63). The other related knockout is of Itmap1 (also known as Cuzd1), a CUB and ZP domain containing protein expressed in the exocrine pancreas. Although encoded by a distinct gene, Itmap1 resembles a truncated version of Muclin: it is comprised of two CUB domains, a single ZP domain, a transmembrane domain, and a short C-terminus cytosolic tail (27). Itmap1 knockout mice do not have a spontaneous phenotype but they do exhibit greater severity of experimentally-induced pancreatitis, especially when the severe choline-deficient, ethionine-supplemented diet model was used (24). It may be that these related proteins have overlapping roles such that loss of a single one does not result in an obvious spontaneous phenotype in the pancreas.
A common theme from studies of the protein products of the DMBT1 gene is that they are able to bind to a variety of biological molecules. Muclin is concentrated in pancreatic zymogen granules where it binds to digestive enzymes stored in the granules. We suggested that Muclin is important in protein sorting and packaging in the regulated secretory pathway of the acinar cell (4; 9). We also showed that when Muclin was expressed in AR42J cells which lack the regulated pathway, the cells developed granules whose secretion could be stimulated by caerulein (11).
In the Muclin-deficient mouse, exocrine cell function is impaired and stimulated protein secretion is less. A closer examination of protein trafficking in the regulated secretory pathway shows that the rate at which newly made secretory proteins are transported to a stimulus-releasable pool is significantly retarded. We have proposed a model for the role of Muclin in the regulated secretory pathway (4; 9; 11). Briefly, Muclin is synthesized on the rough endoplasmic reticulum as a type I membrane protein. It becomes N- and O-glycosylated as it passes through the secretory pathway to the TGN. In the TGN it becomes sulfated on its O-linked oligosaccharides and thus acquires fixed negative charges (8). The luminal environment of the TGN and secretory granules is mildly acidic, and this fosters the aggregation of regulated secretory proteins as well as their binding to the TGN and nascent zymogen granule membrane via Muclin’s sulfates (4; 9). Either at the TGN or in the immature secretory granule, Muclin is proteolytically cleaved releasing an 80 kDa membrane protein containing the ZP domain (called apactin, see below), which is then removed from the maturing granule and is independently targeted to the acinar cell apical plasma membrane (13). Mature Muclin remains in the zymogen granule and in association with the aggregate of regulated secretory proteins. In this way, Muclin acts as a sorting receptor and helps collect regulated proteins to the TGN/nascent zymogen granule membrane and keep them there as the granule matures (14). This process is less efficient in the absence of Muclin and delivery of proteins to the stimulus-releasable pool is slowed.
The 80 kDa membrane protein which is cleaved off Muclin in the TGN/immature granule was named ‘apactin’ because it associates with the apical actin cytoskeleton of the acinar cell through a type I PDZ (postsynaptic density protein 95/Drosophila disks large/zonula occludens 1) binding domain at its cytosolic C-terminus (55). Apactin may function to modulate the reorganization of the actin cytoskeleton at the apical pole of the acinar cell during and after stimulated protein secretion (55). Preliminary studies in Muclin-deficient cells have not shown obvious abnormalities by FITC-phalloidin staining of the acinar cell actin cytoskeleton (data not shown), but this is something that needs closer examination.
In Muclin-deficient acinar cells, traffic of newly made protein to a stimulus-releasable pool is slower, but stimulated release of digestive enzymes still occurs. Some have proposed that Golgi sorting receptors are not needed in the regulated pathway, and that the pH-dependent formation of aggregates and their hydrophobic association with the TGN/secretory granule membrane are sufficient to form nascent secretory granules [for review, see (1; 14)]. The fact that the acinar cell regulated pathway is affected in the absence of Muclin indicates that such receptors are important.
ACKNOWLEDGMENTS
We thank Dr. Wenhao Xu of the University of Kansas School of Medicine Transgenic & Gene-Targeting Institutional Facility for ES cell work and generating the Muclin chimeric mice (Dr. Xu is currently at the University of Virginia). We thank Oxana Norkina for performing Western blots and for assistance with the pancreatitis studies, Eileen Roach for assistance with figure preparation, Racquel Sewell for animal care, Maureen Flynn for immunohistochemistry, and Hui Wu and Ping Hu for advice during the final phases of BAC sequencing, closure, and finishing.
GRANTS
Supported by National Institutes of Health grant R21 DK60769 (RCD), a pilot grant as part of NIH grant P20 RR016475 from the INBRE Program of the National Center for Research Resources (RCD), NIH-NHGRI (BAR), and the Trans-NIH Mouse Initiative (BAR).
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Arvan P, Zhang BY, Feng LJ, Liu M, Kuliawat R. Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr Opin Cell Biol. 2002;14:448–453. doi: 10.1016/s0955-0674(02)00344-7. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn AC, Hill LZ, Roberts AL, Wang J, Aud D, Jung J, Nikolcheva T, Allard J, Peltz G, Otis CN, Cao QJ, Ricketts RS, Naber SP, Mollenhauer J, Poustka A, Malamud D, Jerry DJ. Genetic mapping in mice identifies DMBT1 as a candidate modifier of mammary tumors and breast cancer risk. Am J Pathol. 2007;170:2030–2041. doi: 10.2353/ajpath.2007.060512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodenteich A, Chissoe S, Wang YF, Roe BA. Shotgun Cloning as the Strategy of Choice to Generate Templates for High-throughput Dideoxynucleotide Sequencing. In: Venter JC, editor. Automated DNA Sequencing and Analysis Techniques. London: Academic Press; 1993. pp. 42–50. [Google Scholar]
- 4.Boulatnikov I, De Lisle RC. Binding of the Golgi sorting receptor Muclin to pancreatic zymogens through sulfated O-linked oligosaccharides. J Biol Chem. 2004;279:40918–40926. doi: 10.1074/jbc.M406213200. [DOI] [PubMed] [Google Scholar]
- 5.Braidotti P, Nuciforo PG, Mollenhauer J, Poustka A, Pellegrini C, Moro A, Bulfamante G, Coggi G, Bosari S, Pietra GG. DMBT1 expression is down-regulated in breast cancer. BMC Cancer. 2004;4:46. doi: 10.1186/1471-2407-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng H, Bjerknes M, Chen HY. CRP-ductin: A gene expressed in intestinal crypts and in pancreatic and hepatic ducts. Anat Rec. 1996;244:327–343. doi: 10.1002/(SICI)1097-0185(199603)244:3<327::AID-AR5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Conde AR, Martins AP, Brito M, Manuel A, Ramos S, Malta-Vacas J, Renner M, Poustka A, Mollenhauer J, Monteiro C. DMBT1 is frequently downregulated in well-differentiated gastric carcinoma but more frequently upregulated across various gastric cancer types. Int J Oncol. 2007;30:1441–1446. [PubMed] [Google Scholar]
- 8.De Lisle RC. Characterization of the major sulfated protein of mouse pancreatic acinar cells: a high molecular weight peripheral membrane glycoprotein of zymogen granules. J Cell Biochem. 1994;56:385–396. doi: 10.1002/jcb.240560315. [DOI] [PubMed] [Google Scholar]
- 9.De Lisle RC. Role of sulfated O-linked glycoproteins in zymogen granule formation. J Cell Sci. 2002;115:2941–2952. doi: 10.1242/jcs.115.14.2941. [DOI] [PubMed] [Google Scholar]
- 10.De Lisle RC. Altered posttranslational processing of glycoproteins in cerulein-induced pancreatitis. Exp Cell Res. 2005;308:101–113. doi: 10.1016/j.yexcr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.De Lisle RC, Norkina O, Roach E, Ziemer D. Expression of pro-Muclin in pancreatic AR42J cells induces functional regulated secretory granules. Am J Physiol Cell Physiol. 2005;289:C1169–C1178. doi: 10.1152/ajpcell.00099.2005. [DOI] [PubMed] [Google Scholar]
- 12.De Lisle RC, Petitt M, Huff J, Isom KS, Agbas A. MUCLIN expression in the cystic fibrosis transmembrane conductance regulator knockout mouse. Gastroenterology. 1997;113:521–532. doi: 10.1053/gast.1997.v113.pm9247472. [DOI] [PubMed] [Google Scholar]
- 13.De Lisle RC, Ziemer D. Processing of pro-Muclin and divergent targeting of its products to zymogen granules and the apical plasma membrane of pancreatic acinar cells. Eur J Cell Biol. 2000;79:892–904. doi: 10.1078/0171-9335-00121. [DOI] [PubMed] [Google Scholar]
- 14.Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol. 2007;177:191–196. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 16.Ericson T, Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983;133:255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- 17.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gronborg M, Bunkenborg J, Kristiansen TZ, Jensen ON, Yeo CJ, Hruban RH, Maitra A, Goggins MG, Pandey A. Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res. 2004;3:1042–1055. doi: 10.1021/pr0499085. [DOI] [PubMed] [Google Scholar]
- 19.Grys TE, Siegel MB, Lathem WW, Welch RA. The StcE protease contributes to intimate adherence of enterohemorrhagic Escherichia coli O157:H7 to host cells. Infect Immun. 2005;73:1295–1303. doi: 10.1128/IAI.73.3.1295-1303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermans KG, Van Alewijk DC, Veltman JA, Van Weerden W, Van Kessel AG, Trapman J. Loss of a small region around the PTEN locus is a major chromosome 10 alteration in prostate cancer xenografts and cell lines. Genes Chromosomes Cancer. 2004;39:171–184. doi: 10.1002/gcc.10311. [DOI] [PubMed] [Google Scholar]
- 21.Holmskov U, Mollenhauer J, Madsen J, Vitved L, Gronlund J, Tornoe I, Kliem A, Reid KB, Poustka A, Skjodt K. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci USA. 1999;96:10794–10799. doi: 10.1073/pnas.96.19.10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 23.Imai MA, Moriya T, Imai FL, Shiiba M, Bukawa H, Yokoe H, Uzawa K, Tanzawa H. Down-regulation of DMBT1 gene expression in human oral squamous cell carcinoma 39. Int J Mol Med. 2005;15:585–589. [PubMed] [Google Scholar]
- 24.Imamura T, Asada M, Vogt SK, Rudnick DA, Lowe ME, Muglia LJ. Protection from pancreatitis by the zymogen granule membrane protein integral membrane-associated protein-1. J Biol Chem. 2002;277:50725–50733. doi: 10.1074/jbc.M204159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd LM, Andringa A, Rubio CA, Spicer Z, Shull GE, Miller ML. Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(−/−)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol. 2005;20:1266–1278. doi: 10.1111/j.1440-1746.2005.03867.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Nielsen O, Fenger C, Leslie RG, Holmskov U, Reid KB. Induction of DMBT1 expression by reduced ERK activity during gastric mucosa differentiation-like process and its association with human gastric cancer 38. Carcinogenesis. 2005 doi: 10.1093/carcin/bgi045. [DOI] [PubMed] [Google Scholar]
- 27.Kasik JW. A cDNA cloned from pregnant mouse uterus exhibits temporo-spatial expression and predicts a novel protein. Biochem J. 1998;330(Pt 2):947–950. doi: 10.1042/bj3300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, Goggins MG, Maitra A, Pandey A. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3:715–728. doi: 10.1074/mcp.M400015-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Li X-J, Snyder SH. Molecular cloning of Ebnerin, a von Ebner’s gland protein associated with taste buds. J Biol Chem. 1995;270:17674–17679. doi: 10.1074/jbc.270.30.17674. [DOI] [PubMed] [Google Scholar]
- 30.Ligtenberg AJ, Bikker FJ, Blieck-Hogervorst JM, Veerman EC, Nieuw Amerongen AV. Binding of Salivary Agglutinin (SAG) to IgA. Biochem J Pt. 2004 doi: 10.1042/BJ20040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ligtenberg TJM, Bikker FJ, Groenink J, Tornoe I, Leth-Larsen R, Veerman EC, Amerongen Nieuw AV, Holmskov U. Human salivary agglutinin binds to lung surfactant protein-D and is identical with scavenger receptor protein gp-340. Biochem J. 2001;359:243–248. doi: 10.1042/0264-6021:3590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen J, Tornoe I, Nielsen O, Lausen M, Krebs I, Mollenhauer J, Kollender G, Poustka A, Skjodt K, Holmskov U. CRP-ductin, the mouse homologue of gp-340/deleted in malignant brain tumors 1 (DMBT1), binds gram-positive and gram-negative bacteria and interacts with lung surfactant protein D. Eur J Immunol. 2003;33:2327–2336. doi: 10.1002/eji.200323972. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita F, Miyawaki A, Mikoshiba K. Vomeroglandin/CRP-ductin is strongly expressed in the glands associated with the mouse vomeronasal organ: Identification and characterization of mouse vomeroglandin. Biochem Biophys Res Commun. 2000;268:275–281. doi: 10.1006/bbrc.2000.2104. [DOI] [PubMed] [Google Scholar]
- 34.McVay LD, Keilbaugh SA, Wong TM, Kierstein S, Shin ME, Lehrke M, Lefterova MI, Shifflett DE, Barnes SL, Cominelli F, Cohn SM, Hecht G, Lazar MA, Haczku A, Wu GD. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006 doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollenhauer J, Deichmann M, Helmke B, Mueller H, Kollender G, Holmskov U, Ligtenberg T, Krebs I, Wiemann S, Bantel-Schaal U, Madsen J, Bikker F, Klauck SM, Otto HF, Moldenhauer G, Poustka A. Frequent downregulation of DMBT1 and galectin-3 in epithelial skin cancer. Int J Cancer. 2003;105:149–157. doi: 10.1002/ijc.11072. [DOI] [PubMed] [Google Scholar]
- 36.Mollenhauer J, Helmke B, Muller H, Kollender G, Lyer S, Diedrichs L, Holmskov U, Ligtenberg T, Herbertz S, Krebs I, Wiemann S, Madsen J, Bikker F, Schmitt L, Otto HF, Poustka A. Sequential changes of the DMBT1 expression and location in normal lung tissue and lung carcinomas. Genes Chromosomes Cancer. 2002;35:164–169. doi: 10.1002/gcc.10096. [DOI] [PubMed] [Google Scholar]
- 37.Mollenhauer J, Wiemann S, Scheurlen W, Korn B, Hayashi Y, Wilgenbus KK, von Deimling A, Poustka A. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3−26.1 is deleted in malignant brain tumours. Nature Genet. 1997;17:32–39. doi: 10.1038/ng0997-32. [DOI] [PubMed] [Google Scholar]
- 38.Norkina O, Graf R, Appenzeller P, De Lisle RC. Caerulein-induced acute pancreatitis in mice that constitutively overexpress Reg/PAP genes. BMC Gastroenterol. 2006;6:16. doi: 10.1186/1471-230X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–G1041. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 40.Pang JCS, Dong ZQ, Zhang R, Liu YH, Zhou LF, Chan BW, Poon WS, Ng HK. Mutation analysis of DMBT1 in glioblastoma, medulloblastoma and oligodendroglial tumors. Int J Cancer. 2003;105:76–81. doi: 10.1002/ijc.11019. [DOI] [PubMed] [Google Scholar]
- 41.Petersen S, Rudolf J, Bockmühl U, Deutschmann N, Dietel M, Petersen I. Analysis of the DMBT1 gene in carcinomas of the respiratory tract. Int J Cancer. 2000;88:71–76. doi: 10.1002/1097-0215(20001001)88:1<71::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.Prakobphol A, Xu F, Hoang VM, Larsson T, Bergstrom J, Johansson I, Frangsmyr L, Holmskov U, Leffler H, Nilsson C, Boren T, Wright JR, Stromberg N, Fisher SJ. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem. 2000;275:39860–39866. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 43.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renner M, Bergmann G, Krebs I, End C, Lyer S, Hilberg F, Helmke B, Gassler N, Autschbach F, Bikker F, Strobel-Freidekind O, Gronert-Sum S, Benner A, Blaich S, Wittig R, Hudler M, Ligtenberg AJ, Madsen J, Holmskov U, Annese V, Latiano A, Schirmacher P, Amerongen AV, D’Amato M, Kioschis P, Hafner M, Poustka A, Mollenhauer J. DMBT1 confers mucosal protection in vivo and a deletion variant is associated with Crohn’s disease. Gastroenterology. 2007;133:1499–1509. doi: 10.1053/j.gastro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Roe BA. Shotgun library construction for DNA sequencing. Methods Mol Biol. 2004;255:171–187. doi: 10.1385/1-59259-752-1:171. [DOI] [PubMed] [Google Scholar]
- 46.Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Folsch UR, Helmke B, Autschbach F, Schirmacher P, Kioschis P, Hafner M, Poustka A, Mollenhauer J, Schreiber S. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 2007;178:8203–8211. doi: 10.4049/jimmunol.178.12.8203. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki M, Huang SF, Chen MF, Jan YY, Yeh TS, Ishikawa A, Mollenhauer J, Poustka A, Tsuneyama K, Nimura Y, Oda K, Nakanuma Y. Expression of deleted in malignant brain tumor-1 (DMBT1) molecule in biliary epithelium is augmented in hepatolithiasis: possible participation in lithogenesis. Dig Dis Sci. 2003;48:1234–1240. doi: 10.1023/a:1024186504893. [DOI] [PubMed] [Google Scholar]
- 48.Schulz BL, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochem J. 2002;366:511–520. doi: 10.1042/BJ20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulz BL, Sloane AJ, Robinson LJ, Sebastian LT, Glanville AR, Song Y, Verkman AS, Harry JL, Packer NH, Karlsson NG. Mucin glycosylation changes in cystic fibrosis lung disease are not manifest in submucosal gland secretions. Biochem J. 2005;387:911–919. doi: 10.1042/BJ20041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoddard E, Cannon G, Ni H, Kariko K, Capodici J, Malamud D, Weissman D. gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J Immunol. 2007;179:3126–3132. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 51.Sun FS, Kaur S, Ziemer D, Banerjee S, Samuelson LC, De Lisle RC. Decreased gastric bacterial killing and upregulation of protective genes in the small intestine in the gastrin deficient mouse. Dig Dis Sci. 2003;48:976–985. doi: 10.1023/a:1023068116934. [DOI] [PubMed] [Google Scholar]
- 52.Takito J, Al Awqati Q. Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin 14. J Cell Biol. 2004;166:1093–1102. doi: 10.1083/jcb.200405159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takito J, Hikita C, Al-Awqati Q. Hensin, a new collecting duct protein involved in the in vitro plasticity of intercalated cell polarity. J Clin Invest. 1996;98:2324–2331. doi: 10.1172/JCI119044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takito J, Yan LB, Ma J, Hikita C, Vijayakumar S, Warburton D, Al-Awqati Q. Hensin, the polarity reversal protein, is encoded by DMBT1, a gene frequently deleted in malignant gliomas. Am J Physiol Renal Physiol. 1999;277:F277–F289. doi: 10.1152/ajprenal.1999.277.2.F277. [DOI] [PubMed] [Google Scholar]
- 55.Tandon C, De Lisle RC. Apactin is involved in remodeling of the actin cytoskeleton during regulated exocytosis. Eur J Cell Biol. 2004;83:79–89. doi: 10.1078/0171-9335-00361. [DOI] [PubMed] [Google Scholar]
- 56.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 57.Vijayakumar S, Takito J, Gao X, Schwartz GJ, Al-Awqati Q. Differentiation of columnar epithelia: the hensin pathway. J Cell Sci. 2006;119:4797–4801. doi: 10.1242/jcs.03269. [DOI] [PubMed] [Google Scholar]
- 58.Wagner M, Greten FR, Weber CK, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid RM. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weighardt H, Mages J, Jusek G, Kaiser-Moore S, Lang R, Holzmann B. Organ-specific role of MyD88 for gene regulation during polymicrobial peritonitis. Infect Immun. 2006;74:3618–3632. doi: 10.1128/IAI.01681-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White MR, Crouch E, Van EM, Hartshorn M, Pemberton L, Tornoe I, Holmskov U, Hartshorn KL. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L831–L840. doi: 10.1152/ajplung.00365.2004. [DOI] [PubMed] [Google Scholar]
- 61.Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2006;22:498–504. doi: 10.1097/01.mog.0000239863.96833.c0. [DOI] [PubMed] [Google Scholar]
- 62.Wu Z, Van Ryk D, Davis C, Abrams WR, Chaiken I, Magnani J, Malamud D. Salivary agglutinin inhibits HIV Type 1 infectivity through interaction with viral glycoprotein 120. AIDS Res Hum Retroviruses. 2003;19:201–209. doi: 10.1089/088922203763315704. [DOI] [PubMed] [Google Scholar]
- 63.Yu S, Michie SA, Lowe AW. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. J Biol Chem. 2004;279:50274–50279. doi: 10.1074/jbc.M410599200. [DOI] [PubMed] [Google Scholar]