Abstract
Human alcoholics display dramatic disruptions of circadian rhythms that may contribute to the maintenance of excessive drinking, thus creating a vicious cycle. While clinical studies cannot establish direct causal mechanisms, recent animal experiments have revealed bidirectional interactions between circadian rhythms and ethanol intake, suggesting that the chronobiological disruptions seen in human alcoholics are mediated in part by alterations in circadian pacemaker function. The present study was designed to further explore these interactions using C57BL/6J (B6) and DBA/2J (D2) inbred mice, two widely employed strains differing in both circadian and alcohol-related phenotypes. Mice were maintained in running-wheel cages with or without free-choice access to ethanol and exposed to a variety of lighting regimens, including standard light–dark cycles, constant darkness, constant light, and a “shift-lag” schedule consisting of repeated light–dark phase shifts. Relative to the standard light–dark cycle, B6 mice showed reduced ethanol intake in both constant darkness and constant light, while D2 mice showed reduced ethanol intake only in constant darkness. In contrast, shift-lag lighting failed to affect ethanol intake in either strain. Access to ethanol altered daily activity patterns in both B6 and D2 mice, and increased activity levels in D2 mice, but had no effects on other circadian parameters. Thus, the overall pattern of results was broadly similar in both strains, and consistent with previous observations that chronic ethanol intake alters circadian activity patterns while environmental perturbation of circadian rhythms modulates voluntary ethanol intake. These results suggest that circadian-based interventions may prove useful in the management of alcohol use disorders.
Keywords: Circadian rhythm, Ethanol intake, Inbred mice, Environmental lighting
1. Introduction
Several lines of evidence have revealed reciprocal interactions between the circadian timing system and alcohol intake at both the physiologic and molecular-genetic levels [1–4]. Thus, human alcoholics display dramatic disruptions of sleep–wake cycles and other circadian rhythms, including hormone secretion, body temperature and motor activity [4–6], while in turn, these disruptions may contribute to the maintenance of excessive drinking, thus creating a vicious cycle [7–9]. Further, polymorphisms in circadian clock genes are associated with alcohol and drug abuse [3,10–12]. Despite this evidence, human studies are unable to identify the causal neurobiological mechanisms underlying the chronobiological effects of ethanol, and it remains unclear to what extent these effects are mediated by alterations in circadian clock function, as opposed to disruption of effector systems located “downstream” from the circadian pacemaker [1].
It is therefore significant that animal experiments have revealed direct pharmacological effects of ethanol on underlying circadian pacemaker function. Thus, acute and chronic ethanol exposures modify free-running circadian period and responsiveness to both photic and non-photic phase shifting stimuli [13–21], variables that reflect fundamental parameters of the circadian pacemaker. These effects are mediated in part by the effects of ethanol on neural activity [19–21] and gene expression [22–24] within the suprachiasmatic nucleus (SCN), site of the “master” circadian pacemaker. Conversely, circadian disruption evoked by environmental perturbation [25–27] or clock gene mutation [11,28,29] alters voluntary ethanol intake. Finally, selective breeding for ethanol preference alters circadian phenotype [30,31], further implicating close genetic linkages between these two functions.
In the present study, we utilized two widely-used inbred mouse strains, characterized by divergent ethanol-related and circadian phenotypes. Thus, C57BL/6 (B6) mice display high levels of ethanol preference as well as relative insensitivity to ethanol withdrawal, while DBA/2 (D2) mice display very low ethanol preference and high withdrawal sensitivity [32,33]. With regard to circadian rhythms, B6 mice display consistently longer free-running periods in constant darkness than D2 mice [34–36]. On the other hand, studies of daily activity patterns and overall activity levels in these strains have been much less consistent [37–40].
Despite the ready availability of numerous inbred mouse strains, previous chronobiological studies of ethanol in mice have all used the B6 strain [16,20,21,41]. Thus, we expected that comparison of B6 and D2 mice could reveal potential strain differences in these effects. Animals were maintained under a variety of lighting regimens to evaluate the effects of environmental manipulation of the circadian pacemaker on voluntary ethanol intake. In addition, we evaluated the effects of long-term ethanol drinking on circadian activity patterns by comparing ethanol-exposed and non-exposed groups of animals.
2. Methods
2.1. Subjects and apparatus
Male C57BL/6J (B6) and DBA/2J (D2) mice (N=30 per strain) were obtained from the Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age. Upon arrival in the laboratory, mice were placed individually in commercial running-wheel cages (Coulbourn Instruments, Whitehall, PA; model ACT 551). Cages were made of polycarbonate, with approximate dimensions of 33×18×14 cm, L×W×H; the circumference of the running wheel was 36 cm. In turn, the running-wheel cages were housed within sound-attenuated and light-controlled cabinets. Wood shavings were used as bedding material, and bedding was replaced weekly. Wheel turns were monitored continuously via cam-activated microswitches mounted outside the cage and data were logged and analyzed using the ClockLab interface system (Coulbourn Instruments, Whitehall, PA).
2.2. Procedures
All animals were initially maintained for 3 weeks under a standard light–dark cycle (LD 12:12) with food and water freely available. Following this 3 week acclimation period, half of the animals (N=15 per strain) were provided with both 10% v/v ethanol solution and plain water via separate drinking bottles throughout the remainder of the experiment, while the other animals were continued on water only. Water and ethanol intakes were monitored and bottle positions reversed on a weekly basis for animals in the ethanol groups, while fluid measurements were not taken for the water-only groups. These measurements were used to derive weekly values for 10% ethanol intake, water intake, and ethanol preference (i.e., the volume of 10% ethanol divided by total fluid consumption). Since animals were not repeatedly weighed throughout the course of the experiment, ethanol consumption is reported in volumetric units rather than as grams per kilogram.
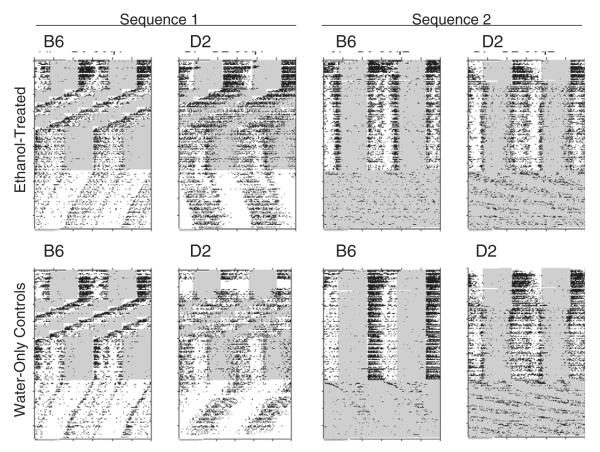
Approximately half (7–8) of the ethanol-exposed and water-only animals of each strain were exposed to each of two different sequences of experimental conditions, as follows: Sequence 1: LD 12:12 (5 weeks), chronic shift-lag (described below; 9 weeks), LD 12:12 (4 weeks), constant darkness (DD; 9 weeks); Sequence 2: LD 12:12 (18 weeks); constant light (LL; 9 weeks). Thus, while Sequence 1 animals were exposed to the shift-lag regimen, Sequence 2 animals were maintained continuously under the original LD 12:12 cycle, and while Sequence 1 animals were exposed to DD, Sequence 2 animals were exposed to LL. The shift-lag procedure was designed to mimic the changes in environmental lighting that would accompany repeated episodes of eastward travel, and consisted of a total of 8 6-hour phase advances of the LD cycle, repeated at weekly intervals. These sequences and conditions can be visualized by inspection of the circadian activity records shown in Fig. 1.
Fig. 1.
Double-plotted (48-hour span) raster-style circadian activity records for one representative animal from each strain (B6: C57BL/6J; D2: DBA/2J) and treatment group (Ethanol-treated vs. Water-only controls; Sequence 1 vs. Sequence 2). See text for details on experimental sequences. Shaded areas indicate times of light exposure.
2.3. Data analyses
2.3.1. Effects of lighting conditions on ethanol and water intake
The effects of shift-lag on fluid intake parameters were first evaluated using a 2 (strains) by 2 (treatments: LD vs. shift-lag) between-groups ANOVA; this analysis compared intake under the shift-lag schedule to that seen in parallel controls kept under a normal LD cycle. As a secondary analysis, we also used a strain by treatment repeated measures ANOVA to compare intake under shift-lag to that seen in the same animals under the immediately preceding LD conditions.
The effects of LL and DD on intake parameters were evaluated using a 2 (strains) by 2 (sequences) by 2 (successive treatments: LD vs. LL/DD) repeated-measures ANOVA, which allowed for both between- and within-groups evaluation of the effects of lighting conditions on fluid intake.
2.3.2. Effects of ethanol drinking on circadian activity rhythms
Several aspects of circadian activity patterns were evaluated in both ethanol-exposed and water-only control mice. These include the shape of the circadian activity waveform under LD conditions, the efficacy of adaptation to the shift-lag schedule, free-running circadian periods in DD and LL, spectral peak magnitude in LD, DD, and LL, and total daily activity level in LD, DD and LL. Analyses of circadian waveforms and daily activity levels were based on the last 3 weeks of LD immediately prior to DD and LL conditions, and included only animals that did not undergo the shift-lag protocol, in order to ensure that these measures reflected steady-state behavior patterns. Efficacy of entrainment under the shift-lag regimen was evaluated by estimating the overall circadian period and spectral peak magnitude for this 9-week treatment, and comparing the expressed period to the 23.14 hour mean period of the shift-lag schedule (i.e., the weekly 6-hour phase advance shortens the period of the otherwise 24-hour LD cycle by approximately 0.86 h per day). Circadian periods and spectral peak magnitudes under both shift-lag and free-running conditions were determined using the Lomb–Scargle periodogram routine included in the ClockLab analysis package, and statistical significance of these parameters was evaluated by 2 (strains) by 2 (alcohol vs. water-only) ANOVA.
2.4. Ethics
These experiments were approved by the University of Maine Institutional Animal Care and Use Committee, and were conducted under the NIH Guide for the Care and Use of Laboratory Animals.
3. Results
3.1. Qualitative features of activity patterns
Fig. 1 shows typical running-wheel activity records for a single representative animal from each of the eight treatment groups. When maintained under LD conditions, B6 mice showed generally well-integrated nocturnal activity patterns, with activity onset occurring at or immediately following dark onset. Several B6 mice showed moderate to high levels of activity extending into the early hours of the light phase; this “dawn” activity typically emerged only after 20–50 days of running-wheel access, and was unrelated to the ethanol treatment since similar patterns were seen in both ethanol-treated and control mice. Under the shift-lag protocol, B6 mice continued to display robust nocturnal activity patterns, and typically appeared to show complete or near-complete phase adjustment prior to each successive LD shift. In DD, B6 mice displayed clear free-running rhythms, with periods consistently shorter than 24 h, but in LL, B6 mice showed little or no evidence for coherent free-running rhythms.
Activity patterns in D2 mice differed consistently in several features from those observed in B6. Thus, in addition to their nocturnal activity, most D2 mice exhibited moderate to high levels of both late-light and early-light phase activity, which again typically emerged between experimental days 20–50 and was unrelated to ethanol access. Under the shift-lag protocol, D2 mice generally displayed less well-integrated activity patterns than those seen in B6, and seldom appeared to approach complete re-synchronization following LD phase shifts. In DD, D2 mice expressed highly variable free-running periods that were usually but not always longer than 24 h, while in LL, D2 mice displayed much clearer free-running rhythms than those seen in B6 mice.
In sum, while B6 and D2 mice showed consistent strain differences in circadian organization, no consistent ethanol-related differences were apparent in the activity patterns of either strain.
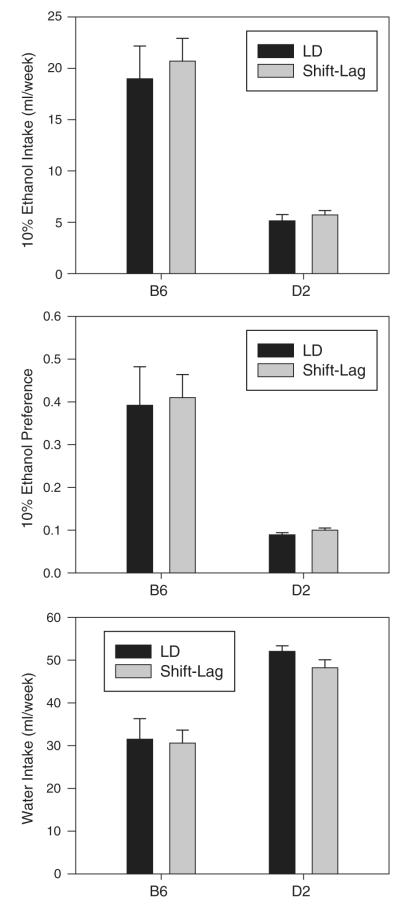
3.2. Effects of shift-lag on ethanol and water intake
Separate strain by treatment between-groups ANOVAs conducted on ethanol intake, water intake and ethanol preference detected significant main effects of strain for each parameter (ethanol intake: F1,26=60.310, p<0.001; water intake: F1,26=42.649, p<0.001; ethanol preference: F1,26=41.182, p<0.001), but no effects of treatment nor any interaction (Fig. 2). Similarly, comparison of shift-lag animals to their own immediately preceding LD conditions also revealed significant main effects of strain on ethanol intake (F1,13=27.254, p<0.001), water intake (F1,13=338.089, p<0.001) and ethanol preference (F1,13=22.374, p<0.001), but no effects of lighting conditions. Thus, while B6 mice consumed more ethanol and less water, and had higher ethanol preferences than D2 mice, shift-lag had no significant effect on either ethanol or water intake in either strain.
Fig. 2.
Ethanol intake, ethanol preference, and water intake in B6 and D2 mice during exposure to either a standard 12:12 light–dark cycle (LD) or to shift-lag lighting (see text for details of shift-lag procedure).
3.3. Effects of DD and LL on ethanol and water intake
Fig. 3 shows fluid intake under the standard LD cycle and under subsequent free-running conditions (either DD or LL in different groups) in B6 and D2 mice. ANOVA revealed significant strain effects for all fluid intake parameters (ethanol intake: F1,26=28.730, p<0.001; water intake: F1,26=84.774, p<0.001; ethanol preference: F1,26=32.177, p<0.001), indicating that B6 mice consumed more ethanol and less water than D2 mice during this phase of the experiment. In addition, mice consumed less ethanol (F1,26=26.738, p<0.001) and displayed lower ethanol preference (F1,26=25.365, p<0.001) under free-running conditions than under the LD cycle, while condition by strain interactions showed that this effect was more robust in B6 than D2 mice (ethanol intake: F1,26=16.581, p<0.001; ethanol preference: F1,26=16.241, p<0.001). Finally, the reduction in ethanol intake under free-running conditions was more pronounced under DD than under LL (F1,26=4.770, p=0.038). These interactions were explored further by both within-groups and between-groups post-hoc pairwise tests, which showed that ethanol intake was reduced under both DD and LL in B6 mice, but only under DD in D2 mice, and that ethanol intake and ethanol preference were lower under DD than under LL in D2 but not in B6 mice. In sum, DD reduced ethanol intake and preference in both strains, but LL reduced ethanol intake only in B6 mice.
Fig. 3.
Ethanol intake, ethanol preference, and water intake in B6 and D2 mice during exposure to a standard 12:12 light–dark cycle (LD) and to subsequent free-running conditions. LD–LL: standard LD cycle followed by constant light (LL); LD–DD: standard LD cycle followed by constant darkness (DD). *, LL or DD significantly different from preceding LD; #, LL significantly different from DD.
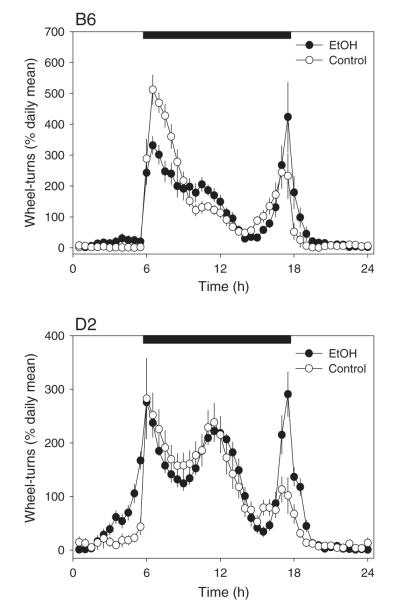
3.4. Effects of ethanol access on circadian waveforms
Fig. 4 shows circadian waveforms for ethanol-maintained and control mice under long-term steady-state exposure to LD. These waveforms have each been normalized to their own means in order to illustrate strain- and ethanol-related effects on the patterning of daily activity, unconfounded by differences in overall activity levels. B6 mice showed a strongly bimodal distribution of nocturnal activity, with major peaks occurring in the first and last hours of the dark phase, while in contrast, D2 mice showed a pronounced tri-modal pattern with an additional prominent activity peak near the middle of the dark phase. The waveform data were analyzed using a 2 (strains) by 2 (ethanol vs. control) by 48 (half-hour time bins) repeated-measures ANOVA. As expected, no main effects of strain or ethanol treatment were detected, presumably due to the normalization of the waveforms as relative activity. More importantly, ANOVA detected a main effect of time-bin (F47,1222 =76.969, p<0.001) as well as time by strain (F47,1222 =8.552, p<0.001), time by ethanol (F47,1222 =4.780, p<0.001), and time by strain by ethanol (F47,1222 =1.690, p=0.003) interactions. These results show that waveforms differed between strains and as a function of ethanol access, and that ethanol access resulted in strain-differentiated effects on the waveform. While ethanol increased the relative magnitude of the “dawn” activity peak in both strains, it reduced the magnitude of the “dusk” peak only in B6 mice. In addition, ethanol access increased relative activity levels during the first 2 h and last 2 h of the light phase, and this effect was especially prominent in the D2 mice.
Fig. 4.
Averaged daily activity waveforms under initial light–dark conditions in ethanol-exposed (EtOH, black symbols) and water-only control animals (white symbols). The black horizontal bars indicate the hours of darkness under the light–dark cycle. Activity levels in each 30-minute bin were normalized to each individual animal’s daily mean prior to averaging across animals in order to eliminate the effects of individual and/or strain differences in activity levels.
3.5. Effects of ethanol access on adaptation to shift-lag
In B6 mice, overall circadian periods measured under the shift-lag protocol were almost exactly 23.14 h, thus matching the average period of the lighting schedule and suggesting effective synchronization of the activity rhythm (Fig. 5). In contrast, however, circadian periods were somewhat longer in D2 mice, suggesting that animals in this strain had more difficulty in successfully adapting to the rotating shift schedule. Analysis of circadian period under the shift-lag schedule showed a significant main effect of strain (F1,24=5.635, p=0.026), but no effect of ethanol nor any strain by ethanol interaction. In addition, B6 mice showed stronger spectral peaks than D2 mice in this condition (F1,24=9.740, p=0.005), but there were no effects of ethanol on this parameter either.
Fig. 5.

Circadian period and spectral magnitude in ethanol-exposed (EtOH, gray bars) and water-only control animals (black bars) during exposure to the shift-lag lighting regimen.
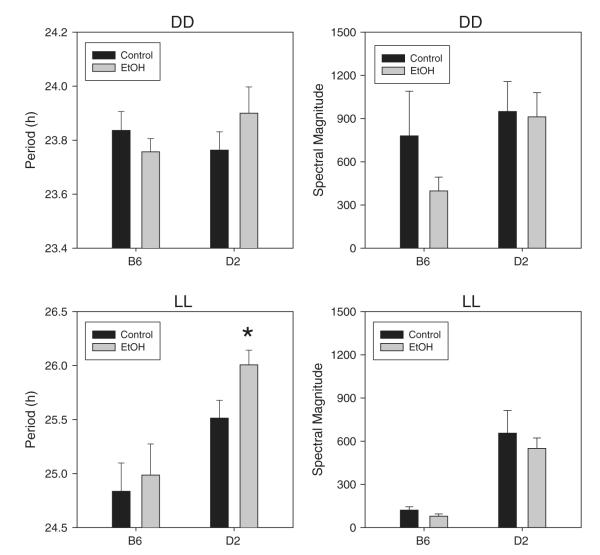
3.6. Effects of ethanol access on free-running circadian period
ANOVA revealed significant main effects of strain (F1,49=14.242, p<0.001) and lighting condition (F1,26=168.755, p<0.001) and a strain by lighting interaction (F1,26=12.086, p=0.001), indicating that D2 mice showed longer free-running periods and were more sensitive to the period-lengthening effect of LL than B6 mice (Fig. 6). In contrast, however, there were no significant main effects or interactions involving ethanol on free-running period. Nevertheless, an exploratory post-hoc comparison showed that ethanol significantly lengthened free-running period in LL in D2 mice.
Fig. 6.
Circadian period and spectral magnitude in ethanol-exposed (EtOH, gray bars) and water-only control animals (black bars) during exposure to constant darkness (DD, top panels) or constant light (LL, bottom panels).
3.7. Effects of ethanol access on spectral peak magnitude
Ethanol-exposed mice showed slightly lower spectral magnitudes than non-exposed animals, while D2 mice showed somewhat larger spectral magnitudes than B6 mice, and spectral magnitudes were somewhat lower in LL than DD (Fig. 6). Nevertheless, ANOVA failed to detect significant effects of any of these variables on spectral magnitude.
3.8. Effects of ethanol access on level of daily locomotor activity
Separate analyses were conducted to determine the effects of strain and ethanol on total daily wheel-turns under steady-state LD conditions and under free-running conditions (DD and LL). Under LD, ANOVA showed significant main effects of strain (F1,26=8.637, p=0.007) and ethanol access (F1,26=13.390, p=0.001) and a strain by ethanol interaction (F1,26=9.537, p=0.001). This interaction was explored further by post-hoc pairwise tests which showed that ethanol access significantly increased daily activity in D2 but not in B6 mice (Fig. 7). Under free-running conditions, daily activity varied by strain (F1,50=10.573, p=0.002; D2 mice were more active than B6) and lighting conditions (F1,50=34.854, p<0.001; mice were more active in DD than LL), but there were no effects of ethanol access (Fig. 7).
Fig. 7.

Daily wheel-turns in ethanol-exposed (EtOH, gray bars) and water-only control animals (black bars) under the second exposure to the standard light–dark cycle (LD) and subsequent exposure to constant darkness (DD) or constant light (LL).
4. Discussion
This study examined potential bidirectional interactions between circadian activity rhythms and voluntary ethanol intake in two inbred mouse strains known to exhibit divergent circadian and ethanol-related phenotypes. These phenotypic differences were generally replicated in the present study, in that B6 mice showed higher ethanol drinking and shorter circadian periods than D2 mice. Nevertheless, the two strains displayed broadly similar responses to our experimental manipulations. Thus, relative to the standard light–dark cycle, B6 mice showed reduced ethanol intake in both DD and LL, while D2 mice showed reduced ethanol intake in DD. In contrast, shift-lag lighting failed to affect ethanol intake in either strain. Access to ethanol altered daily activity patterns in both B6 and D2 mice, and increased activity levels in D2 mice, but had no effects on other circadian parameters. Of course, since D2 mice ingested much less ethanol than B6 mice, it seems likely that D2 mice are much more sensitive to the chronobiological effects of ethanol, consistent with their characterization as a generally ethanol-sensitive strain [42–44].
We found no evidence that chronic exposure to a shift-lag lighting regimen affected ethanol intake in either B6 or D2 mice. These observations are similar to those of Trujillo et al. [41], who reported no effect of either long (26 h) or short (22 h) LD cycles on ethanol drinking in B6 mice. While previous studies have reported both higher [25] and lower [26,27] ethanol intake in shift-lag exposed rats, it should be noted that the shift-lag regimen employed in the present study did not dramatically disrupt circadian activity rhythms. Thus, both B6 and D2 mice generally synchronized to the shift-lag schedule, and there was no evidence for either non-entrainment or “splitting” of activity rhythms in either strain. It remains possible, therefore, that larger or more frequent LD shifts would be able to successfully disrupt circadian activity rhythms and alter voluntary ethanol drinking in mice.
In contrast to the lack of shift-lag effects, we found that maintenance under DD reduced ethanol drinking in both B6 and D2 mice. These results contrast with those of Trujillo et al. [41], who saw no effect of DD on ethanol intake in selectively-bred high (HAP) and low (LAP) alcohol preferring mice, but are consistent with previous studies in rats [44] and hamsters [45]. Surprisingly, B6 mice also showed reduced ethanol drinking in LL, despite the fact that these mice displayed robust activity rhythms in DD but were nearly arrhythmic in LL. While these results are consistent with a previous study showing reduced ethanol intake under both DD and LL in rats [44], D2 mice in the present study showed reduced ethanol drinking only in DD. Taken together, these observations confirm that while environmental lighting conditions can alter voluntary ethanol intake, these effects appear to be somewhat variable across genetic backgrounds and are not strongly correlated with their effects on circadian activity rhythms.
Instead, discrepant results could be related to differences in melatonin production among species and strains. Indeed, while alterations in melatonin secretion have been implicated in the effects of lighting conditions on ethanol intake in rats and hamsters [45–47], B6 and D2 mice – like several other widely used inbred mouse strains – exhibit a profound genetic deficiency in melatonin synthesis [48]. Further, exposure to DD has essentially opposite effects of brain levels of alcohol dehydrogenase in rats and mice [49,50], which could also account for species differences in photoperiodic effects on ethanol preference.
Ethanol access had no detectable effect on adaptation to shift-lag lighting in either strain. In addition, while ethanol access appeared to induce modest lengthening of free-running circadian period in D2 mice under DD and LL, there were no significant differences between ethanol-treated and water-only controls in either free-running period or spectral peak magnitude in either strain. This result contrasts with previous findings of ethanol-induced alterations in free-running period in hamsters [13], rats [14] and mice [16]. It should be noted, however, that the reported period-altering effects of ethanol intake have been somewhat inconsistent, and include both shortening and lengthening of circadian period, even in the same species [14]. Further, in our previous study using B6 mice [16], ethanol-induced period shortening was no longer significant after about 15 weeks of ethanol access, which is approximately when animals were transferred from LD into DD or LL in the present study.
In contrast to its lack of effect on circadian period and spectral magnitude, ethanol clearly altered the daily activity waveform in both B6 and D2 mice under steady-state LD entrainment. Thus, ethanol-drinking animals of both strains displayed a relative shift of nocturnal activity from the early to the late night phase of the cycle, as well as a relative increase in light-phase activity. Importantly, these changes were independent of alterations in total daily activity, which was unchanged in B6 mice but significantly increased by ethanol in D2 mice. While free-running circadian period is considered to directly reflect the period of the underlying circadian pacemaker, the relationship between the circadian pacemaker and the shape of the daily activity pattern is completely unknown. Indeed, while all measured circadian functions in a given individual display essentially identical free-running periods when assessed concurrently, each rhythmic variable (e.g., activity, temperature, hormone secretion) is characterized by its own, unique waveform. It is likely, therefore, that the circadian activity waveform reflects multiple ethanol-sensitive processes, including mechanisms located downstream from the central pacemaker.
In this study, running-wheel activity was used as an assay of circadian pacemaker function, as in many previous experiments. It has become increasingly clear, however, that running wheel access is not a behaviorally inert procedure, and housing with running wheels alters numerous neurobehavioral parameters, including both circadian activity rhythms [51,52] and voluntary ethanol intake [53,54], in both mice and rats. Further, it is plausible that running-wheel access interacts with strain to alter these processes [35]. Thus, it is possible that some of the findings reported in this study are dependent on the use of running wheels, and that somewhat different patterns of effects could have emerged in sedentary animals.
To summarize, the major positive findings of this study are that voluntary ethanol intake is altered by exposure to both constant darkness and constant light, while ethanol intake alters daily activity patterns. These effects were similar in the two strains tested, and may therefore be readily generalizable. These observations support a growing literature on the chronobiology of ethanol, and suggest that circadian-based interventions may be developed to mitigate problems associated with alcohol use disorders.
HIGHLIGHTS.
▶ Lighting conditions affect ethanol intake and preference.
▶ Ethanol access alters circadian activity pattern.
▶ Both effects are strain-dependent.
Acknowledgments
Supported by the Integrative Neuroscience Initiative on Alcoholism (INIA-Stress), NIAAA U01 AA13641.
References
- [1].Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms. Human and animal models. Alcohol Res Health. 2001;25:126–35. [PMC free article] [PubMed] [Google Scholar]
- [2].Danel T, Touitou Y. Chronobiology of alcohol: from chronokinetics to alcohol-related alterations of the circadian system. Chronobiol Int. 2004;21:923–35. doi: 10.1081/cbi-200036886. [DOI] [PubMed] [Google Scholar]
- [3].Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcohol Clin Exp Res. 2005;29:1550–7. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- [4].Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–43. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- [6].Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiol Int. 2010;29:35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- [8].Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–25. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- [9].Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- [10].Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, et al. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol. 2010;45:303–11. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- [11].Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, et al. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry. 2011;168:1090–8. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- [12].Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neurosci Biobehav Rev. 2010;34:1249–55. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [13].Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Behav. 1992;43:159–65. doi: 10.1016/0091-3057(92)90652-v. [DOI] [PubMed] [Google Scholar]
- [14].Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005;84:537–42. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [15].Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Int. 2005;22:227–36. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- [16].Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–12. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol. 2009;296:R411–8. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297:R729–37. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34:1266–73. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol Clin Exp Res. 2011;35:1467–74. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci. 1997;17:1302–19. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–54. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- [24].Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2011;17:351–64. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcohol Clin Exp Res. 1997;21:817–25. [PubMed] [Google Scholar]
- [26].Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light–dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcohol Clin Exp Res. 2007;31:1699–706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- [27].Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light–dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010;44:229–37. doi: 10.1016/j.alcohol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [28].Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- [29].Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiol Int. 2011;28:664–72. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30:81–5. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- [31].Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [32].Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–25. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- [33].Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, et al. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–90. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- [34].Possidente B, Stephan FK. Circadian period in mice: analysis of genetic and maternal contributions to inbred strain differences. Behav Genet. 1988;18:109–17. doi: 10.1007/BF01067080. [DOI] [PubMed] [Google Scholar]
- [35].Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–94. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hofstetter JR, Mayeda AR, Possidente B, Nurnberger JI., Jr Quantitative trait loci (QTL) for circadian rhythms of locomotor activity in mice. Behav Genet. 1995;25:545–56. doi: 10.1007/BF02327578. [DOI] [PubMed] [Google Scholar]
- [37].Ono T, Shimizu T, Yoshida M. Characteristics of wheel-running activity in nine inbred strains of male mice under 12 L–12D cycle. J Mamm Soc Jpn. 1992;16:47–58. [Google Scholar]
- [38].Kopp C. Locomotor activity rhythm in inbred strains of mice: implications for behavioural studies. Behav Brain Res. 2001;125:93–6. doi: 10.1016/s0166-4328(01)00289-3. [DOI] [PubMed] [Google Scholar]
- [39].Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–6. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- [40].de Visser L, van den Bos R, Stoker AK, Kas MJ, Spruijt BM. Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res. 2007;177:290–7. doi: 10.1016/j.bbr.2006.11.019. [DOI] [PubMed] [Google Scholar]
- [41].Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR. Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol. 2011;45:147–59. doi: 10.1016/j.alcohol.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodriguez LA, Plomin R, Blizard DA, Jones BC, McClearn GE. Alcohol acceptance, preference, and sensitivity in mice. II. Quantitative trait loci mapping analysis using BXD recombinant inbred strains. Alcohol Clin Exp Res. 1995;19:367–73. doi: 10.1111/j.1530-0277.1995.tb01517.x. [DOI] [PubMed] [Google Scholar]
- [43].Browman KE, Crabbe JC. Quantitative trait loci affecting ethanol sensitivity in BXD recombinant inbred mice. Alcohol Clin Exp Res. 2000;24:17–23. [PubMed] [Google Scholar]
- [44].Goodwin FL, Amir S, Amit Z. Environmental lighting has a selective influence on ethanol intake in rats. Physiol Behav. 1999;66:323–8. doi: 10.1016/s0031-9384(98)00302-3. [DOI] [PubMed] [Google Scholar]
- [45].Reiter RJ, Blum K, Wallace JE, Merritt JH. Pineal gland: evidence for an influence on ethanol preference in male Syrian hamsters. Comp Biochem Physiol A Comp Physiol. 1974;47:11–6. doi: 10.1016/0300-9629(74)90045-0. [DOI] [PubMed] [Google Scholar]
- [46].Blum K, Merritt JH, Reiter RJ, Wallace JE. A possible relationship between the pineal gland and ethanol preference in the rat. Curr Ther Res Clin Exp. 1973;15:25–30. [PubMed] [Google Scholar]
- [47].Burke LP, Kramer SZ. Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacol Biochem Behav. 1974;2:459–63. doi: 10.1016/0091-3057(74)90004-5. [DOI] [PubMed] [Google Scholar]
- [48].Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–3. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- [49].Messiha FS, Hartman T, Geller I. Darkness-induced alterations in alcohol dehydrogenase activity in specific brain regions of the rat. Res Commun Chem Pathol Pharmacol. 1975;10:399–402. [PubMed] [Google Scholar]
- [50].Messiha FS. Darkness, ethanol and cerebral activity. Neurobehav Toxicol Teratol. 1985;7:149–53. [PubMed] [Google Scholar]
- [51].Yamada N, Shimoda K, Takahashi K, Takahashi S. Relationship between free-running period and motor activity in blinded rats. Brain Res Bull. 1990;25:115–9. doi: 10.1016/0361-9230(90)90261-w. [DOI] [PubMed] [Google Scholar]
- [52].Edgar DM, Martin CE, Dement WC. Activity feedback to the mammalian circadian pacemaker: influence on observed measures of rhythm period length. J Biol Rhythms. 1991;6:185–99. doi: 10.1177/074873049100600301. [DOI] [PubMed] [Google Scholar]
- [53].McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- [54].Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–52. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]