Abstract
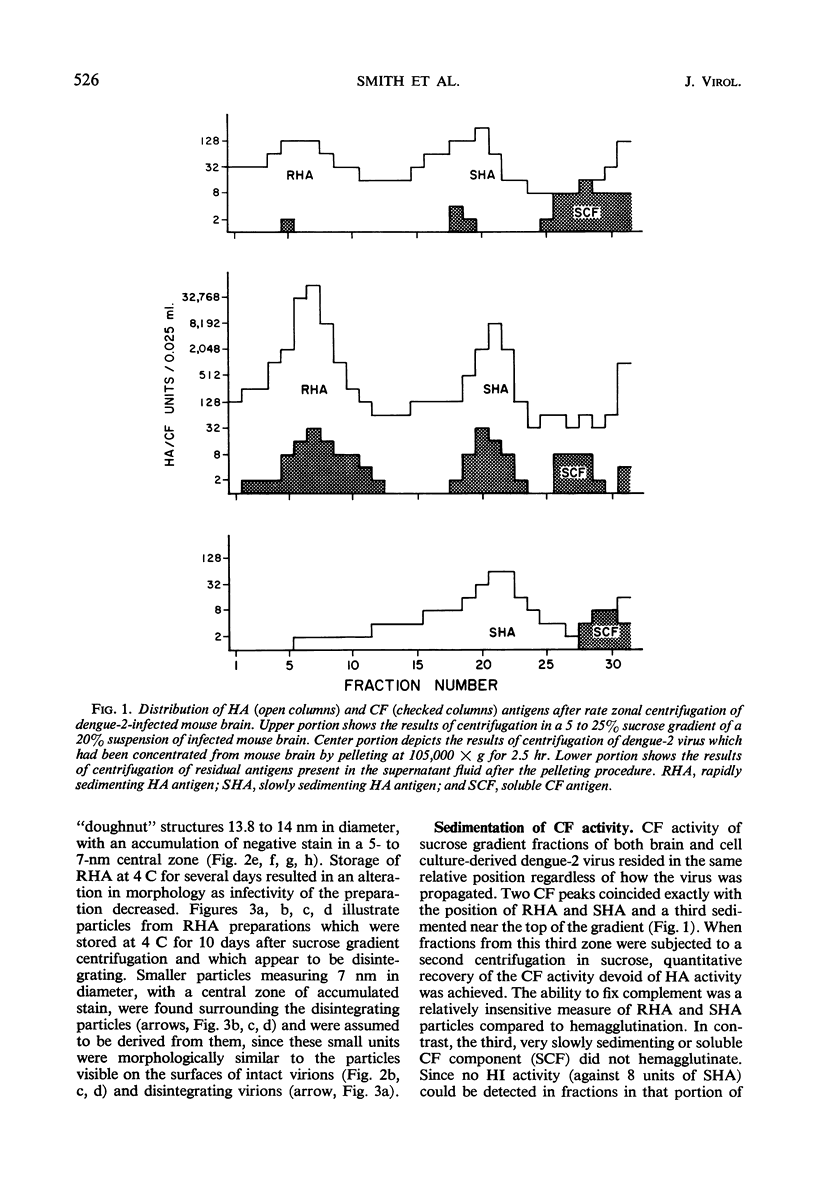
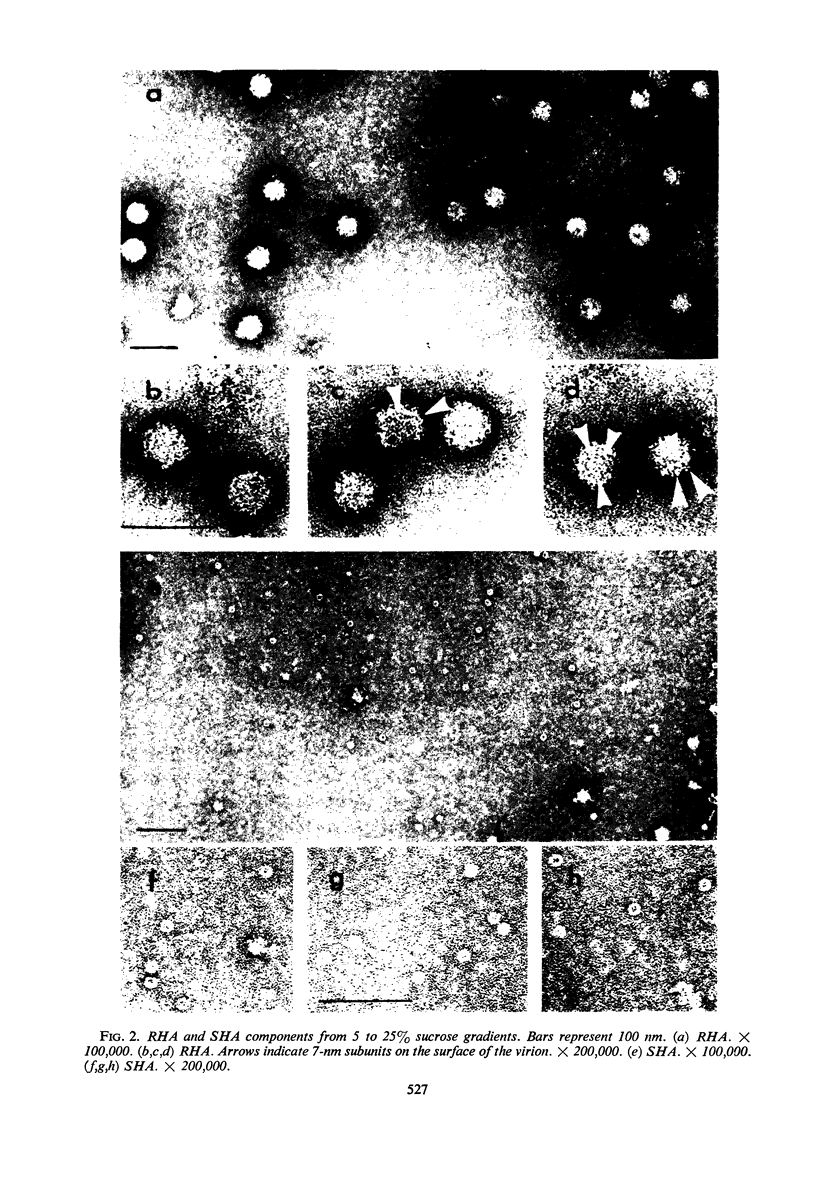
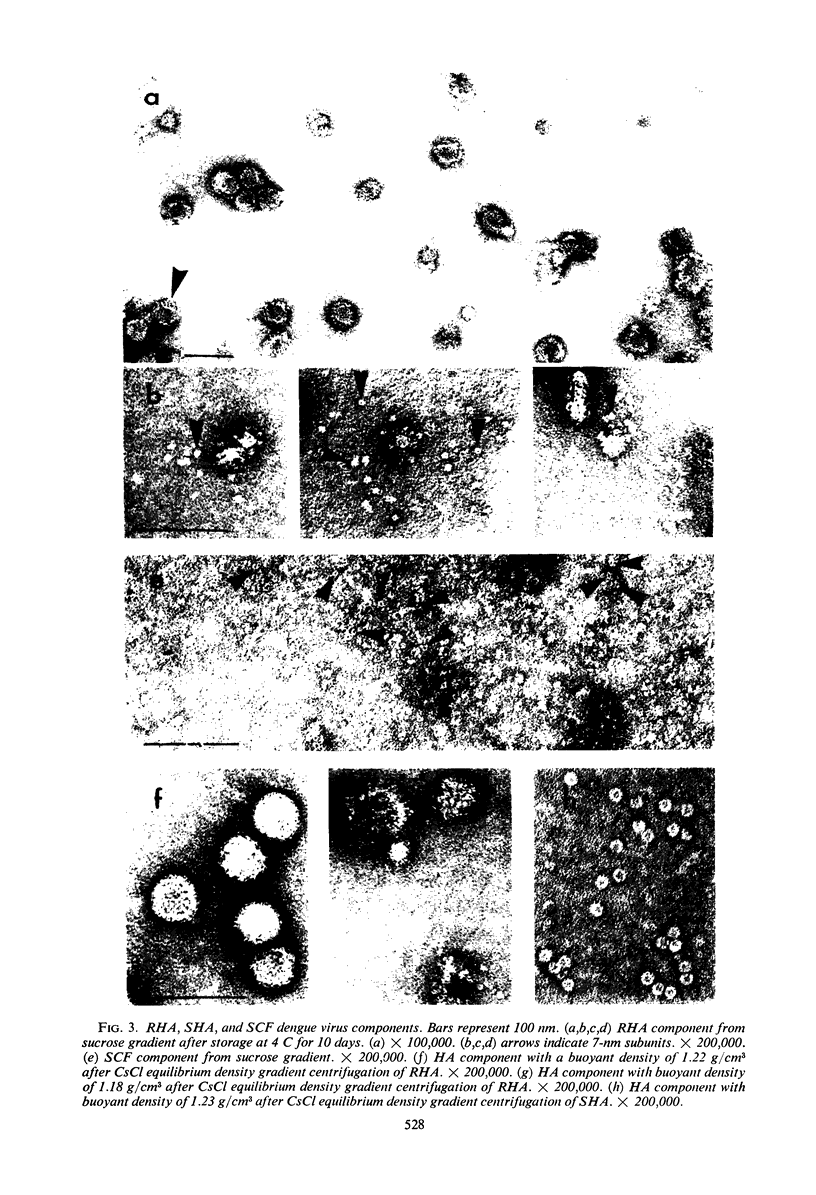
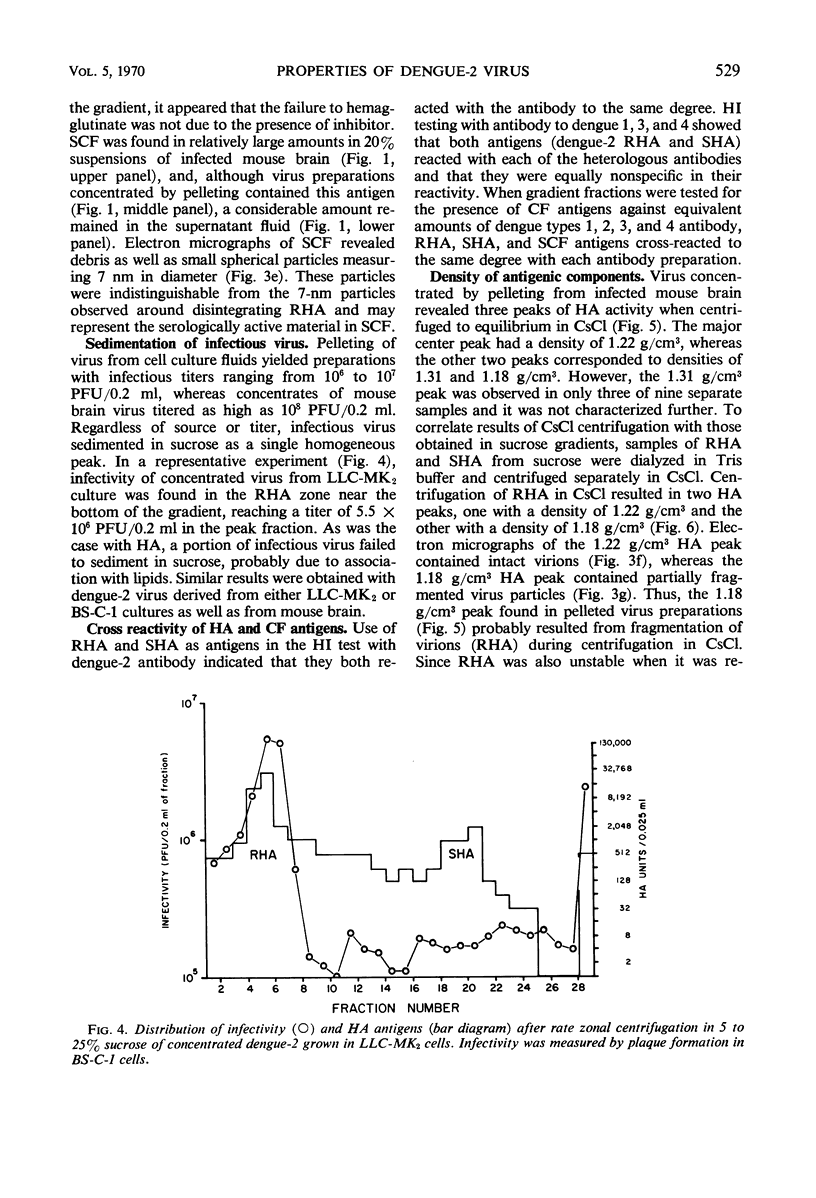
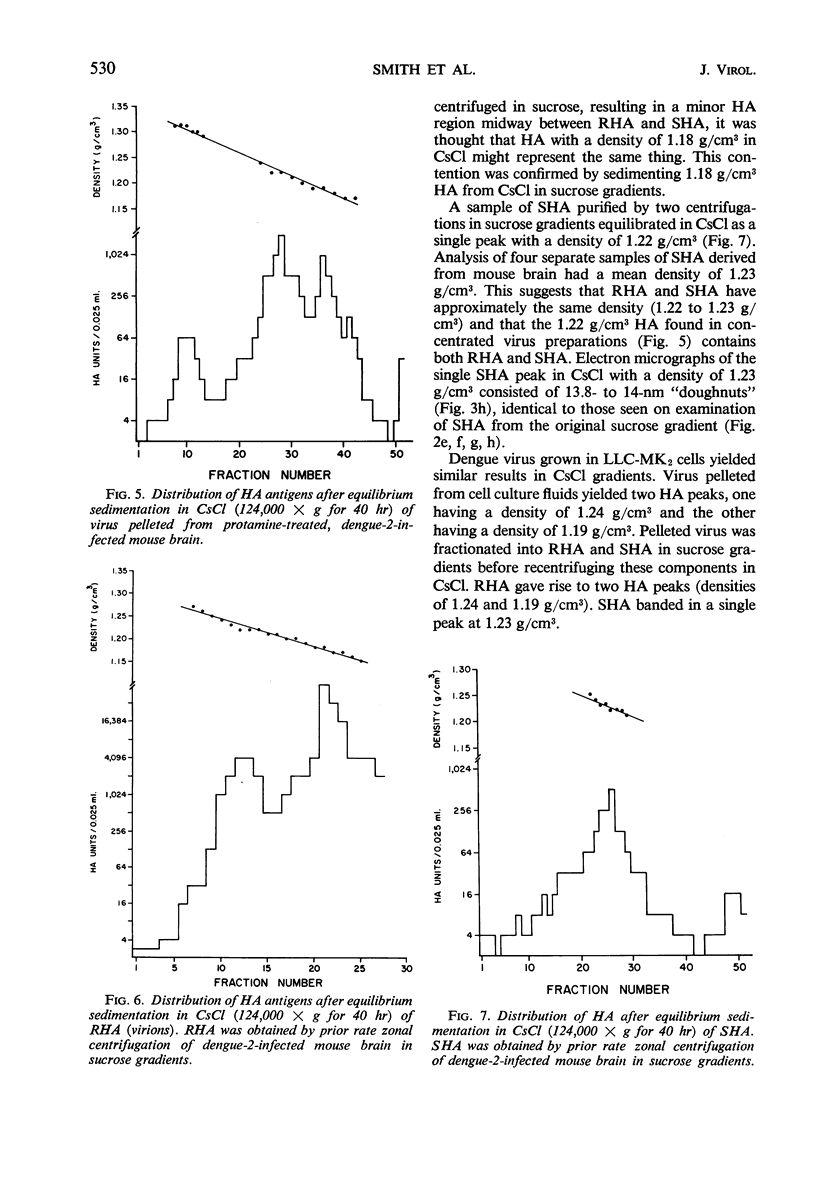
Dengue virus suspensions from mouse brain and cell culture were fractionated into three components by rate zonal centrifugation in sucrose gradients. Infectious virus sedimented in a single zone and possessed hemagglutinating (HA) and complement fixing (CF) activity. Electron micrographs showed the virion to be a spherical particle 48 to 50 nm in diameter with 7-nm spherical structures on its surface. Buoyant density in CsCl of virions from mouse brain was estimated at 1.22 g/cm3 and from cell culture at 1.24 g/cm3. During centrifugation of virions in CsCl, an additional HA component appeared with a buoyant density of 1.18 g/cm3. It was shown in electron micrographs to consist of virion fragments. A noninfectious component with HA and CF activity sedimented in sucrose more slowly than intact virus, had a buoyant density of 1.23 g/cm3 in CsCl, and appeared as “doughnut” forms measuring 13.8 to 14 nm in diameter. A third component, with CF activity and no HA activity, sedimented very little in sucrose gradients. Particles of the same size and shape as the spherical subunits on the surface of the virion were observed in electron micrographs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Hoffman E. J., Brown A. Fractionation of Eastern equine encephalitis virus by density gradient centrifugation in CsCl. J Virol. 1968 Oct;2(10):972–978. doi: 10.21236/ad0832598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUESCHER E. L., SCHERER W. F., GROSSBERG S. E., CHANOCK R. M., PHILPOT V., Jr Immunologic studies of Japanese encephalitis virus in Japan. I. Antibody responses following overt infection of man. J Immunol. 1959 Dec;83:582–593. [PubMed] [Google Scholar]
- Brandt W. E., Buescher E. L., Hetrick F. M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967 May;16(3):339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- CHENG P. Y. Some physical properties of hemagglutinating and complement-fixing particles of Semliki Forest virus. Virology. 1961 May;14:132–140. doi: 10.1016/0042-6822(61)90140-4. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Takehara M., Hotta S. Some fundamental characteristics of dengue and yellow fever viruses: inhomogeneity and characterization of partially purified viral particles. Kobe J Med Sci. 1967 Dec;13(4):273–293. [PubMed] [Google Scholar]
- Mussgay M., Horzinek M. Investigations on complement-fixing subunits of a group A arbo virus (Sindbis). Virology. 1966 Jun;29(2):199–204. doi: 10.1016/0042-6822(66)90026-2. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967 Aug;99(2):291–296. [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]