Abstract
Background:
The aim of this study was to compare the effects of diode laser irradiation on wound healing in oral rat mucosa and also to measure the amount of inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS) on oral wound healing. Healing was assessed by histology and the amounts of eNOS and iNOS were measured by real-time polymerase chain reaction (PCR).
Materials and Methods:
Twenty-four standardized incisions were carried out on the buccal mucosa of 12 male Wistar rats; each rat received two incisions on the opposite sides of the buccal mucosa by using a steel scalpel. On the right side (test side), a diode laser (660 nm) was employed on the incision for 10 seconds on days 1-4 and 6-9. The left side (control side) did not receive any laser. Histological and real-time PCR analysis were done on tissue samples after 2, 7, 14, and 21 days.
Results:
Histological analysis showed that the tissue healing after seven days on the laser irradiated side was better than the control side, but there was no significant difference between the two sides on days 2, 14, and 21 after surgery. Paired t-test analysis showed that there was no significant difference in the amount of eNOS between the groups. The difference in the amounts of iNOS between the groups was significant; it was more in the laser-irradiated side than the control side.
Conclusion:
Histological findings showed that diode laser needs several repeated irradiations for the acceleration of wound healing. The iNOS amount showed that increases are associated with better healing.
Keywords: Endothelial nitric oxide synthase, inducible nitric oxide synthase, polymerase chain reaction, real-time polymerase chain reaction
INTRODUCTION
The use of lowlevel laser as a therapeutic device started with Mester in 1971,[1] who studied its effect on wound healing in rats.
High intensity lasers use heat for making incisions and vaporizing tissues and are identified as surgical lasers. In a different way, low-intensity lasers have the power of biostimulation without an increase in temperature. The most important effects of low-level laser therapy (LLLT) are pain relief and improvement of wound healing.[2]
Diode lasers are portable inexpensive devices, which in medicine and dentistry have been used for LLLT or biostimulation;[3] however, there is controversy surrounding the effectiveness of some of these procedures.[4,5]
The present study is done to know the effects of diode laser irradiation on wound healing in oral rat mucosa.
Acute wounds normally heal in a very orderly and efficient manner characterized by four distinct but overlapping phases: Hemostasis, inflammation, proliferation, and remodeling.[6,7]
Nitric oxide is an intracellular messenger molecule with important immune functions.[8] It is produced by a group of isoenzymes collectively termed NOS (NO synthase).[9] Three distinct isoforms of NOS have been explained: Endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS).
The effects of iNOS in the inflammatory response are particularly complex, and the enzyme appears to be involved in both promotion and resolution of inflammation.[10]
Here, to further examine the role of iNOS and eNOS in oral wound healing, we measured them by reverse transcriptase polymerase chain reaction (PCR).
The aim of this study was to assess the effects of diode laser irradiation on wound healing in oral rat mucosa and also to measure the amount of iNOS and eNOS in oral wound healing.
MATERIALS AND METHODS
Laser devices
In the present study, a continuous mode diode laser (AZOR-2K, Moscow, Russia) emitting at 660 nm was employed. Laser radiation was employed on the oral mucosa of rats at 25 mW output power to evaluate the healing process after 2, 7, 14, and 21 days. The laser tip was in touch with the epithelial surface during the laser exposure (for 10 seconds) on days 1-4 and 6-9.
Animal model and experimental groups
Classification
Twelve one-to two-year-old Wistar male white rats were kept in metal cages at room temperature, with 12 hours of light per day and 37% relative humidity. They received a standard laboratory diet and water ad libitum. Before the experimental procedures, the rats were randomly divided into four groups of three rats each. Two parallel incisions by steel scalpel (Bard-Parker number 15) and approximately 10 mm in length were performed in the buccal mucosa of each rat. On one side, incision diode laser at 25 mW was employed for 10 seconds. The incisions were not sutured. The rats were killed at intervals of 2, 7, 14, and 21 days after the surgical procedure, and the tissues were analyzed to compare histological and real-time PCR alterations in each group at different times:
Group 1: With histological and real-time PCR analysis after two days
Group 2: With histological and real-time PCR analysis after seven days
Group 3: With histological and real-time PCR analysis after 14 days
Group 4: With histological and real-time PCR analysis after 21 days
Presurgical treatment and surgical procedure
The buccal mucosa was selected for the oral wounds because of its accessibility. After weighing, each animal received an anesthetic injection of 10% ketamine (80 mg/kg) and 1% acepromazine (2.5 mg/kg).
After anesthesia, the intraoral surgical field together with the handpiece and fiber of the laser device were sterilized with Butadiene solution (Behsa, Arak, Iran).
On both sides of the buccal mucosa, the tissues were incised using a steel scalpel (Bard Parker number 15). On the right side, a diode laser with continuous output power of 25 mW and wavelength 660 nm (visible red) was used. The energy density was 1 j/cm². The laser tip beam was kept perpendicular to the irradiated tissue surface. All of the surgical procedures were performed by the same operator under aseptic conditions. The rats were then returned to their cages, without limitations of activity.
Postsurgical and histological procedures
To prevent postsurgical infections, antibiotics (penicillin 0.5 mL intramuscular [IM]) were administered on the day of the surgical procedure. The rats were sacrificed by means of an overdose of chloroform at intervals of 2, 7, 14, and 21 days after the surgical procedure, for groups 1 and 2 and groups 3 and 4, respectively. Specimens measuring approximately 5 × 10 mm were then removed from the control (left) and test (right) sides of each animal. The histological sections were stained with hematoxylin and eosin by using standard procedures and then were examined by light microscopy. Finally, the remaining tissues were centrifuged and managed for eNOS and iNOS molecular analysis.
eNOS and iNOS biochemical procedures
Reverse transcriptase PCR was done by Applied Bios stems Step one. The sequences of the primers used were 5’-TGTCTGTCTGCTGCTAG-3’(sense), 5’-CTCTCCAGGCACTTCAGGC-3’ (antisense) for eNOS, and 5’-AGTGATGGCAAGCACGACTTC-3’ (sense) and 5’-TCTGTCACTCGCTCACCACGG-3’ (antisense) for iNOS.
Histomorphometry and statistics
After staining with H and E, the specimens were analyzed by Olympus optical microscope (CX 21, Japan). The healing process on various group tissues was classified as grade 1 (very light), grade 2 (moderate), grade 3 (advanced), and grade 4 (well organized).
Statistical analysis
Histological results were displayed as healing grade and processed for Wilcoxon analysis. Real-time PCR results were displayed as mean and standard deviation and processed for statistical analysis. Repeated measure analysis of variance (ANOVA) test was performed between all groups. A probability of a null hypothesis of < 5% (P < 0.05) was regarded as statistically significant.
RESULTS
eNOS and iNOS levels in healthy tissues of two rats were measured and their values were as follows: eNOS: 0.55 and 0.96; ions: 1.4 and 1.8.
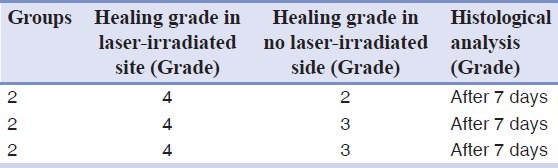
Histological analysis showed that the tissue healing after seven days on the laser irradiated side was better than the control side, but there was no difference between the two sides on days 2, 14, and 21 after surgery. The difference between all groups was not statistically significant (P < 0.05) because of limited number of samples. The healing process in the second group of tissues is reported in Table 1.
Table 1.
Healing at different times based on the histological differences in second group
The real-time PCR analysis showed that there was no significant difference in the amount of eNOS between groups (P > 0.05). The difference in the amount of iNOS between the groups was significant (P < 0.05). The amount of iNOS in the laser-irradiated side was more than the control side The results are shown in Tables 2 and 3 and in Figures 1 and 2.
Table 2.
Real-time PCR analysis for eNOS
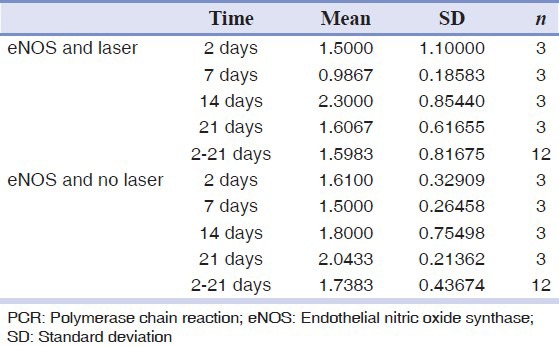
Table 3.
Real-time PCR analysis for iNOS
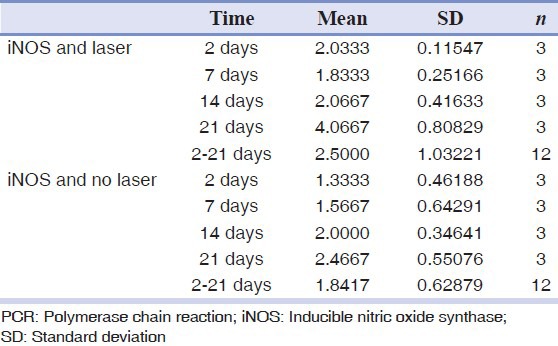
Figure 1.
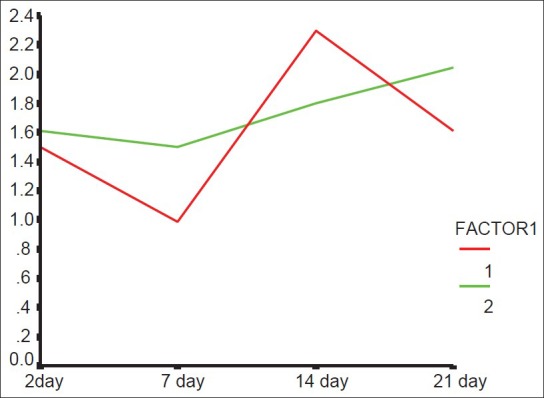
eNOS averages according to postsurgical time and different groups (1: Laser-irradiated side, 2: Control side)
Figure 2.
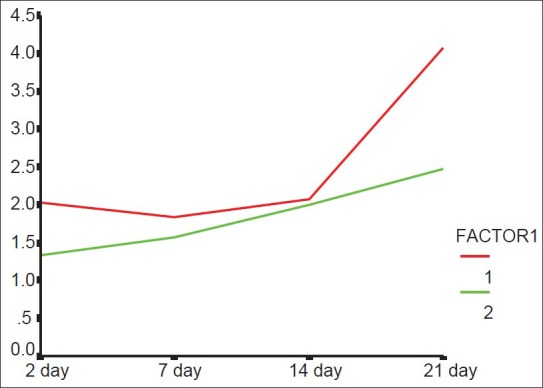
iNOS averages according to postsurgical time and different groups (1: Laser.irradiated side, 2: Control side)
DISCUSSION
Promotion of healing is of paramount importance in medicine, particularly in diabetic and immune-compromised patients.[11] Several studies have been done on LLLT; however, conflicting results and little research on the mouth was an incentive for us to do this research.
A diode laser was used in this study and the results showed that seven days after the surgical procedure, healing was better on the side receiving laser radiation. This result was similar to the results of some previous studies,[11,12,13,14] although the results were in contrast to many other studies.[15,16,17,18]
In the previous studies, researchers believed that the differences between fluency energy level in tissues,[11,19,20] frequency of radiation,[21] systemic effect,[13,22,23] and the type of ulcer[14] would influence the results of low-level laser exposures and generate conflicting results.
In this study, the test of choice for assessing the healing grade was histological observation. We also measured iNOS and eNOS levels; so, we could evaluate their variations in the laser receiving sides and control sides. Because there is not enough information about eNOS and iNOS and their role in the healing process in the literature, this study will open the way for further investigation on their role; so, we can have more accurate assessment for measuring the healing grade.
In this study, the healing was compared in periods of 2, 7, 14, and 21 days after the surgical procedure. In group 2, there was histologically better healing on the irradiated side compared to the control side. Improvement in the healing in the second group compared to the first group on the laser irradiated side could be due to the fact that in the first group, the rats did not receive enough laser radiation and according to the high rate of repair in rats, there was complete healing in groups 3 and 4 and there was no difference between the groups. This study was consistent with the researches of Parirokh, et al.[11] and Sandra, et al.[24]
Schlager, et al.[25] did not observe any beneficial effect of laser therapy, which is inconsistent with this study. Ten consecutive exposures might inhibit the effect of laser. The eNOS levels in the different groups were not significantly different. This is probably due to small size of the sample. In group 2, the eNOS level was reduced on both sides but further reduction was seen in the laser exposed side. The reduction in eNOS can be attributed to a better healing, although these changes were not statistically significant.
The amount of iNOS on both sides and in all groups had significant differences. In the laser exposed side, the iNOS level was more than the control side in all the groups. It can be inferred from these findings that increased iNOS may play a role in speeding repair, but it may be due to its role in the inflammatory process. This finding is consistent with the findings of Schwentker, et al.[26] They showed that reduced iNOS is associated with weaker repair.
The present findings are inconsistent with the findings of Jude, et al.,[27] Camillo, et al.,[28] Nergis, et al., 29 and Farhad, et al.[30] They showed that decreased iNOS is associated with better healing.
Therefore, further research to evaluate variations in iNOS and eNOS levels during wound healing and the interpretation of these variations is recommended. Also, more research in the field of laser effects in wound healing in humans is recommended.
CONCLUSION
Histological findings showed that diode laser needs several repeated irradiations for acceleration of wound healing. The amount of iNOS showed that increase is associated with better healing.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSc degree in Oral medicine. (#389333 proposal code). The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University
Conflict of Interest: None declared
REFERENCES
- 1.Merster E. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–5. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- 2.Carla AD, Sebastião L, Adriana CP, Euloir P. Clinical evaluation of the effects of low intensity laser (GAALAS) on wound healing after gingivoplasty in humans. J Appl Oral Sci. 2004;12:133–6. doi: 10.1590/s1678-77572004000200010. [DOI] [PubMed] [Google Scholar]
- 3.Goldman L, Goldman B, Van Lieu N. Current laser dentistry. Lasers Surg Med. 1987;6:559–62. doi: 10.1002/lsm.1900060616. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth AJ. Lasers and wound healing. Dermatol Clin. 1993;11:783–9. [PubMed] [Google Scholar]
- 5.Basford JR. Low intensity laser therapy: Still not an established clinical tool. Lasers Surg Med. 1995;16:331–42. doi: 10.1002/lsm.1900160404. [DOI] [PubMed] [Google Scholar]
- 6.Richardson M. Acute wounds: an overview of the physiological healing process. Nurs Times. 2004;100:50–3. [PubMed] [Google Scholar]
- 7.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 8.Carew JF, Ward RF, LaBruna A, Torzilli PA, Schley WS. Effects of scalpel, electrocautery, and CO2 and KTP lasers on wound healing in rat tongues. Laryngoscope. 1998;108:373–80. doi: 10.1097/00005537-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sinha UK, Gallagher LA. Effects of steel scalpel, ultrasonic scalpel, CO 2 laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa. Laryngoscope. 2003;113:228–36. doi: 10.1097/00005537-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Vallance P, Leiper J. Blocking NO synthesis: How, where and why? Nat Rev Drug Discov. 2002;1:939–50. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- 11.Parirokh M, Dabiri SH. Effect of low power laser on incisional wound healing. Iranian endod J. 2006:1156–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Saperia D, Glassberg E, Lyons RF, Abergel RP, Baneux P, Castel JC, et al. Demonstration of elevated type I and type III procollagen mRNA levels in cutaneous wounds treated with He-Ne laser. Biochem Biophys Res Commun. 1986;138:1123–8. doi: 10.1016/s0006-291x(86)80399-0. [DOI] [PubMed] [Google Scholar]
- 13.Belkin M, Schwartz M. Photochemical effects upon the cornea, skin and other tissues. Health Phys. 1989;56:687–90. doi: 10.1097/00004032-198905000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Anneroth G, Hall G, Rydenh Z. The effect of low-energy infra-red laser radiation on wound healing. Br J Oral Maxillofac Surg. 1988;26:12–7. doi: 10.1016/0266-4356(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 16.Colver GB, Priestley GC. Failure of a He-Ne laser to affect components of wound healing in vitro. Br J Dermatol. 1989;121:179–86. doi: 10.1111/j.1365-2133.1989.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 17.Strange R, Moseley H, Carmaicheal A. Soft lasers-have they place in dentistry? Br Dent J. 1988;24:221–5. doi: 10.1038/sj.bdj.4806564. [DOI] [PubMed] [Google Scholar]
- 18.Masse JF, Landry RG, Rochette, Dufour L, Morency R, D’Aoust P. Effectiveness of soft laser treatment in periodontal surgery. Int Dent J. 1993;43:121–7. [PubMed] [Google Scholar]
- 19.Neiburger EJ. Accelerated healing of gingival incisions by the He-Ne diode laser: A prelim inary study. Gen Dent. 1997;45:166–70. [PubMed] [Google Scholar]
- 20.Yu HS, Chang KL, YU CL, Chen JW, Chen GS. Low-Energy Helium-Neon Laser Irradiation Stimulates Interleukin-1 and Interleukin-8 Release from Cultured Human Keratinocytes. J Invest Dermatol. 1996;107:593–6. doi: 10.1111/1523-1747.ep12583090. [DOI] [PubMed] [Google Scholar]
- 21.Braekt MM, van Alphen FA, Kuijpers- Jagtman AM, Maltha JC. Effect of low level laser therapy on wound healing after palatal surgery in beagle dogs. Lasers Surg Med. 1991;11:462–70. doi: 10.1002/lsm.1900110512. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Nishiaka J, Hukuda S. Suppressed tuberculin reaction in guinea pigs following laser irradiation. Lasers Surg Med. 1989;9:271–5. doi: 10.1002/lsm.1900090310. [DOI] [PubMed] [Google Scholar]
- 23.Wilder-Smith P. The soft laser therapeutic tool or popular placebo? Oral Surg. 1988;66:654–66. doi: 10.1016/0030-4220(88)90311-8. [DOI] [PubMed] [Google Scholar]
- 24.Rezende SB, Ribeiro MS, Núñez SC, Garcia VG, Maldonado EP. Effects of a single near-infrared laser treatment on cutaneous wound healing: Biometrical and histological study in rats. J Photochem Photobiol B. 2007;87:145–53. doi: 10.1016/j.jphotobiol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Schlager AK, Oehler KU, Huebner M, Schmuth L, Spoetl L. Healing of burns after treatment with 670-nanometer low-power laser light. Plast Reconstr Surg. 2000;105:1635–9. doi: 10.1097/00006534-200004050-00006. [DOI] [PubMed] [Google Scholar]
- 26.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: Role of cytokines? Nitric oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 27.Jude EB, Boulton AJ, Fergusen MW, Appleton I. The role of nitric oxide synthase isoforms and arginase in the pathogenesis of diabetic foot ulcers: Possible modulatory effects by transforming growth factor beta1. Diabetologia. 1999;42:748–57. doi: 10.1007/s001250051224. [DOI] [PubMed] [Google Scholar]
- 28.Camillo DA, Franca Di Nardo Di Matio, Gianni DP, Monica B, Serigo C. A preliminary study of diode laser versus scalpel incisions in rat oral tissue: A comparison of clinical, histological, and immunohistochemical results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:764–73. doi: 10.1016/j.tripleo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Nergiz Y, Samet I. Effects of polyglecaprone 25, silk and catgut suture materials on normal mucosa wound healing in diabetic rats: An evaluation of nitric oxide dynamics. Med Patol Oral Cir Bucal. 2010;15:e526–30. [PubMed] [Google Scholar]
- 30.Farhad AR, Razavi M, Alavi Nejad P. The use of aminoguanidine, a selective inducible nitric oxide synthase inhibitor, to evaluate the role of nitric oxide on periapical healing. DRJ. 2011;8:187–202. doi: 10.4103/1735-3327.86038. [DOI] [PMC free article] [PubMed] [Google Scholar]


