Abstract
Malignant melanoma is a potentially aggressive tumor of melanocytic origin. Primary oral malignant melanoma is a rare neoplasm, accounting for 0.5% of all oral malignancies. The present case occurred in a 60-year-old female patient, as a pedunculated growth involving the palate and alveolar ridge and histologically showing a desmoplastic differentiation. The article discusses the distinct clinico-pathologic presentation of this case and emphasizes on the need to identify and report such cases for further understanding of their biologic behavior.
Keywords: Desmoplastic melanoma, malignant melanoma, oral mucosal melanoma
INTRODUCTION
Malignant melanoma is a potentially aggressive tumor of melanocytic origin.[1] that arises from a benign melanocytic lesion or de novo from melanocytes within otherwise, normal skin or mucosa.[2] The incidence of head and neck mucosal melanomas is approximately 4/10 million population per year in the USA.[3] Only about 1% of all melanomas arise in the oral mucosa and these account for 0.5% of all oral malignancies.[2,4] These rare lesions may present themselves in yet uncommon ways which need to be identified and reported to understand their biologic behavior, and hence this case report is an attempt do the same.
CASE REPORT
A 60-year-old female patient reported with the complaint of a growth on the palate and inability to wear the denture since 3 months. On clinical examination, the lesional mass was seen involving the entire hard palate as a pedunculated mass, measuring approximately 3 cm × 3 cm, attached to the palatal mucosa with a narrow stalk. The base of the pedunculated mass, the palatal mucosa and the surrounding alveolar mucosa showed areas of brownish black pigmentation. The mucosa on the oral side of the lesion showed a pale whitish color [Figure 1]. On palpation the lesion was smooth, non-tender, well defined, firm to hard in consistency with induration of the posterior margins.
Figure 1.

Clinical picture showing a pedunculated lesion involving the palate
On computed tomography an enhancing, infiltrative soft tissue mass arising from the hard palate with subtle bony erosion noted over the mid portion of the hard palate [Figure 2] and bilateral submandibular lymphadenopathy were evident.
Figure 2.
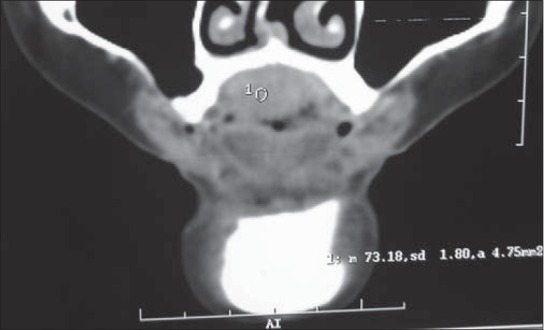
Computed tomography image showing subtle bony erosion noted over the mid portion of the hard palate
A provisional diagnosis of malignant melanoma was considered and an incisional biopsy was performed which confirmed the diagnosis. The patient underwent total maxillectomy with neck dissection.
The histopathological examination of the excised tissue revealed cells with a clear cytoplasm and hyper chromatic nuclei resembling atypical melanocytes seen proliferating from dermal-epidermal junction into the connective tissue. The atypical melanocytes were epitheloid in the superficial region [Figure 3], and spindle shaped in the deeper regions of the connective tissue. The spindle cell type predominated showing an irregular branching pattern with intervening fibrous septae [Figure 4], these cells appeared fusiform to round in different orientations [Figure 5]. Atypical features like mitosis, multi-nucleation, pleomorphism, hyper chromatism, increased nuclear cytoplasmic ratio and prominent nucleoli were seen [Figure 6]. Pigmented nests of atypical melanocytes along with melanophages were seen in the superficial region. The tumor-stroma interface showed a marked inflammatory response and dense fibrosis. The evaluation the harvested submandibular lymph node showed metastatic involvement. The histopathology confirmed the diagnosis of malignant melanoma with metastatic level I nodes. The spindle cell predominance with collagenous stroma was suggestive of desmoplastic malignant melanoma.
Figure 3.

Superficial dermis showing pigmented epitheloid and spindle tumor cells (H and E, ×40)
Figure 4.

Deep dermis showing the tumor with predominant spindle cell type arranged in irregular branching pattern with intervening fibrous septae (H and E, ×10)
Figure 5.
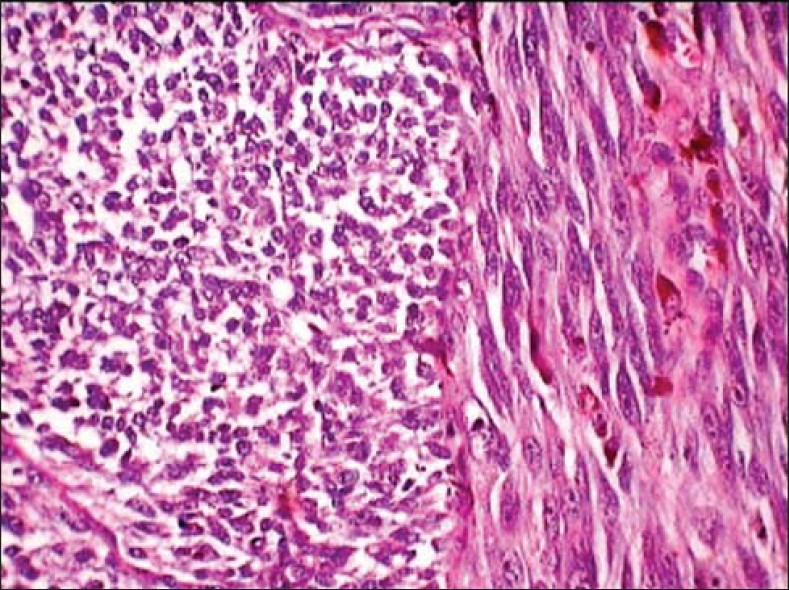
Spindle tumor cells appear fusiform to rounded in different orientations (H and E, ×40)
Figure 6.

Tumor cells showing atypical features like mitosis, pleomorphism, hyperchromatism, increased nuclear cytoplasmic ratio and prominent nucleoli (H and E, ×40)
The patient underwent reconstruction, prosthetic rehabilitation, and has been on regular follow with no recurrence till date.
DISCUSSION
Mucosal melanomas of the head and neck comprise just over 1% of all melanomas and of these about 50% arise in the oral cavity. Oral mucosal melanomas are therefore, rare representing about 0.5% of oral malignancies and less than 0.01% of all oral biopsies. They arise in adults with an average age of about 55, but with a uniform age distribution from years 20 to 80 years. Very rare cases have been reported in children. In most large series there is a male predominance in a ratio of about 3:1. No etiological factors are known to be associated with oral melanoma.[5] Prognosis for malignant melanoma in the oral region is poorer than its counterpart in cutaneous regions because of anatomic considerations and delayed diagnosis.[6] Mucosal melanomas are so rare that there are no large data bases compared to those for cutaneous melanomas; therefore, pathologic micro staging has not been possible, and the fine-tuning of the prognosis that has been useful in cutaneous melanomas (Breslow thickness) has so far not been possible in mucosal melanomas.[7]
Typically, an oral melanoma is composed of sheets or islands of epithelioid melanocytes, which may be arranged in an organoid, or alveolar pattern. The cells have pale cytoplasm and large open nuclei with prominent nucleoli and occasionally they may be plasmacytoid. Sheets and fascicles of spindle cells may also be seen, but are usually a minor part of the lesion. Occasional lesions may be predominantly or wholly spindled.[5]
Desmoplastic melanoma (DM) is a rare variant of malignant melanoma first described by Conley et al. in 1971 as an invasive melanoma composed of spindle cells surrounded by abundant collagen or fibrous stroma.[8,9] It is a histological subtype of melanoma that has been reported to account for approximately 1% of all cases of melanoma.[10] In DM the spindle-shaped melanocytes, which often resemble fibroblasts and are usually non-pigmented, are found in and between mature collagen bundles. The latter may be thickened and/or associated with a mild to marked stromal fibrosis. The distribution of spindle cells is usually haphazard but occasionally they form parallel bundles or storiform areas. The overlying epidermis may be thinned or thickened. The cytological atypia of the spindle cells usually varies from mild to moderate.[8] Similar observations were made in the present case.
It is most likely that the desmoplastic cells are derived from melanocytes that have undergone adaptive fibroplasia. Some authors have suggested that the desmoplasia occurs because of a fibroblastic stromal response and neurofibrosarcomatous differentiation of the tumor cells.[7] Electron microscopic examination of the spindle cells shows that a few contain melanosomes and most show a fibroblastic, neurotrrophic or myofibroblstaic transformation. The dermal component of DM is usually negative for Melan A and Human melanoma black HMB45. Both the epidermal component and dermal atypical spindle melanocytes are positive for S100 immunostain.[11] Immunohistochemical evaluation shows S 100 positivity in 93% cases while HMB 45 only in 7% cases.[12]
Despite the improvement of surgical techniques and the introduction of new chemotherapeutic agents, prognosis of this malignancy remains poor. The generally advanced stage of the tumor at initial diagnosis leads to a poorer survival of patients with mucosal melanomas as compared with patients with cutaneous melanomas and presence of vertical growth phase are associated with median survival rate.[13]
The prognosis for oral melanoma is poor with an overall median survival of about 2 years and 5-year survival of less than 20%. Stage is a predictor of survival but even localized tumors (stage I) show a 5-year survival of less than 50%. Depth of invasion (Breslow thickness and Clark's levels) is of limited value in oral lesions. Nevertheless, lesions thicker than 5 mm may have a significantly worse prognosis. Other factors associated with poor prognosis include, vascular invasion, necrosis, a polymorphous tumor cell population, and increasing age.[5]
DM has a propensity for neurotropic metastases, which may lead to local recurrence despite excision with apparently disease-free margins.[14] Recurrences are common especially after incomplete excision, marginal excision < 10 mm or if neurotropism is present. The conflicting results regarding the risk of regional node field metastases and prognosis of DM patients may be due to a heterogeneity of tumors classified as DM and failure to account for tumor thickness. Regional nodal metastases appear to very uncommon in paucicellular DMs with prominent fibrosis and are associated with longer survival. Otherwise, disease free survival rates are similar to other melanomas of comparable thickness. Neurotropism, HMB45 positivity, high mitotic rate, male gender, thickness, ulceration and site all appear to affect survival which overall is 79% at 5 years. Of patients with a recurrence, 78.2% experienced it within 2 years. Wide local excision is the treatment of choice. Radiation therapy has been effective in some cases.[8]
CONCLUSIONS
Oral mucosal melanomas are rare oral malignancies. Vigilant comprehensive analysis of published cases and recognition of new ones may be helpful in establishing definite classification and proposing clinical features that would facilitate its early diagnosis, as a prerequisite for timely treatment and better prognosis of this rare pathology.[15]
Melanomas in the palate and the maxillary alveolar ridge may sometimes present as a polypoid mass, however the presentation as a pedunculated mass in extremely rare, with only a few reported cases, as present in this case. In addition to the pedunculated morphology, the present case showed a tumor mass, comprising of a predominant spindle cell component and fibrosis suggestive a spindle cell-desmoplastic variant of malignant melanoma. The unusual clinic-pathologic presentation of the case and the conflicting biologic behavior makes it essential that such cases be reported and studied further to evaluate their long term behavior and prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.van der Waal RI, Snow GB, Karim AB, van der Waal I. Primary malignant melanoma of the oral cavity: A review of eight cases. Br Dent J. 1994;176:185–8. doi: 10.1038/sj.bdj.4808406. [DOI] [PubMed] [Google Scholar]
- 2.Neville BD, Damm DD, Carl MA, Bouquot JE. Melanoma-Epithelial Pathology. 2nd ed. Philadelphia: Saunders; 2005. pp. 376–80. [Google Scholar]
- 3.Gu GM, Epstein JB, Morton TH., Jr Intraoral melanoma: Long-term follow-up and implication for dental clinicians. A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:404–13. doi: 10.1016/s1079-2104(03)00320-2. [DOI] [PubMed] [Google Scholar]
- 4.Hicks MJ, Flaitz CM. Oral mucosal melanoma: Epidemiology and pathobiology. Oral Oncol. 2000;36:152–69. doi: 10.1016/s1368-8375(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 5.Speight PM. Lyon: IARC Press; 2005. Mucosal malignant melanoma. World Health Organization Classification of Tumours, Pathology and Genetics of Head and Neck Tumours; pp. 206–7. [Google Scholar]
- 6.Uchiyama Y, Murakami S, Kawai T, Ishida T, Fuchihata H. Primary malignant melanoma in the oral mucosal membrane with metastasis in the cervical lymph node: MR appearance. AJNR Am J Neuroradiol. 1998;19:954–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff K, Johnson RA, Suurmond D. United States: The McGraw-Hill Companies; 2009. Melanoma precursors and primary cutaneous melanoma Fitzpatrick's color atlas and synopsis of clinical dermatology. Part I: Disorders presenting in the skin and mucous membranes-section 12; pp. 300–28. [Google Scholar]
- 8.McCarthy SW, Crotty KA, Scolyer RA. Pathology and genetics of skin tumours. Lyon: IARC Press; 2006. Desmoplastic melanoma and desmoplastic neurotropic melanoma. World Health Organization Classification of Tumours; pp. 76–8. [Google Scholar]
- 9.Manganoni AM, Farisoglio C, Bassissi S, Braga D, Facchetti F, Ungari M, et al. Desmoplastic melanoma: Report of 5 cases. Dermatol Res Pract. doi: 10.1155/2009/679010. doi: 10.1155/2009/679010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livestro DP, Muzikansky A, Kaine EM, Flotte TJ, Sober AJ, Mihm MC, Jr, et al. Biology of desmoplastic melanoma: A case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739–46. doi: 10.1200/JCO.2005.04.515. [DOI] [PubMed] [Google Scholar]
- 11.Bandarchi B, Ma L, Navab R, Seth A, Rasty G. From melanocyte to metastatic malignant melanoma. Dermatol Res Pract 2010. 2010 doi: 10.1155/2010/583748. pii: 583748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perniciaro C. Dermatopathologic variants of malignant melanoma. Mayo Clin Proc. 1997;72:273–9. doi: 10.4065/72.3.273. [DOI] [PubMed] [Google Scholar]
- 13.Bhullar RP, Bhullar A, Vanaki SS, Puranik RS, Sudhakara M, Kamat MS. Primary melanoma of oral mucosa: A case report and review of literature. Dent Res J (Isfahan) 2012;9:353–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Hasney C, Butcher RB, 2nd, Amedee RG. Malignant melanoma of the head and neck: A brief review of pathophysiology, current staging, and management. Ochsner J. 2008;8:181–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Bindal R, Shetty DC, Singh HP. Primary oral malignant melanoma: Clinicopathological series of four cases. Dent Res J (Isfahan) 2012;9:338–44. [PMC free article] [PubMed] [Google Scholar]


