Abstract
Heart disease is a major cause of morbidity and mortality in the developed world. Patient recovery after cardiac injury is hampered by the extremely limited regenerative capacity of the adult mammalian heart. In addition to cell-based approaches and in situ cardiac reprogramming, significant interest has been focused on genetic and small molecule–based strategies to enhance endogenous cardiomyocyte proliferative potential. A recent study in Nature suggests, for the first time, that microRNAs may have the ability to induce cardiomyocyte proliferation and cardiac regeneration in adult mice.
The overwhelming majority of cardiomyocytes in the mammalian heart lose their proliferative capacity shortly after birth. Acute or chronic injury to the myocardium often results in extensive loss of cardiomyocytes and a non-regenerative healing response characterized by scar formation, both of which contribute to a permanent loss of cardiac function. Despite some evidence that the rate of cardiomyocyte renewal may increase slightly after injury,1 this response is insufficient to replace the approximately 1 billion cardiomyocytes that may be lost during a typical myocardial infarction. The failure of cardiac repair results in progressive cardiac dysfunction, and individuals suffering from end-stage heart failure are currently limited to orthotopic cardiac transplant. It is, therefore, of great clinical importance to develop therapeutic strategies that could enhance the normal regenerative potential of the adult mammalian heart.
Recently, a study published in Nature by Eulalio et al.2 took a novel approach to this problem. Using a screening approach, the authors interrogated the potential of a class of genes called microRNAs (miRNAs) to induce cell-cycle reentry in postnatal cardiomyocytes. miRNAs are small non-coding RNAs that negatively regulate the translation or stability of their target mRNAs. While miRNA targeting of mRNAs occurs in a sequence-specific manner, perfect base-pair complementarity is not required for effective silencing. Thus, a single miRNA may have hundreds of cellular targets, making them powerful regulators of myriad biological processes. Eulalio et al. showed that administration of several different miRNA species in multiple contexts resulted in cardiomyocyte proliferation and cardiac regeneration. Initially, they screened 875 miRNA mimics for ones that could enhance proliferation in primary rat neonatal cardiomyocytes. Surprisingly, they identified 204 miRNAs that increased proliferation more than two-fold over a control mimic. Of the identified miRNAs, roughly 20 % (40) also enhanced proliferation in mouse neonatal cardiomyocytes.
For further characterization and in vivo studies, the authors selected two candidates, miR-199a-3p and miR-590-3p, that most effectively promoted proliferation in the mouse and rat studies, respectively. When introduced into the neonatal rat heart, these miRNAs induced cardiomyocyte hyperplasia. A comparable effect was observed when cardiotropic viral vectors encoding the miRNAs were administered systemically to neonatal mice. Perhaps more excitingly, each of the two miRNAs promoted cardiac regeneration in an adult mouse model of myocardial infarction. When viruses encoding miR-199-3p or miR-590-3p were injected in the peri-infarct area immediately after ligation of the left anterior descending coronary artery, the authors observed a dramatic decrease in subsequent scar size as well as impressive functional improvement, in comparison with animals treated with a control miRNA. Assuming these results are reproducible, the findings have exciting scientific and therapeutic implications.
Over the last decade, a growing body of work has challenged the once held view that the mammalian heart completely lacks regenerative capabilities. Studies utilizing radiocarbon isotope dating suggest that the normal turnover rate of cardiomyocytes in the human heart hovers around 1% for young adults.3 While studies using alternate techniques estimate turnover rates to be even higher.4 Thus, while mammals may lack the robust regenerative abilities seen in amphibians and teleost fishes, the adult mammalian heart slowly but steadily renews itself.
More recently, Porrello et al.5 showed that neonatal mammals mount a regenerative response after cardiac injury more akin to lower vertebrates than adult mammals. This study demonstrated that neonatal mice fully regenerate portions of their ventricles after resection and that this response is lost within the first postnatal week. Furthermore, the loss of regenerative potential is correlated with an upregulation in the expression of miR-15 family members6 highlighting the powerful role miRNAs may play in repressing cardiomyocyte proliferation in the neonatal heart.
These findings lead to an interesting paradigm in which the regenerative potential of a cardiomyocyte is not so much species-specific as it is age-restricted. It also poses an interesting biological question. Can mature adult cardiomyocytes regain the regenerative properties of immature cardiomyocytes? The work of Eulalio et al. is insightful because it demonstrates that normal mechanisms that restrict the proliferative potential of cardiomyocytes can be overcome by forced expression of a single miRNA, suggesting that, in fact, mature cardiomyocytes can be coaxed into a more “immature” or regenerative state.
The in vitro data presented by Eulalio et al. suggest that the regenerative effect of the miRNAs is due to their effect on cardiomyocytes and not other cardiac cell types. However, controversy remains about the source of new cardiomyocytes during normal turnover as well as post-injury in vivo. While some contribution of a resident cardiac stem cell has not been ruled out, several recent studies have shown that a majority of new cardiomyocytes in the adult heart are derived from pre-existing cardiomyocytes, further challenging the notion that adult cardiomyocytes have permanently exited the cell cycle. These findings are consistent with the model proposed in neonatal mouse cardiac regeneration, as well as that in adult zebrafish, where cardiac regeneration is now thought to occur through proliferation of existing cardiomyocytes.7,8 While the exogenous expression of the miRNAs described by Eulalio et al. promoted impressive recovery of cardiac function post-infarction, it will be important to definitively determine which target cell type in the adult heart is responsible for mediating the regenerative effect through careful lineage tracing experiments.
miRNAs have been optimized by evolution to be highly efficient regulators of many biological processes. Not only can a single miRNA target multiple genes within a single pathway, but multiple pathways within a greater network may be coordinately regulated. It has been appreciated for several years that, in the mammalian heart, precise expression patterns and dosages of miRNAs are required for normal development and homeostasis9–11 (reviewed in 12,13). Additionally, the introduction of several miRNAs in combination is sufficient to reprogram non-myocyte fibroblasts into a cardiomyocyte-like cell,14 demonstrating that miRNAs are not only powerful regulators of normal cardiac function but also, like transcription factors, can regulate major cell fate decisions.
To determine which genes may be contributing to the enhanced cardiomyocyte proliferation seen upon overexpression of miR-199a-3p or miR-590-3p, the authors performed an RNA sequencing analysis and compared the expression patterns of cardiomyocytes with or without expression of the candidate miRNA. An siRNA screen of putative target genes that were down-regulated (>600 genes targeted) revealed 45 candidates whose downregulation alone was sufficient to reactivate the cell-cycle in neonatal cardiomyocytes to some extent. Notably, no single candidate gene knockdown was as effective as miRNA overexpression in promoting proliferation, suggesting that the identified miRNAs likely mediate their effects through the combined targeting of multiple genes. Interestingly, only three of the miR-199a-3p and miR-590-3p target genes overlapped: Homer1, Hopx and Clic5. While, Hopx is known to inhibit embryonic cardiomyocyte proliferation (reviewed in 15), Homer1 and Clic5 have not previously been implicated in cardiomyocyte proliferation. Future studies will be required to determine the mechanisms by which these genes normally act to restrict proliferation in the adult cardiomyocyte.
In conclusion, the work presented by Eulalio et al. is notable because it adds to the growing body of work suggesting that the regenerative capacity of the heart can be enhanced in vivo. Moreover, the authors identified specific miRNA sequences and their downstream targets that can promote regeneration. Like most ground-breaking studies, this work raises many new questions. What is the mechanism of cell cycle re-entry? How is the number of cell divisions controlled? Can we be certain that the new myocytes are derived from pre-existing ones in the absence of rigorous lineage tracing experiments? Do the findings translate to the human system? While these and other questions will undoubtedly be explored in the future, miR-199-3p and miR-590-3p and their targets may prove interesting therapeutic targets and further work on these miRNAs may indicate scientific avenues to explore for cardiac regenerative approaches.
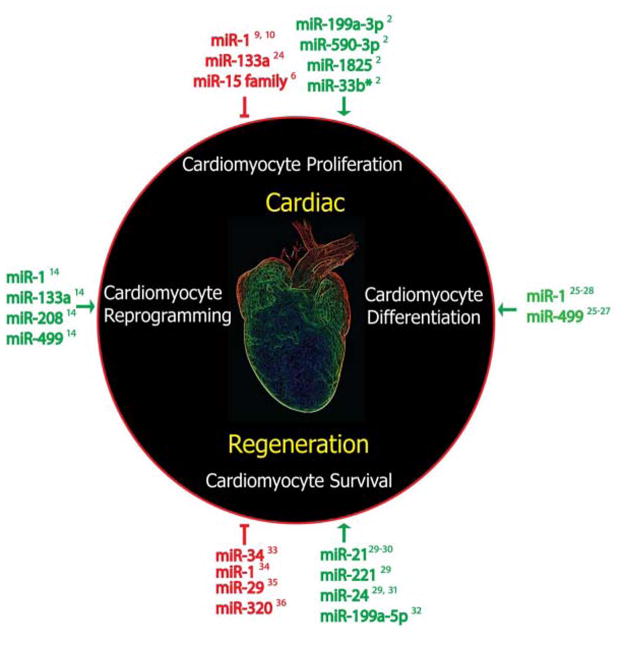
It is gratifying that, with new tools at our disposal, we are beginning to attack heart disease from multiple angles, including the induction of cardiomyocyte proliferation and survival16–18, cardiomyocyte reprogramming14,19–21, and stimulation of endogenous repair mechanisms through cell therapy.22,23 Furthermore miRNAs represent a novel class of biological tools implicated in the regulation of many of these regenerative strategies (Figure 1)24–36 With the recent successes, there is every reason to be hopeful that someday patients with end-stage heart failure will have more options than the highly limited one of heart transplant.
Figure 1. MicroRNA regulation of cardiac regeneration.
miRNAs regulate many processes in cardiac regeneration, including cardiomyocyte proliferation, differentiation, survival, and reprogramming.
Acknowledgments
The authors are grateful to G. Howard and B. Taylor for editorial services.
Sources of Funding
D.S. was supported by grants from NHLBI/NIH (U01 HL098179, R01 HL057181, P01 HL089707), the California Institute for Regenerative Medicine (CIRM), the William Younger Family Foundation, the L.K. Whittier Foundation, and the Eugene Roddenberry Foundation. A.J.H. is supported by the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Disclosures
None
References
- 1.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eulalio A, Mano M, Ferro MD, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the Adult Human Heart. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam Y-J, Matkovich SJ, Dorn GW, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circulation Research. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DYR, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jopling C, Sleep E, Raya M, MEM, Raya A, Belmonte JCIUA. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Ransom JF, Li A, Vedantham V, Drehle von M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of Cardiogenesis, Cardiac Conduction, and Cell Cycle in Mice Lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature Publishing Group. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 12.Cordes KR, Srivastava D. MicroRNA Regulation of Cardiovascular Development. Circulation Research. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circulation Research. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circulation Research. 2012 doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kook H, Epstein JA. Hopping to the beat. Hop regulation of cardiac gene expression. Trends in Cardiovascular Medicine. 2003;13:261–264. doi: 10.1016/s1050-1738(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 16.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Kühn B, del Monte F, Hajjar RJ, Chang Y-S, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 18.Engel FB, Hsieh PCH, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proceedings of the National Academy of Sciences. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ieda M, Fu J-D, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu J-D, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 23.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes & Development. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluijter JPG, van Mil A, van Vliet P, Metz CHG, Liu J, Doevendans PA, Goumans MJ. MicroRNA-1 and -499 Regulate Differentiation and Proliferation in Human-Derived Cardiomyocyte Progenitor Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 26.Wilson KD, Hu S, Venkatasubrahmanyam S, Fu J-D, Sun N, Abilez OJ, Baugh JJA, Jia F, Ghosh Z, Li RA, Butte AJ, Wu JC. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circ Cardiovasc Genet. 2010;3:426–435. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J-D, Rushing SN, Lieu DK, Chan CW, Kong C-W, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ, Li RA. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2011;6:e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivey KN, Muth A, Arnold J, King FW, Yeh R-F, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. MicroRNA Regulation of Cell Lineages in Mouse and Human Embryonic Stem Cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu S, Huang M, Nguyen PK, Gong Y, Li Z, Jia F, Lan F, Liu J, Nag D, Robbins RC, Wu JC. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124:S27–34. doi: 10.1161/CIRCULATIONAHA.111.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. Journal of Biological Chemistry. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian L, van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. Journal of Experimental Medicine. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circulation Research. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, Horrevoets AJG, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Müller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013 doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 34.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, Wang J, Xie L, Lu Y, Yang B. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovascular Research. 2010;87:535–544. doi: 10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- 36.Ren X-P, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan G-C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]