Abstract
OBJECTIVES
To evaluate objective physical performance measures as predictors of survival and subsequent disability in older patients with cancer.
DESIGN
Longitudinal cohort study.
SETTING
Health, Aging and Body Composition (Health ABC) Study.
PARTICIPANTS
Four hundred twenty-nine individuals diagnosed with cancer during the first 6 years of follow-up of the Health ABC Study.
MEASUREMENTS
The associations between precancer measures of physical performance (20-m usual gait speed, 400-m long-distance corridor walk (LDCW), and grip strength) and overall survival and a short-term outcome of 2-year progression to disability or death were evaluated. Cox proportional hazards and logistic regression models, stratified for metastatic disease, respectively, were used for outcomes.
RESULTS
Mean age was 77.2, 36.1% were women, and 45.7% were black. Faster 20-m usual walking speed was associated with a lower risk of death in the metastatic group (hazard ratio = 0.89, 95% confidence interval (CI) = 0.79–0.99) and lower 2-year progression to disability or death in the nonmetastatic group (odds ratio (OR) = 0.77, 95% CI = 0.64–0.94). Ability to complete the 400-m LDCW was associated with lower 2-year progression to disability or death in the nonmetastatic group (OR = 0.24, 95% CI = 0.10–0.62). There were no associations between grip strength and disability or death.
CONCLUSION
Lower extremity physical performance tests (usual gait speed and 400-m LDCW) were associated with survival and 2-year progression to disability or death. Objective physical performance measures may help inform pretreatment evaluations in older adults with cancer.
Keywords: physical performance, elderly, cancer, disability, survival
Approximately 60% of cancers are diagnosed in persons aged 65 and older.1 In general, older adults with cancer have lower survival and higher morbidity with treatment than younger people.2 Some older cancer patients can benefit from intensive therapies,3,4 but there is limited information regarding the clinical characteristics that influence outcomes.
Although clinical outcomes vary based on tumor type and treatment, patient-specific characteristics are also important predictors of survival and tolerance of therapy.5–8 Identifying specific measurable characteristics to better predict acute treatment-related toxicity, future disability, and survival is critical to inform decision-making for older cancer patients.9,10 Physical function is a key element in the evaluation of frailty in geriatric populations and may help predict clinical outcomes in older patients with cancer.11
In oncology practice, physical function is typically estimated using the Karnofsky or Eastern Cooperative Oncology Group (ECOG) performance scales.12 These scales lack sensitivity, are subjective, and do not address specific physical tasks. Many older adults with “good” performance scores have meaningful impairments in physical function that may reduce reserve capacity.13 Whereas these scales are predictive of outcomes in older adults with substantial functional impairment at diagnosis,14,15 they are less useful for most older patients with cancer, who do not have substantial disability at the time of cancer diagnoses.8,13 Thus, more-sensitive, -objective, and -task-focused measures of physical function are needed for this population.
Physical performance measures (e.g., walking speed and grip strength) predict future disability, hospitalizations, and mortality in the general geriatric population.16–19 Assessment of physical performance to evaluate frailty is currently included in the National Comprehensive Cancer Network Guidelines for Senior Adult Oncology,20 but the predictive value of these tests in older patients with cancer has not been evaluated. In the present analysis, it was hypothesized that measures of physical performance (usual walking speed over 20 m, 400-m long-distance corridor walk (LDCW), and grip strength) obtained close to the time of cancer diagnosis in older adults with malignancy would predict overall survival and short-term outcome (2-year progression to disability or death).
METHODS
Setting
The Health, Aging and Body Composition (Health ABC) Study is an observational study designed to investigate the effect of changes in body composition on physical function and the subsequent development of disability in healthy older adults.21–23 The study enrolled 3,075 well-functioning black and white community-dwelling older adults. Participants were recruited from a random sample of white Medicare beneficiaries and all age-eligible black residents in designated ZIP code areas in and around Pittsburgh, Pennsylvania, and Memphis, Tennessee, between March 1997 and July 1998. Eligibility criteria for the Health ABC Study were aged 70 to 79; no difficulty performing activities of daily living, walking one-quarter or a mile, or climbing 10 steps without resting; no reported need of assistive devices (e.g., cane, walker); no active treatment for cancer in the prior 3 years; no life-threatening illness; and no plans to leave the area for 3 years. All participants provided written informed consent. The institutional review boards of the study sites approved the protocol.
Study participants were contacted every 6 months by telephone or in person and interviewed about health status, hospitalizations, outpatient procedures, and new cancer diagnoses. Information regarding incident cancer diagnoses was also obtained from hospital or clinic records.
Study Population
Four hundred thirty-one 431 adjudicated cancer diagnoses in the Health ABC cohort were identified during the first 6 years of follow-up. Cancer diagnoses were confirmed according to pathology reports or other medical record information. Two participants were excluded from analyses because of lack of follow-up data.
Measures
Measures of Physical Performance
For all physical performance tests, values from the visit most closely preceding cancer diagnosis were used as baseline for each participant to most closely approximate physical performance testing at the time of diagnosis. The mean times between measurement of physical performance and cancer diagnosis for 20-m gait speed, 400-m LDCW, and grip strength were 9.5, 18.1, and 10.3 months, respectively.
Gait speed was assessed annually during the Health ABC Study, with participants instructed to walk at their usual pace over a 20-m course. For multivariable analyses, 20-m gait speed was assessed as a continuous variable using 0.1 m/s (m/s) as the unit of change.24
The 400-m LDCW was performed during Years 1, 2, 4, and 6 of the Health ABC Study.22,23 Participants were instructed to walk 400m in a corridor on a 20-m-per-segment course for 10 laps after a 2-minute warm-up. Instructions were to “walk as quickly as you can, without running, at a pace you can maintain.” Medical exclusions included potentially acute electrocardiogram abnormalities, uncontrolled hypertension (≥200/110 mmHg), resting heart rate greater than 120 beats per minute or less than 40 beats per minute, recent exacerbation of chest pain, shortness of breath, or recent cardiac event or procedure. Participants could stop during the test for fatigue or other symptoms or tachycardia (>135 beats per minute) according to a heart monitor. For all analyses, the 400-m LDCW was evaluated as a categorical variable (excluded, stopped, completed).22
Isometric grip strength was measured during Years 1, 2, 4, and 6 of the Health ABC Study using a hand-held dynamometer (JAMAR Technologies, Inc., Hatfield, PA). Two trials were performed for each hand. An average of the trials performed on the strongest hand was used for analyses. Grip strength was analyzed as a continuous variable for all multivariate models, using 10 kg as the unit of change.
Outcome Measures
The primary outcome for this analysis was overall survival from the date of cancer diagnosis. The secondary outcome was a combined measure of functional decline, which captures progression to disability or death within 2 years after cancer diagnosis. This variable was intended to capture a clinically relevant decline in functional status over a short period. Disability was defined as requiring a cane or walker for ambulation, self-reported inability to walk one-quarter of a mile or climb 10 steps, or requiring assistance with an activity of daily living (transfer, dressing, or bathing).
Participant follow-up was conducted every 6 months (in-person examinations alternating with telephone follow-up). A Health ABC committee adjudicated hospital records, death certificates, informant interviews, and autopsy data to confirm causes of death. Median follow-up time for overall survival was 3.8 years.
Covariates
Any characteristic present at cancer diagnosis that might confound the relationship between physical performance and survival or 2-year progression to disability or death was considered a covariate. For all covariates except demographics and smoking status, the value available that most closely preceded the cancer diagnosis date was used to establish a baseline for each participant.
Data on demographics (age, sex, race, educational level) and health behaviors were collected using standard self-report assessments. Age at cancer diagnosis was used as a continuous variable. Smoking status was classified as current, former, or never. Body mass index (BMI, kg/m2) was calculated yearly from measured height and weight. Physical activity was assessed using a calculated variable for kcal/kg per week total walking based on questionnaire data available yearly.
Comorbid illnesses considered as confounding variables were knee arthritis, depression, diabetes mellitus, cardiovascular disease, pulmonary disease, and cognitive dysfunction. Self-reported knee pain, collected yearly, was used as a surrogate measure for knee arthritis. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression (CES-D) scale,25 collected at Years 1 and 3 to 6. A categorical variable was created using a standard cutoff score of 16 for the 20-item test and 10 for the 10-item test. Cardiovascular disease, including coronary artery disease and stroke, was assessed from self-report and hospitalization records. Diabetes mellitus was assessed from self-report and fasting glucose levels. Pulmonary disease was assessed using forced expiratory volume in 1 second less than 80% of predicted (available at Years 1 and 5). If pulmonary function testing was not conducted, self-reported obstructive pulmonary disease at Year 1 was used. Modified Mini-Mental State Examination score was used as a measure of cognitive function.26 The four most common cancer types (breast, colorectal, prostate, lung) were considered as covariates.
Statistical Analyses
Means and proportions were used to describe the baseline characteristics of the cohort. Proportions were used to describe cancer diagnoses and 2-year progression to disability or death.
Survival Analysis
Overall survival was estimated using the Kaplan-Meier method. The unadjusted association between each physical performance measure (20-m walk, 400-m LDCW, and grip strength) and overall survival was evaluated using the log-rank test. For this analysis, categorical variables were created for 20-m walk and grip strength using a cut-off of 1 m/s and 33 kg, respectively.16 Grip strength tertiles were also evaluated.18
After assessing the proportionality assumption, Cox proportional hazards regression was used to assess the association between each physical performance measure and overall survival. Separate models were fit for metastatic and nonmetastatic disease because of the profound effect of metastatic disease on mortality.
All covariates were evaluated separately as potential confounders before inclusion in the analysis. P < .25 was used for inclusion of covariates in the final model. Depressive symptoms, BMI, and knee pain were not associated with survival and were excluded from the final Cox proportional hazards regression models.
Two models are presented for each performance measure, stratified according to presence of metastatic disease: unadjusted and adjusted for covariates. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported.
Analysis of 2-Year Progression to Disability or Death
Logistic regression was used to investigate the relationship between physical performance measures and 2-year progression to disability or death. Twenty-six participants were excluded because of missing disability data at baseline or at the 2-year follow-up. An additional 32 subjects were excluded because of baseline disability.
Physical activity, knee pain, CES-D score, and BMI were not associated with 2-year progression to disability or death in univariate analyses and were not included in the final multivariable logistic regression models.
Odds ratios (ORs), with 95% CIs, were estimated for each performance measure and presented in unadjusted and fully adjusted models. Goodness of fit for each model was tested using the Hosmer-Lemershow test.27 All analyses were conducted using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline characteristics for the study cohort are presented in Table 1. The four most common cancer types made up the majority (61.7%) of cases. Metastatic disease was present in 37.1% at the time of diagnosis.
Table 1.
Participant Characteristics Preceding Cancer Diagnosis
| Characteristic | Total Cohort (n = 429) |
Nonmetastatic (n = 268)* |
Metastatic (n = 159) |
|---|---|---|---|
| Demographic | |||
| Age at diagnosis, mean ± SD | 77.2 ± 3.3 | 77.1 ± 3.3 | 77.5 ± 3.4 |
| Black, % | 45.7 | 42.9 | 50.3 |
| Female, % | 36.1 | 32.8 | 41.5 |
| Education, % | |||
| Less than high school | 24.8 | 20.2 | 33.1 |
| High school graduate | 30.4 | 27.6 | 34.4 |
| Postsecondary | 44.7 | 52.2 | 32.5 |
| Health status | |||
| Diabetes mellitus, % | 22.1 | 25.6 | 21.0 |
| Cardiovascular disease, % | 32.9 | 30.2 | 37.1 |
| Knee pain, % | 21.0 | 21.6 | 20.1 |
| Impaired pulmonary function, % | 25.9 | 23.9 | 28.9 |
| Depression, % | 8.0 | 8.2 | 7.6 |
| Smoking, % | |||
| Never | 34.5 | 35.1 | 33.3 |
| Current | 12.4 | 11.6 | 13.8 |
| Former | 53.2 | 53.4 | 52.8 |
| Body mass index, kg/m2, mean ± SD | 27.0 ± 4.7 | 26.7 ± 4.4 | 27.5 ± 5.1 |
| Functional status | |||
| 20-m gait speed, m/s, mean ± SD | 1.17 ± 0.2 | 1.20 ± 0.2 | 1.12 ± 0.2 |
| Grip strength, kg, mean ± SD | 33.4 ± 11.9 | 34.0 ± 12.1 | 32.4 ± 11.4 |
| 400-m walk, % | |||
| Excluded | 24.1 | 25.3 | 21.9 |
| Stopped | 13.7 | 13.4 | 14.4 |
| Completed | 62.2 | 61.3 | 63.7 |
| Physically active, %† | 53.2 | 51.7 | 55.6 |
| Modified Mini-Mental State Examination score, mean ± SD (range 0–100) | 89.4 ± 8.4 | 90.2 ± 8.5 | 88.2 ± 8.0 |
| Self-reported disability, %‡ | 7.5 | 7.5 | 7.5 |
| Incident cancer site of origin, % | |||
| Prostate | 23.2 | 29.0 | 13.8 |
| Colorectal | 14.6 | 10.8 | 21.3 |
| Lung | 12.5 | 8.9 | 18.8 |
| Breast | 11.4 | 13.8 | 7.5 |
| Other | 38.3 | 37.5 | 38.6 |
Metastatic disease status was unknown in two participants.
Physical activity was recorded as kcal/kg per week of walking; participants who reported any physical activity are presented as physically active.
Self-reported disability defined as requiring assistance with transfers, bathing, or dressing or reporting difficulty walking one-quarter of a mile or walking up a flight of stairs.
SD = standard deviation.
The overall median survival time for the entire cohort was 4.6 years (7.7 years for nonmetastatic and 1.1 years for metastatic). The proportion of participants who progressed to disability or death within 2 years after cancer diagnosis was 45.7% in the overall cohort (33.6% nonmetastatic and 66.2% metastatic).
Survival Analysis
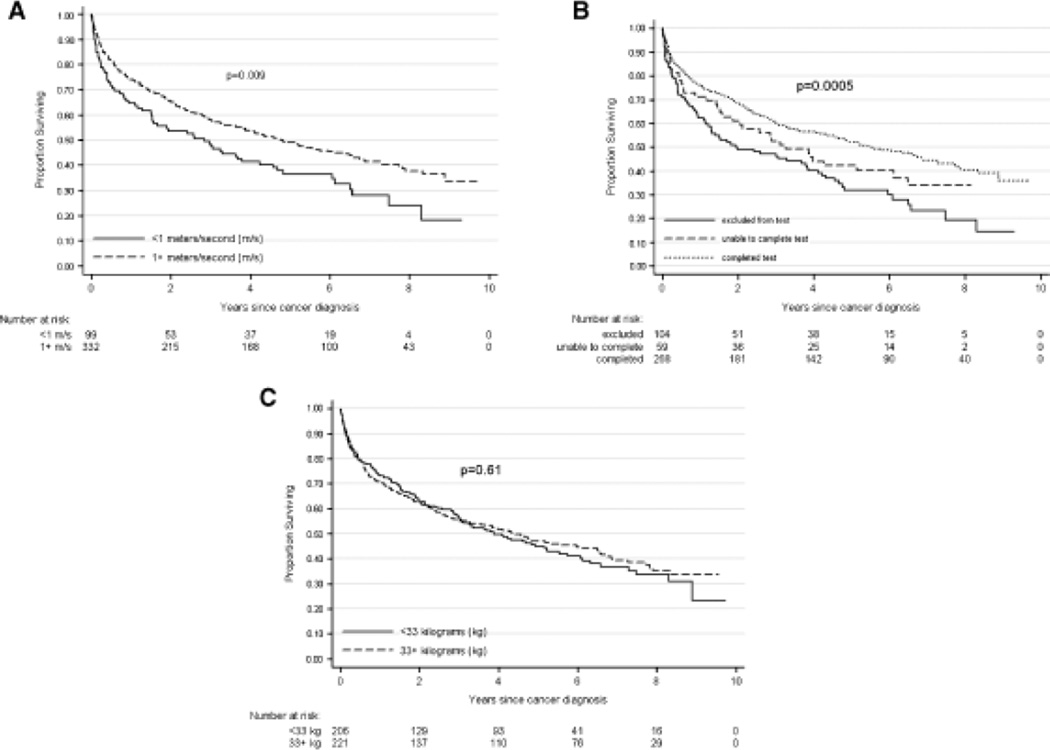
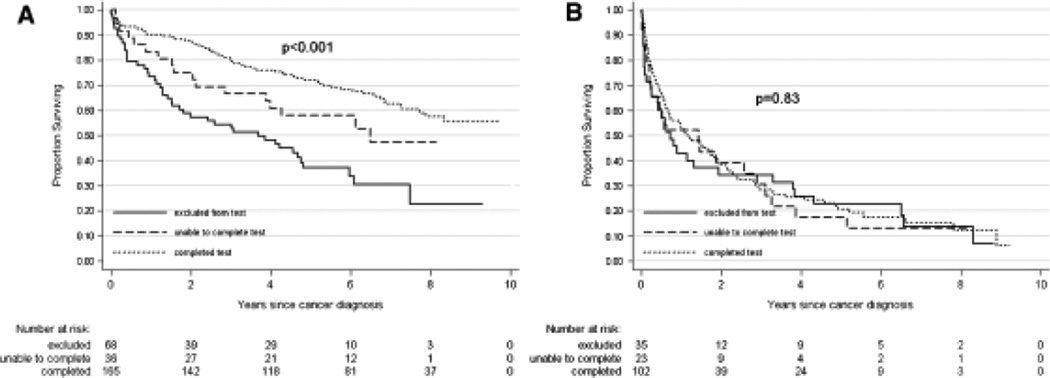
The unadjusted association between each physical performance measure and overall survival is illustrated in Figure 1A–C. The 20-m walk and 400-m LDCW were associated with overall survival, whereas grip strength was not. Results did not change when evaluating survival according to grip strength tertiles (data not shown). Both measures were significantly associated with survival in the nonmetastatic cohort but not in the metastatic cohort, when evaluated as categorical variables. Figure 2 illustrates the results for the 400-m LDCW in participants with and without metastasis.
Figure 1.
Association between physical performance measures and overall survival in older adults with cancer. (A) Overall survival stratified according to gait speed on the 20-m walk test. (B) Overall survival stratified according to ability to complete the 400-m long-distance corridor walk. (C) Overall survival stratified according to grip strength. Log-rank P-value is presented with each Kaplan-Meier curve.
Figure 2.
Association between ability to complete the 400-m long-distance corridor walk and overall survival, stratified according to diagnosis of nonmetastatic (A) versus metastatic (B) disease in older adults with cancer. Log-rank P-value is presented with each Kaplan-Meier curve.
A faster pace on the 20-m walk test was associated with lower risk of death in unadjusted analyses for the metastatic and nonmetastatic groups (Table 2, Cox proportional hazards regression). This association was attenuated in the nonmetastatic group after adjusting for covariates, although in the metastatic group, faster walking speed remained statistically associated with survival after adjustment. In this model, a 0.1-m/s faster walking speed was associated with an 11% reduction in the risk of death (HR = 0.89, 95% CI = 0.79–0.99).
Table 2.
Association Between Physical Performance Measures and Overall Survival After Cancer Diagnosis
| Nonmetastatic | Metastatic | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| Performance Measure | N | Unadjusted | Adjusted* | N | Unadjusted | Adjusted* |
| 20-m gait speed (per 0.1 m/s) | 257 | 0.91 (0.84–0.98) | 1.02 (0.92–1.13) | 153 | 0.91 (0.84–0.99) | 0.89 (0.79–0.99) |
| 400-m long-distance corridor walk | 264 | 157 | ||||
| Stopped | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Excluded | 1.7 (0.97–3.00) | 0.98 (0.5–1.90) | 0.97 (0.55–1.72) | 1.10 (0.57–2.13) | ||
| Completed | 0.64 (0.37–1.10) | 0.56 (0.31–1.01) | 0.87 (0.53–1.42) | 1.01 (0.54–1.89) | ||
| Grip strength (per 10 kg) | 263 | 0.96 (0.83–1.12) | 1.03 (0.81–1.33) | 156 | 0.93 (0.80–1.09) | 0.93 (0.73–1.19) |
The full models included demographics, smoking status, physical activity, major disability at baseline, cognitive screen (modified Mini-Mental State Examination score), diabetes mellitus, cardiovascular disease, obstructive pulmonary disease, and cancer type (breast, lung, prostate, colorectal).
HR = hazard ratio; CI = confidence interval.
In the nonmetastatic group, subjects who completed the 400-m LDCW without stopping had a lower hazard of death than those who stopped during the test. This association persisted after adjustment (HR = 0.56, 95% CI = 0.31–1.01). In the metastatic group, there was no statistically significant difference in the hazard of death between those who were excluded, stopped during the test, and completed the test.
There was no statistically significant association between grip strength and survival in the nonmetastatic or metastatic group.
2-Year Progression to Disability or Death
A 0.1-m/s faster usual gait speed on the 20-m course was associated with a 23% lower odds of progression to disability or death at 2 years in the nonmetastatic group after adjustment (OR = 0.77, 95% CI = 0.64–0.94) (Table 3). In addition, individuals who completed the 400-m LDCW had 76% lower odds of 2-year progression to disability or death than those who stopped during the test (OR = 0.24, 95% CI = 0.10–0.62). Results were not statistically significant in adjusted analyses for the metastatic group (OR = 0.33, 95% CI = 0.07–1.67). There was no association between grip strength before cancer diagnosis and subsequent 2-year progression to disability or death.
Table 3.
Association Between Physical Performance Measures and Progression to Disability or Death 2 Years After Cancer Diagnosis
| Nonmetastatic | Metastatic | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||||
| Performance Measure | N | Unadjusted | Adjusted* | N | Unadjusted | Adjusted* |
| 20-m gait speed (per 0.1 m/s) | 232 | 0.75 (0.65–0.87) | 0.77 (0.64–0.94) | 131 | 0.88 (0.74–1.06) | 0.88 (0.70–1.12) |
| 400-m long-distance corridor walk | 237 | 133 | ||||
| Stopped | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Excluded | 1.5 (0.40–2.20) | 0.88 (0.31–2.50) | 0.45 (0.11–1.92) | 0.46 (0.07–2.92) | ||
| Completed | 0.26 (0.20–0.60) | 0.24 (0.10–0.62) | 0.26 (0.07–0.95) | 0.33 (0.07–1.67) | ||
| Grip strength (per 10 kg) | 236 | 0.90 (0.71–1.13) | 0.83 (0.56–1.25) | 133 | 0.86 (0.64–1.17) | 0.85 (0.47–1.52) |
The final model was adjusted for demographics (age, sex, race, and education level), obstructive pulmonary disease, cardiovascular disease, diabetes mellitus, cognitive screen (modified Mini-Mental State Examination score), smoking status, and cancer type (breast, colorectal, prostate, lung).
OR = odds ratio; CI = confidence interval.
DISCUSSION
In this study, measures of lower extremity physical performance predicted overall survival and 2-year progression to disability or death in older adults with cancer. A faster 20-m usual gait speed was associated with greater overall survival in subjects with metastatic disease. Alternatively, completion of the 400-m LDCW was associated with greater overall survival in nonmetastatic subjects. Higher performance on the 20-m usual gait speed and the 400-m LDCW predicted lower risk of progression to disability or death 2 years after diagnosis in the nonmetastatic subjects only. No association was found between grip strength and overall survival or 2-year progression to disability or death in this study.
This study suggests that assessment of select lower extremity performance measures may help predict survival and future disability in older adults with a new cancer diagnosis. These measures may provide objective evidence of an older adult’s reserve capacity, which multiple factors, including chronic disease, physiological changes of aging, nutrition, fitness, psychosocial well-being, and motivation, influence. Some of these factors can be difficult to assess clinically. Thus, physical performance could provide insight into an older adult’s resilience after a new cancer diagnosis.
Overall, these results are consistent with previous reports in geriatric populations that physical performance measures of lower extremity function predict future disability and mortality.16,22,28–30 For example, usual gait speed has been a powerful predictive measure in multiple studies and more accurately predicts decline in physical function and mortality than self-report measures.30,31 In addition, gait speed alone may perform as well as a summary measure of gait speed, balance, and chair stands, making this an attractive screening test.31
The lack of association with handgrip and either outcome is not consistent with most of the literature in other elderly populations.18,29,32,33 Most prior studies have evaluated the relationship between grip strength and all-cause mortality. One explanation for the results of the current study is that the association between hand grip and mortality may be altered in the presence of specific medical conditions such as cancer. No prospective studies have evaluated grip strength in a population restricted to cancer patients, although a study of the relationship between grip strength and cause-specific mortality in 919 disabled women in the Women’s Health and Aging cohort found no association between handgrip and cancer mortality.18 Similar results were seen in the Japanese Adult Health Study when evaluating cancer-specific mortality,34 consistent with the results of the current study.
Another explanation may be that grip strength is a better marker of outcomes occurring over decades, as evaluated in most previous studies, rather than outcomes occurring within several years, as evaluated in the current analysis. It is also possible that grip strength may change acutely at the time of cancer diagnosis, as tumor burden increases. In this case, measurements obtained before cancer diagnosis that were available for this analysis may not adequately reflect the predictive value of this performance measure at the time of diagnosis.
Finally, the differing results between performance measures reinforce the notion that each test is measuring a different construct. Grip strength assesses upper extremity strength, whereas the 400-m LDCW and usual gait speed capture cardiopulmonary fitness and mobility, respectively. Strength, fitness, and mobility may not be equally important predictors of susceptibility to toxicity or survival in older patients with cancer. Mobility and cardiopulmonary fitness may be more important markers of resilience to the acute stresses of tumor burden, surgeries, chemotherapy, and radiation. Furthermore, differing results between the metastatic and nonmetastatic groups highlight the variable utility of performance measures in different clinical populations. Subjects with metastatic disease have a high short-term mortality rate associated with tumor burden and repeated treatments. The cancer diagnosis is often terminal, and physical function can be expected to decline more sharply and consistently over time. Declines in functional status related to refractory disease or treatment toxicity subsequently limit additional treatment options. Mobility, as measured using usual gait speed, may predict survival in this population by identifying individuals most susceptible to rapid functional decline, but a diagnosis of nonmetastatic cancer is an acute event from which patients often recover; it may or may not drive mortality. Thus, usual gait speed may reflect susceptibility to short-term disability related to the cancer diagnosis (e.g., tumor burden, surgeries, chemotherapy, and radiation), whereas cardiopulmonary fitness may better predict longer-term overall survival.
This is the first study to evaluate the predictive value of physical performance in a cohort of older patients with cancer. In addition, several attributes of the Health ABC database strengthen the analysis—multiple, repeated performance tests of fitness, strength, and mobility and baseline (pre-diagnosis) measures. The Health ABC cohort also received careful evaluation of health conditions, enabling these measures to be evaluated in the context of comorbidity and health status.
There are also several limitations of this analysis. The small sample size of the metastatic cohort limits statistical power. The high mortality rate also limits evaluation of disability alone as an outcome. The heterogeneity of cancer diagnoses (and thus cancer burden and predicted outcomes) limits generalizability of the findings to other clinical settings. Finally, treatment information was not available for this analysis. This is an important limitation; participants with better physical performance may have been more likely to receive aggressive treatments, which could explain a positive association between physical performance and better clinical outcomes.
This study provides an important first step in supporting the hypothesis that objective physical performance tests may be useful adjuncts to clinical measures in the evaluation of older adults with malignancy. Physical performance testing could improve pretreatment assessments of many older adults with a new cancer diagnosis who present without overt disability. To translate this research into clinical practice, performance measures obtained at the time of cancer diagnosis will need to be evaluated prospectively in a cohort of older adults. The predictive value of these assessments on short-and long-term clinical outcomes will need to be assessed within cancer type, stratifying for stage of disease and controlling for treatments administered. If a simple measure such as gait speed can independently predict toxicity or survival, it could be a valuable addition to standard pretreatment assessment evaluations for older adults with cancer.
ACKNOWLEDGMENTS
Supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106), the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG-021332), Atlantic Philanthropies, American Society of Hematology, John A. Hartford Foundation, and Association of Specialty Professors.
Sponsor’s Role: The funders were not involved in any aspect of study design, data acquisition, data analysis, interpretation of data, or manuscript preparation.
Footnotes
Presented in part at the annual meetings of the American Geriatrics Society, Washington, DC, May 2008; the American Society of Clinical Oncology, Chicago, Illinois, May 2008; and the Society of International Geriatric Oncology, Montreal, Canada, October 2008.
Conflict of interest: The authors have no conflicts of interest to report.
Author Contributions: Heidi D. Klepin: study concept and design, analysis and interpretation of data, preparation of manuscript. Ann M. Geiger: interpretation of data and preparation of manuscript. Janet A. Tooze: analysis and interpretation of data, preparation of manuscript. Anne B. Newman, Lisa H. Colbert, Douglas C. Bauer, Suzanne Satterfield, and Juliessa Pavon: editing of manuscript. Stephen Kritchevsky: data acquisition, interpretation of data, editing of manuscript.
REFERENCES
- 1.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R. Cancer burden in the aged: An epidemiologic and demographic overview. Cancer. 1997;80:1273–1283. [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 5.Extermann M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients? J Clin Oncol. 2000;18:1709–1717. doi: 10.1200/JCO.2000.18.8.1709. [DOI] [PubMed] [Google Scholar]
- 6.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO Study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 7.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 8.Wedding U, Rohrig B, Klippstein A, et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–671. doi: 10.1007/s00432-006-0115-7. Epub 2006 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: A critical issue in geriatric oncology. Crit Rev Oncol Hematol. 2003;46:127–137. doi: 10.1016/s1040-8428(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 10.Ershler WB, Longo DL. A report card for geriatric oncology: Borderline pass, improvement needed. J Gerontol A Biol Sci Med Sci. 2006;61A:688. doi: 10.1093/gerona/61.7.688. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SS, Scott CB, Chang VT, et al. Prediction of survival for advanced cancer patients by recursive partitioning analysis: Role of Karnofsky performance status, quality of life, and symptom distress. Cancer Invest. 2004;22:678–687. doi: 10.1081/cnv-200032911. [DOI] [PubMed] [Google Scholar]
- 13.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 14.Gomez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: Results of a multivariate analysis. J Clin Oncol. 1998;16:2065–2069. doi: 10.1200/JCO.1998.16.6.2065. [DOI] [PubMed] [Google Scholar]
- 15.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: Exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 19.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 20.Balducci L, Cohen HJ, Engstrom PF, et al. Senior adult oncology clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:572–590. doi: 10.6004/jnccn.2005.0032. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci MedSci. 2007;62A:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 22.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 23.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56A:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Beekman AT, Deeg DJ, Van Limbeek J, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 27.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Rolland Y, Lauwers-Cances V, Cesari M, et al. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol. 2006;21:113–122. doi: 10.1007/s10654-005-5458-x. [DOI] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 31.Markides KS, Black SA, Ostir GV, et al. Lower body function and mortality in Mexican American elderly people. J Gerontol A Biol Sci Med Sci. 2001;56A:M243–M247. doi: 10.1093/gerona/56.4.m243. [DOI] [PubMed] [Google Scholar]
- 32.Gale CR, Martyn CN, Cooper C, et al. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 33.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki H, Kasagi F, Yamada M, et al. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–342. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]