Abstract
Quaternary ammonium methacryloxy silicate (QAMS), an organically modified silicate (ORMOSIL) functionalized with polymerizable methacrylate groups and an antimicrobial agent with a long lipophilic alkyl chain quaternary ammonium group, was synthesized through a silane-based sol–gel route. By dissolving QAMS in methyl methacrylate monomer, this ORMOSIL molecule was incorporated into an auto-polymerizing, powder/liquid orthodontic acrylic resin system, yielding QAMS-containing poly (methyl methacrylate). The QAMS-containing acrylic resin showed a predominant contact-killing effect on Streptococcus mutans (ATCC 35668) and Actinomyces naeslundii (ATCC 12104) biofilms, while inhibiting adhesion of Candida albicans (ATCC 90028) on the acrylic surface. The antimicrobial activities of QAMS-containing acrylic resin were maintained after a 3 month water-aging period. Bromophenol blue assay showed minimal leaching of quaternary ammonium species when an appropriate amount of QAMS (<4 wt.%) was incorporated into the acrylic resin. The results suggest that QAMS is predominantly co-polymerized with the poly(methyl methacrylate) network, and only a minuscule amount of free QAMS molecules is present within the polymer network after water-aging. Acrylic resin with persistent antimicrobial activities represents a promising method for preventing bacteria- and fungus-induced stomatitis, an infectious disease commonly associated with the wearing of removable orthodontic appliances.
Keywords: Antimicrobial, Biofilm, Ormosil, Orthodontic base polymer, Polymethyl methacrylate
1. Introduction
Uncontrolled biofilm formation is a major concern in individuals receiving medical devices such as implants, removable appliances, intubation tubes and catheters. Microorganisms embedded in a hydrated polymeric matrix of biofilms are much more tolerant to antimicrobial agents than are planktonic microorganisms [1]. Given time and environmentally favorable conditions, most of these biofilms eventually lead to persistent and chronic bacterial infections. Thus, there is exigent demand for developing methods or compounds to combat biofilms growing on medical devices.
Conventional approaches for biofilm control are based on leaching of antimicrobial agents of small molecular weight into the surrounding environment for killing microorganisms. For example, chlorhexidine is added to methacrylate polymeric matrices to produce antimicrobial resin composites [2,3]. However, effective antimicrobial activity is only present during the initial burstrelease phase. The existence of a tail-release phase, which may continue to release an antimicrobial agent for several years without yielding an effective dose of antimicrobial activity, has been criticized for the possible development of microbial resistance [4]. Leaching of antimicrobials from polymers may also exert toxic effects on the host cells, induce a population shift of the microflora or result in deterioration of the mechanical properties of polymers [5].
In terms of methacrylate chemistry, considerable efforts have been devoted to developing antimicrobial resinous materials, wherein antimicrobial compounds are immobilized by forming covalent bonds with the polymer network. The rationale for synthesizing this kind of compound is to combine an antimicrobial agent with a methacrylate functional group, resulting in a polymerizable antimicrobial macromonomer. One excellent example of this concept is 12-methacryloyloxydodecylpyridinium bromide (MDPB), which is a quaternary ammonium dodecylpyridinium bromide-functionalized methacrylate. Because of its bactericidal activity and low cytotoxicity [6], MDPB has been successfully incorporated into primer [7,8], adhesive [9] and resin composite, for use in restoring teeth [5,10]. Another monomethacrylate functionalized QAS is methacryloxyethyl cetyl dimethyl ammonium chloride, which has been employed as a monomer for imparting antimicrobial capability in dental adhesive systems [11]. Recently, a QAS dimethacrylate monomer was synthesized, via a modified Menschutkin reaction [12], and demonstrated antibacterial activity toward Streptococcus mutans after incorporation into a dental nanocomposite [13]. Copolymerization of antimicrobial methacrylates with a methacrylate-based polymer network possesses several advantages. A permanent antimicrobial surface would be highly advantageous since the copolymerized antimicrobial agent will not be released. Unlike antimicrobial coatings in which antimicrobial molecules are grafted on the surface, permanent antimicrobial activities are independent of loss of surface layer, because the antimicrobial methacrylate is incorporated throughout the bulk polymer network. In addition, non-leaching antimicrobial polymers are more environmentally friendly, due to the elimination of pollution issues associated with conventional biocides.
A technique for one-pot synthesis of quaternary ammonium methacryloxy silicate (QAMS) has recently been reported [14]. Through a silane-based sol–gel process, one molecule of 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride (SiQAC) and three molecules of 3-methacryloxypropyltrimethoxysilane (3-MPTS) are attached to an anchoring, core unit – tetraethoxysilane (TEOS). This synthesis technique results in an organically modified silicate (ORMOSIL) with antimicrobial activities conferred by the long, lipophilic, alkyl quaternary ammonium chain derived from SiQAC [15]. The water-insoluble QAMS can be copolymerized with methyl methacrylate monomer, due to the methacrylate groups derived from 3-MPTS. In a previous study, QAMS was incorporated into an orthodontic acrylic resin system [16]. The flexibility of the siloxane backbone as compared with rigid C–C bonds would also benefit the availability of the antimicrobial functionality on the surface of acrylic resin, by enabling the long lipophilic antimicrobial chain of the QAMS molecules to differentially align on the acrylic surface, in spite of the presence of network formation within the bulk of the acrylic resin. The QAMS-copolymerized acrylic resin demonstrated improved fracture toughness without adversely affecting flexural modulus and strength of the orthodontic acrylic. In addition, the QAMS-copolymerized acrylic resin exhibited a contact-killing effect on S. mutans and Actinomyces naeslundii biofilms, while inhibiting adhesion of Candida albicans on the acrylic surface. While these preliminary antimicrobial results are encouraging, it is not known if the antimicrobial activities are retained after the QAMS-containing orthodontic acrylic is subjected to water-aging.
Thus, the purpose of the present study was to investigate the effect of 3 month water-aging on the antimicrobial activities of the aforementioned ORMOSIL-containing orthodontic acrylic resin against three common microbes, often linked with oral diseases: the Gram positive bacteria S. mutans and A. naeslundii, and the fungus C. albicans. The hypothesis tested was that biocidal properties of the orthodontic acrylic are retained after 3 months of water-aging.
2. Materials and methods
2.1. QAMS synthesis and incorporation into orthodontic acrylic resin
QAMS was synthesized through a silane-based sol–gel reaction as described previously [14]. Briefly, the QAMS monomer mixture was prepared by adding TEOS, SiQAC and 3-MPTS in a 1:1:3 molar ratio, in a container with magnetic stirring at 200 rpm, for 1 h at ambient temperature. A stoichiometric amount of acidified MilliQ water (16 moles; pH 2.5) was then added to fully hydrolyze the monomers. Subsequent condensation of the silanol groups was conducted at pH 7.4 by adding sufficient 1 M NaOH to the previously hydrolyzed QAMS. The QAMS precipitate was heated at 100°C and vacuum-stripped to remove the sol-gel by-products (i.e. water and alcohols) from the condensate, yielding a rubbery ORMOSIL.
A commercially available, auto-polymerizing, methyl methacrylate/poly(methyl methacrylate) (MMA/PMMA) orthodontic acrylic resin system (Ortho-Jet; Lang Dental Manufacturing Co. Inc., Wheeling, IL, USA) was used. To incorporate QAMS into PMMA, freshly made, water- and alcohol-free QAMS was dissolved in the MMA liquid, resulting in MMA containing 0 (control), 1, 5, 10 and 15 wt.% of QAMS. Then, MMA monomers with different concentrations of QAMS were mixed with PMMA powder at 2:3 mass ratio, to produce 6 mm diameter and 1 mm thick disks. After polymerization, PMMA containing 0 (control), 0.4, 2, 4 and 6 wt.% of QAMS were produced [16], and subsequently used for antimicrobial activity evaluation and leaching experiments using the bromophenol blue assay.
2.2. Biofilm preparation and antimicrobial activity evaluation
2.2.1. Primary cell suspension
S. mutans ATCC 35668 (ATCC, Manassas, VA, USA) and A. naeslundii ATCC 12104 were cultured in brain heart infusion (BHI) broth (Difco, Becton-Dickinson and Co., Sparks, MD, USA) supplemented with 50 mM sucrose (pH 7.2). C. albicans ATCC 90028 was cultured in yeast nitrogen base (YNB; Difco) supplemented with 50 mM glucose (pH 7.2). Cells were harvested from 24 h fresh cultures by centrifugation at 2500 rpm for 5 min at 4 °C. The respective cell pellet was washed three times with sterile phosphate-buffered saline (PBS, 0.01 M, pH 7.2), resuspended in 100 ml of the respective growth medium and adjusted to a concentration of 107 CFU ml−1 before use. Microbial concentration was determined by measuring the absorbance of the inoculum with a spectrophotometer (Beckman DU530 Life Science UV/vis Spectrophotometer, Beckman Coulter, Inc., Indianapolis, IN, USA) at 660 nm for bacteria and 520 nm for yeast cells. The readings were compared to a regression line derived from McFarland turbidity standards (Pro-Lab Diagnostics, Richmond Hill, ON, Canada) to correspond to a microbial concentration 107 CFU ml−1.
2.2.2. Biofilm formation
Acrylic resin disks prepared from the control and the four QAMS-containing experimental groups (N = 12) were used for biofilm preparation and antimicrobial evaluation. One-half of the acrylic disks from each group were immersed in deionized water at 37 °C for 3 months, while the other half were designated as baseline specimens and used within 24 h after preparation. All acrylic disks were disinfected with UV light for 2 h prior to experimentations.
Sterile, pooled human saliva was used for creating a salivary pellicle on the surface of the un-aged and water-aged acrylic disks. The pooled saliva was obtained by collecting whole saliva from three healthy volunteers, without stimulation (flow rate 0.25 ml min−1; pH 7.3). The pooled saliva was centrifuged at 2000 rpm for 15 min to remove cellular debris, oral microorganisms and particulates. The supernatant was collected and mixed with dithiothreitol (Sigma-Aldrich, St Louis, MO, USA; 2.5 mmol l−1) to reduce salivary protein aggregation. After centrifuging the mixture again for 15 min at 4 °C, the supernatant was collected and filter-sterilized through disposable, sterile 0.22 µm filters (Nalge Numc International, Rochester, NY, USA). The sterile saliva was stored at −20 °C until use.
Each microbe was used individually for the formation of singlespecies biofilms inside an oral biofilm reactor. The reactor consisted of a vessel and 18 recessed holders for insertion of substrate disks, over which microbial biofilms could be grown. The 12 acrylic disks from each group were affixed to the sample ports of the specimen holder, and incubated in thawed, pooled sterile saliva for 1 h at 37 °C, to create a salivary pellicle on the disk surface.
The specimen holder with the pellicle-coated acrylic disks was then transferred to the reactor vessel, which was subsequently filled with a cell suspension (107 CFU ml−1) of S. mutans, A. naeslundii or C. albicans. Each assembly was first incubated for 90 min at 37 °C, in an orbital shaker incubator at 50 rpm placed inside the respective chamber, to develop the adhesion phase of the respective biofilm. Following this phase, the specimen holder was removed, rinsed carefully with 100 ml of PBS (0.01 M, pH 7.2), and transferred aseptically to a new, sterile reactor vessel, which held the disks on a fixed stage in 200 ml of the respective nutrient medium. The assembly was placed over an orbital incubator (37 °C; 50 rpm), and connected to several vessels (nutrient, sucrose solution and waste) and to an infusion pump, to complete the in vitro artificial mouth system. A desired flow rate was obtained before allowing biofilm formation to proceed for 24 h. The flow rate was adjusted according to the chemostat mode at a dilution rate of 0.10 h−1. S. mutans and A. naeslundii biofilms were grown under anaerobic conditions (5% carbon dioxide, 10% hydrogen and 85% nitrogen). C. albicans biofilms were grown under aerobic condition. At the end of the 24 h growth period, the specimen holder with the acrylic discs was aseptically removed, and immersed in 100 ml of sterile PBS to remove non-adherent cells. The acrylic disks with adherent biofilms were then retrieved for further investigation.
2.2.3. Confocal laser scanning microscopy (CLSM)
Biofilms grown on top of the acrylic disks from each of the five groups were stained using a LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Eugene, OR, USA). Live microbes were stained with SYTO-9 to produce green fluorescence, and microbes with compromised membranes were stained with propidium iodide (PI) to produce red fluorescence. The SYTO-9 and PI stock solutions were diluted with PBS buffer (0.01 M) according to the manufacturer’s instructions. Diluted fluorescent dye solutions were incubated with the biofilm-coated disks for 15 min, and then imaged using a CLSM (Fluoview FV1000, Olympus, Tokyo, Japan) at 40× magnification, using excitation wavelengths of 488 and 568 nm for SYTO-9 and PI, respectively. For each of the 12 acrylic resin disks in a group, five image stacks (Z-stack) were obtained at locations that were characteristic of each biofilm on that acrylic disk. Images (field size: 318 × 318 µm) were acquired at a Z-step of 2 µm (i.e. distance between two adjacent images of a stack), beginning from the bottom of the biofilm that was in contact with the acrylic surface, to the top of the biofilm.
Images from each stack were analyzed using image analysis software (BioImageL v2.1; Faculty of Odontology, Malmö University, Malmö, Sweden) [17]. For S. mutans and A. naeslundii biofilms, three-dimensional (3-D) image analysis was performed. Two measurement parameters were employed: biovolume-of-interest and the basal layer of biofilm. The biovolume-of-interest, representing the first 24 µm of each Z-stack (i.e. 1st-13th images), was analyzed for the percentage distribution of live and dead bacteria. Because biofilms vary in thickness, a constant biofilm thickness model for CLSM analysis was used to ensure that the percentage live bacteria data could be statistically analyzed using a general linear model; viability of biofilms in situ was evaluated using CFU counts, as described in the subsequent section. The biomass along the basal layer of each biofilm (contact-surface-biomass – first image of a Z-stack), representing direct contact of the microbes with the acrylic disk surface, was also analyzed to identify the effect of contact-killing by the immobilized antimicrobial agent. Because C. albicans formed biofilms only on the QAMS-free acrylic resin disk surfaces, two-dimensional (2-D) image analysis was adopted for evaluation. A merged image obtained from a representative location of each acrylic disk was used for 2-D analysis of: (a) the percentage distribution of biomass (i.e. both live and dead fungi) within the imaged field, and (b) the percentage distribution of live fungi within the biomass.
2.2.4. Colony forming unit (CFU) counts
Three acrylic disks from each group, after biofilm formation, were placed carefully in a microtube containing 1 ml of phosphate buffered saline (PBS), and vortexed (Maxi Mix vortex mixer, Thermo Scientific, Waltham, MA, USA) for 2 min at high speed to detach the biofilm. Ten-fold serial dilutions were generated in PBS (0.01 mM, pH 7.3), and each dilution was plated (50 µl aliquots) onto Sabouraud dextrose agar plates for C. albicans, and brain heart infusion agar plates for S. mutans and A. naeslundii. The plates were incubated at 37 °C for 48 h in an aerobic chamber for C. albicans and anaerobic chamber for S. mutans and A. naeslundii. After incubation, the CFUs per acrylic disk were calculated manually and statistically analyzed. Three replicates were performed for each acrylic disk in each group (N = 9).
2.2.5. XTT assay
After the formation of biofilms on acrylic surfaces, three additional disks from each group were transferred carefully into separate microtubes containing 4 ml of PBS (0.01 mM, pH 7.3), avoiding any disturbances to the biofilms. 50 µl of 1 mg ml−1 solution of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-car-boxanilide (XTT; Sigma-Aldrich) was then added to each microtube, together with 4 µl of 1 mM menadione (Sigma-Aldrich). The solutions were mixed gently, covered with aluminum foil and incubated for 5 h at 37 °C. After incubation the solution was transferred to a new microtube and centrifuged at 4000 rpm for 10 min at 4 °C The supernatant was placed in a 96-well plate and read at 492 nm using a spectrophotometer (Victor, R&D systems, Minnesota, USA). Two readings were taken for each acrylic disk from each group (N = 6).
2.3. Bromophenol blue assay
Acrylic disks (14.5 mm diameter × 0.4 mm thick; 75 ± 3 mg) from each of the five groups were aged in 2 ml of deionized water in a container at 37 °C under continuous shaking (N = 8). Bromophenol blue is a dye which forms a complex with quaternary ammonium compounds, and results in a shift of λmax from 593 to 603 nm due to complex formation. Briefly, an aqueous solution of 0.001 wt.% bromophenol blue was prepared in deionized water. The solution was buffered with sodium carbonate to pH 7.0 to avoid absorption changes owing to pH fluctuations. A 0.5 ml aliquot of the extract from each acrylic disk was collected at 7 days, mixed with 1.5 ml bromophenol blue solution and agitated gently for 10 min. Absorbance of the mixture was recorded using a UV-vis spectrophotometer (160A UV-Vis spectrometer, Shimadzu, Kyoto, Japan) at 603 nm. The absorption values of known concentrations of SiQAC in deionized water (from 0 to 1.451 mM) were recorded to establish a standard curve, for determining the molar concentration of N+ leachate in the respective aged solutions. Two periods were examined: baseline and 3 months. For the latter, the 3 month water-aged disks were removed from the original aging medium, thoroughly rinsed and immersed in deionized water for 7 days, after which the readings were obtained.
It is known that salivary enzymes (i.e. esterases) play an important role in biodegradation of dental polymers [18,19]. Thus, the bromophenol blue assay was repeated by aging QAMS-free and QAMS-containing acrylic disks in PBS (0.01 mM, pH 7.2), in the presence or absence of porcine liver esterase (PLE, Sigma-Aldrich), to test if PLE accelerates the leaching of QAMS from acrylic resin [20,21]. The PLE solution was prepared by dissolving PLE powder in sterilized PBS at a concentration of 40 U ml−1 and stored at −80 °C until use. Additional acrylic resin disks from the five groups (N = 8) were individually aged in 2 ml of PLE solution in a container at 37 °C under continuous shaking. The used PLE solution was replaced with fresh solution every two days, resulting in 14 ml of leachate-containing PLE solution after 14 days of aging. The method for determining N+ leachate concentration was similar to the aforementioned descriptions, except that the SiQAC standard curve was based on known concentration of SiQAC in PBS instead of deionized water.
2.4. Statistical analyses
For each microorganism, two-factor repeated measures analyses of variances were performed to detect significant differences of various parameters with respect to the factors “QAMS concentration” and “aging time”, as well as the interaction of those two factors. In the event that the normality or the homoscedasticity assumptions of a data set was violated, the data set was logarithmically transformed, and with the normality and homoscedasticity assumptions reconfirmed prior to performing the analysis. Posthoc multiple comparisons were conducted using the Holm-Sidak method. For all analyses, statistical significance was preset at α = 0.05.
3. Results
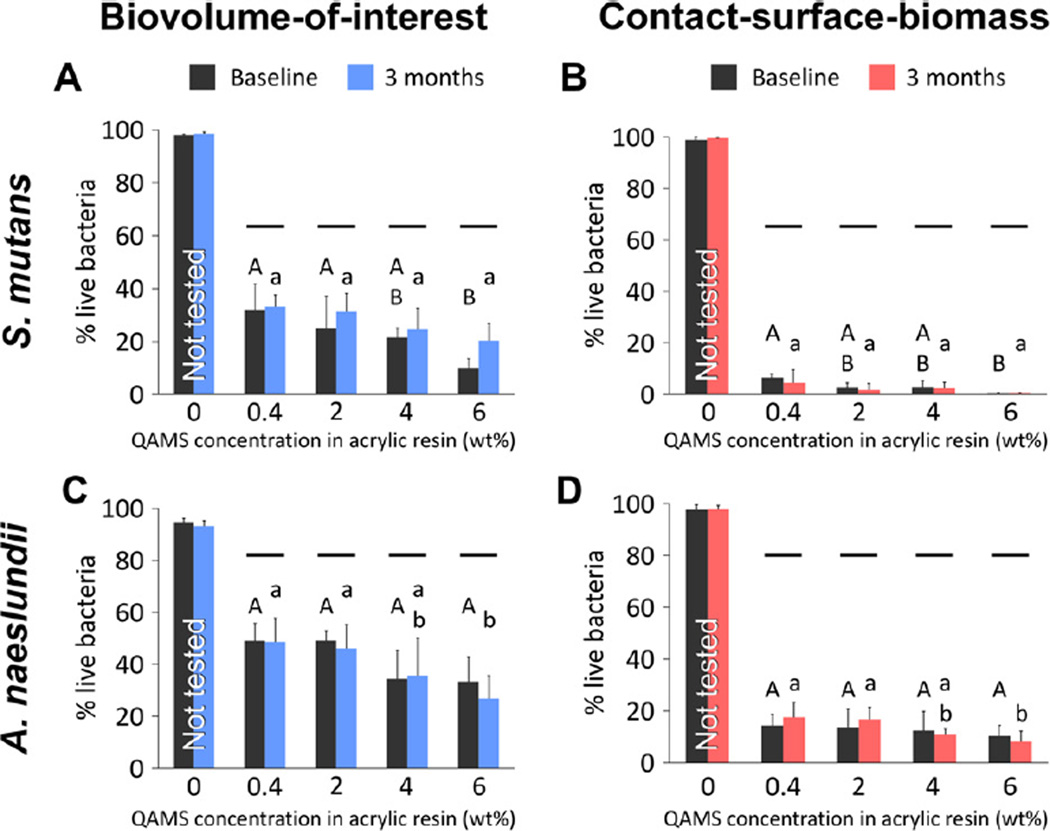
The biocidal effects of QAMS-containing acrylic resin on the biovolume-of-interest, as well as the contact-surface-biomass (biomass on disk surface) of the un-aged and 3 month water-aged acrylic disks are shown in Fig. 1. Because the control group (0 wt.% QAMS) had negligible antibacterial activity, this group was not included in the statistical analyses. For the biovolume-of-interest in S. mutans biofilms, the factor “QAMS concentration” was statistically significant (p = 0.001) while the factor “aging time” was not statistically significant (p = 0.214). The interaction of these two factors was not statistically significant (p = 0.097). Differences among subgroups are shown in Fig. 1A. For the contact-surface biomass of S. mutans biofilms, the factor “QAMS concentration” was statistically significant (p = 0.003) while the factor “aging time” was not statistically significant (p = 0.342). The interaction of these two factors was not statistically significant (p = 0.826). Differences among subgroups are shown in Fig. 1B. For the biovolume-of-interest in A. naeslundii biofilms, the factor “QAMS concentration” was statistically significant (p = 0.002) while the factor “aging time” was not statistically significant (p = 0.411). The interaction of these two factors was not statistically significant (p = 0.724). Differences among subgroups are shown in Fig. 1C. For the contact-surface biomass of A. naeslundii biofilms, the factor “QAMS concentration” was statistically significant (p = 0.036) while the factor “aging time” was not statistically significant (p = 0.708). The interaction of these two factors was not statistically significant (p = 0.538). Differences among subgroups are shown in Fig. 1D. There is extensive reduction of live bacteria within the biofilms, which is dependent upon the concentration of QAMS in the acrylic resin. In addition, antibacterial activities are not significantly decreased after 3 months of water-aging.
Fig. 1.
Percentage of live bacteria within the biovolume-of-interest (i.e. the volume of biofilm from the base to the 24th µm of a z-stack), and within the contact-surface biomass (i.e. within 2 µm from disk surface) of biofilms that were grown on acrylic resin disks from the control (0 wt.% QAMS) and four experimental groups (0.4–6 wt.% QAMS). (A, B) S. mutans biofilms. (C, D) A. naeslundii biofilms. For each parameter (upper case letters for baseline and lower case letters for 3 months), groups with the same letters are not statistically significant (p > 0.05). For comparisons between baseline and 3 months, groups connected with a horizontal bar are not statistically significant (p > 0.05).
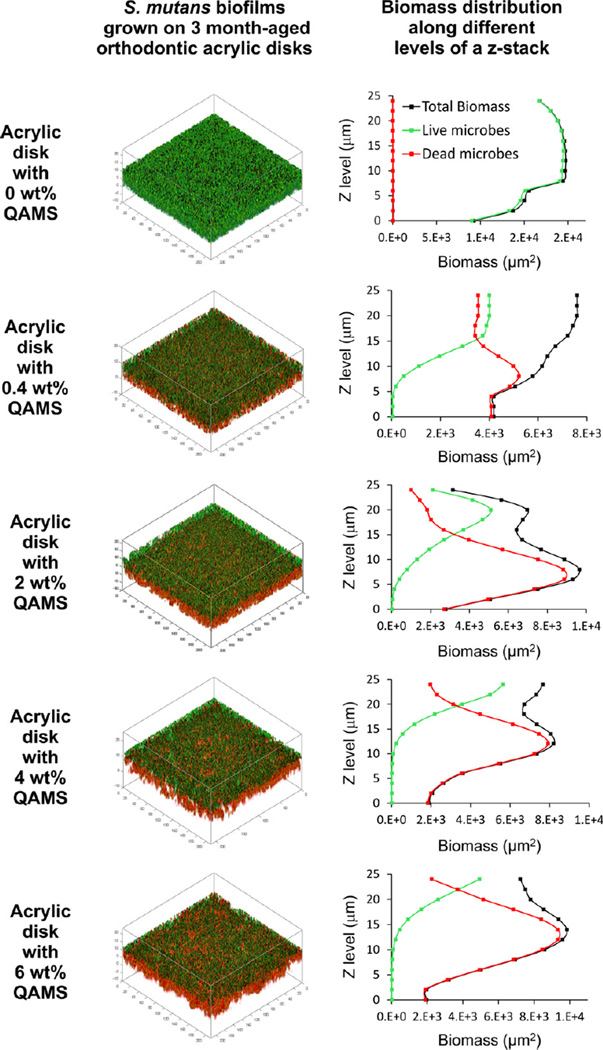
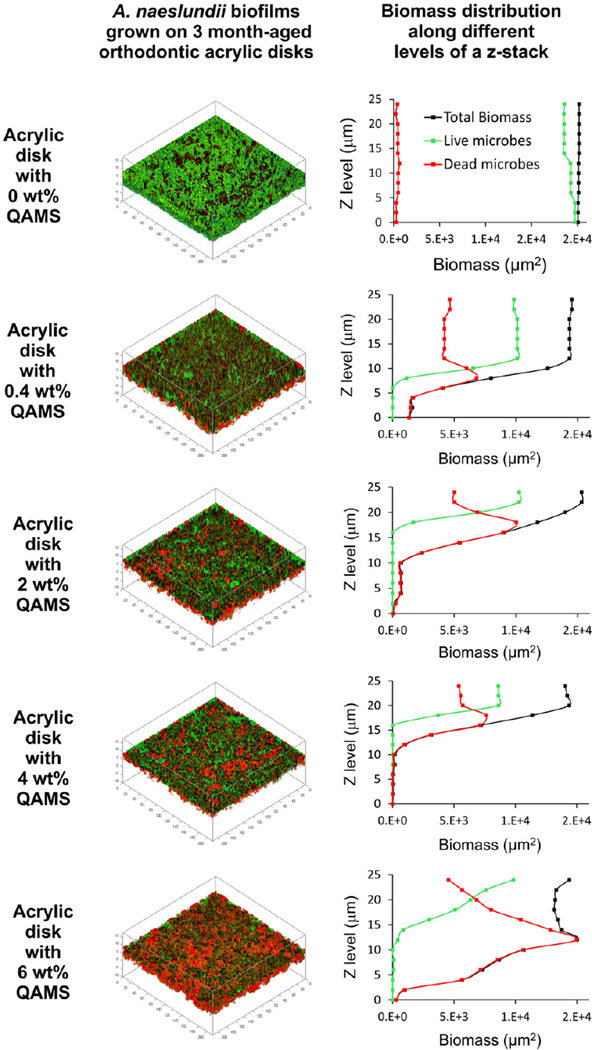
For all QAMS-containing acrylic resins, the percentage of live bacteria at the bottom of the biofilms was lower than those detected within the biovolume-of-interest (i.e. the volume of biofilm from the base to the 24th mm of a z-stack). Figs. 2 (S. mutans) and 3 (A. naeslundii) show representative distribution of live/dead bacteria within biofilms grown on surfaces of 3 month water-aged acrylic disks containing different concentrations of QAMS. The distribution of live (green) and dead (red) bacteria within the bio-volume-of-interest of those biofilms are depicted as 3-D plots in the left column. The percentage distributions of live and dead bacteria from the biomass at each level of a z-stack are summarized in the line plots in the right column.
Fig. 2.
Representative live/dead stained S. mutans biofilms grown on the surfaces of 3 month water-aged acrylic disks with varied QAMS concentrations. Green: live bacteria; red: dead bacteria. Left column represents 3-D plots of the 24 µm thick biovolume-of-interest from each biofilm. Right column represents the biomass of live and dead bacteria as a function of biofilm level (Z step = 2 µm).
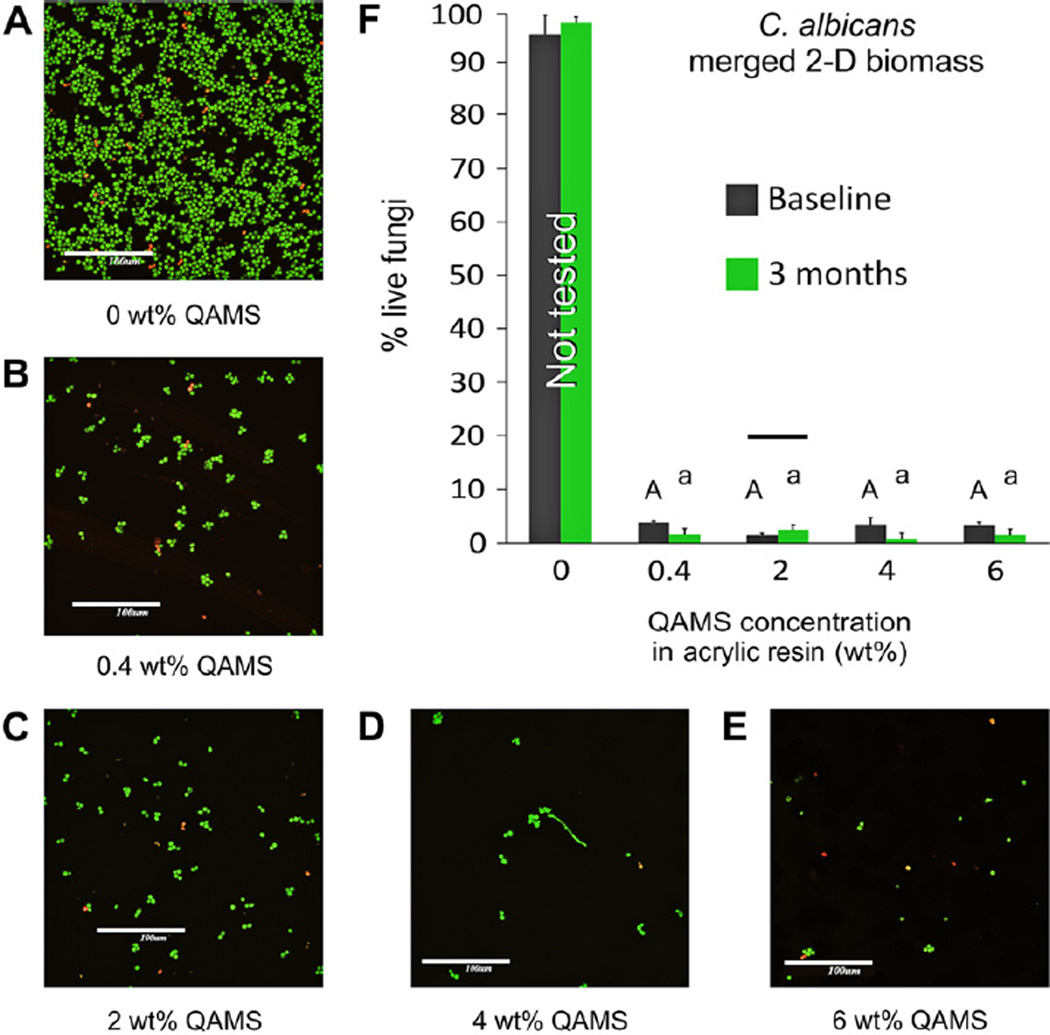
Representative 2-D merged images of C. albicans that adhered and grew on surfaces of 3 month water-aged acrylic resin disks are depicted in Fig. 4A – E. Unlike S. mutans and A. naeslundii, C. albixcans formed biofilms only on the QAMS-free acrylic resin disks (Fig. 4A). Only discontinuous monolayers of fungus cells were apparent on acrylic resins containing QAMS (Fig. 4B – E). The percentage distributions of live C. albicans within the 2-D biomasses are shown in Fig. 4F. Because the control group (0 wt.% QAMS) had negligible antifungal activity, this group was not included in the statistical analysis. Comparison of Groups II–V indicated that the factor “QAMS concentration” was not statistically significant (p = 0.085) while the factor “aging time” was statistically significant (p < 0.001). The interaction of these two factors was statistically significant (p < 0.001). Post-hoc comparisons of the subgroups indicated that difference between the aging times was apparent except for Group III (2 wt.% QAMS; Fig. 4F).
Fig. 4.
Representative merged images showing 2-D biomasses of C. albicans that adhered and grew on surfaces of 3 month water-aged acrylic resin disks containing: (A) 0 wt.% QAMS, (B) 0.4 wt.% QAMS, (C) 2 wt.% QAMS, (D) 4 wt.% QAMS, (E) 6 wt.% QAMS. For all images, bar = 100 µm. (F) Percentage distributions of live fungi within the 2-D biomass on the surfaces of acrylic disks with varied QAMS concentrations. Data obtained from the baseline and 3 month water-aged specimens were included in the statistical analysis. For each parameter (upper case letters for baseline and lower case letters for 3 months), groups with the same letters are not statistically significant (p > 0.05). For comparisons between baseline and 3 months, groups connected with a bar are not statistically significant (p > 0.05).
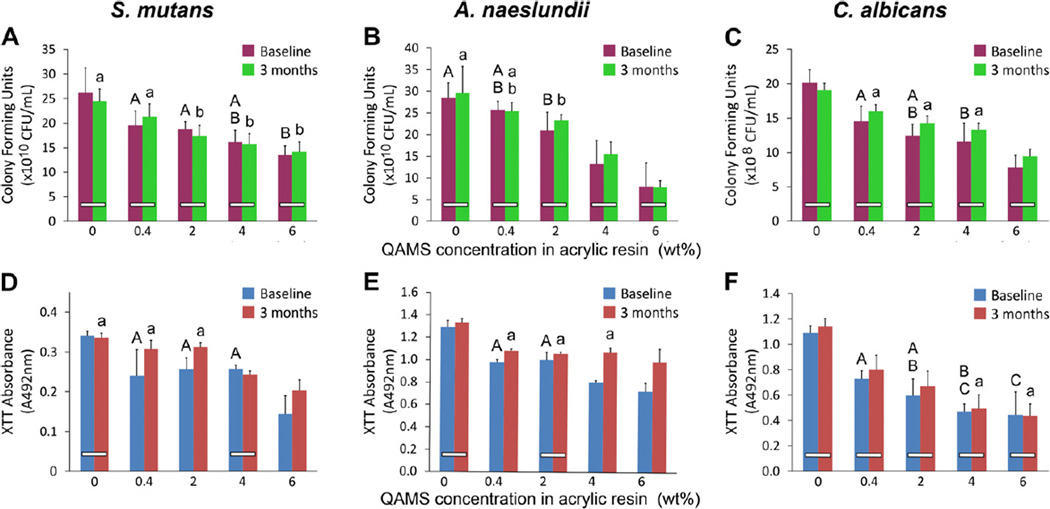
The CFU counts and XTT assay of S. mutans, A. naeslundii and C. albicans biofilms are shown in Fig. 5. Statistical analysis of CFU counts of S. mutans indicated that the factor “QAMS concentration” was statistically significant (p < 0.001) while the factor “aging time” was not statistically significant (p = 0.773). The interaction of these two factors was not statistically significant (p = 0.473). For simplicity, only subgroups that exhibited no significant difference are shown in Fig. 5A. Similarly, analysis of CFU counts of A. naeslundii indicated that the factor “QAMS concentration” was statistically significant (p < 0.001) but the factor “aging time” was not statistically significant (p = 0.238). The interaction of these two factors was not statistically significant (p = 0.78). Subgroups that exhibited no significant difference are shown in Fig. 5B. For C. albicans, analysis of CFU counts indicated that the factor “QAMS concentration” was statistically significant (p < 0.001) but the factor “aging time” was not statistically significant (p = 0.417). The interaction of these two factors was not statistically significant (p = 0.222). Subgroups not demonstrating significant differences are shown in Fig. 5C.
Fig. 5.
Colony forming unit (CFU) cell viability counts and XTT cell metabolism assays of microorganisms grown on orthodontic acrylic resin disks from the control (0 wt.% QAMS) and four experimental groups (0.4–6 wt.% QAMS). (A, D) S. mutans biofilms. (B, E) A. naeslundii biofilms. (C, F) C. albicans. For each parameter (upper case letters for baseline and lower case letters for 3 months), groups with the same letters are not statistically significant (p > 0.05; note – only groups that are not significantly different are labeled). For comparisons between baseline and 3 months, groups connected with a bar are not statistically significant (p > 0.05).
Statistical analysis of the mitochondrial metabolic activities of S. mutans biofilms indicated that both the factors “QAMS concentration” (p < 0.001) and “aging time” had statistically significant effects on the XTT results (p < 0.001). The interaction of these two factors was also statistically significant (p < 0.001). Fig. 5D indicates subgroups not demonstrating significant differences. For A. naeslundii biofilms, both “QAMS concentration” (p < 0.001) “aging time” had statistically significant effects on the XTT results (p < 0.001). The interaction of these two factors was also statistically significant (p < 0.001). Subsets not showing significant differences are shown in Fig. 5E. For C. albicans, the factor “QAMS” concentration” produced statistically significant XTT results (p < 0.001). No statistical significance could be identified for the factor “aging time” (p = 0.161), as well as the interaction between these two factors (p = 0.730). Fig. 5F presents that data, showing sets of groupings that are not significantly different.
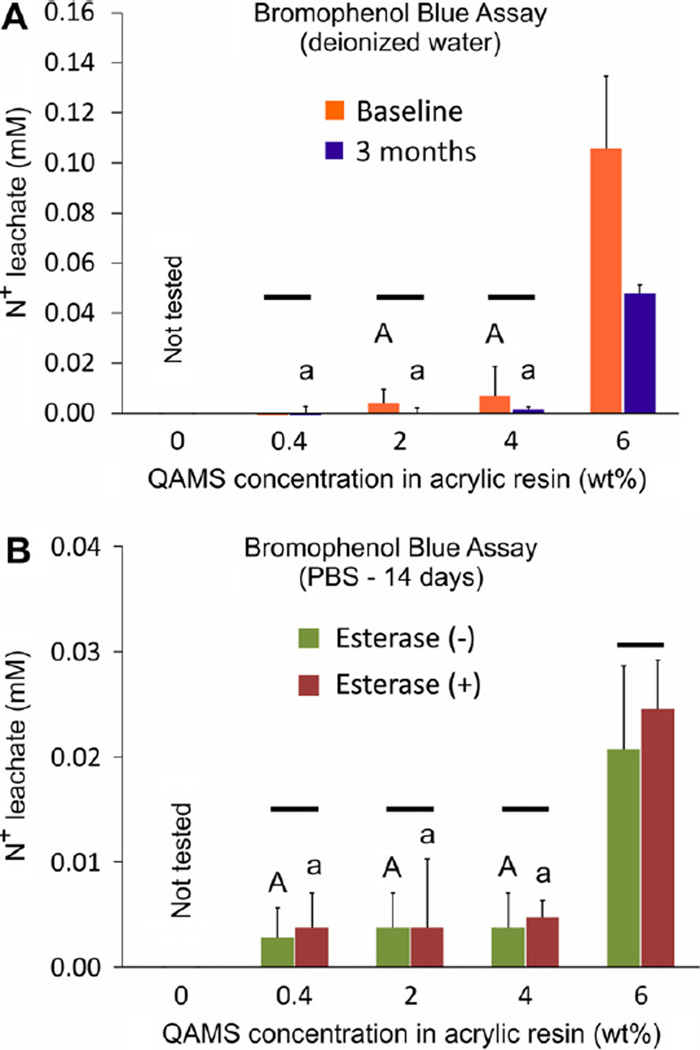
Fig. 6A shows cumulative leaching of quaternary ammonium moieties from acrylic disks containing different concentrations of QAMS during 7 days of water-aging conducted on the un-aged and 3 month water-aged specimens. Because there was no leachable N+ from the QAMS-free acrylic, data from the two control subgroups were not included in the statistical analysis. For the other four experimental groups, statistical significance was observed for the factors “QAMS concentration” (p < 0.001) and “aging time” (p = 0.043). The interaction of these two factors was not statistically significant (p = 0.207). Statistical groupings not showing significant differences are shown. Taken together, the data suggest that there was minimal leaching of N+ from acrylic resin disks containing less than 4 wt.% QAMS. Leaching of N+ species was significantly higher from acrylic resin disks containing 6 wt.% QAMS. After 3 months of water-aging, acrylic resin disks containing 6 wt.% QAMS leached significantly less N+ species than fresh, un-aged acrylic resin disks, and significantly more than disks containing 4 wt.% QAMS or less.
Fig. 6.
Leaching of quaternary ammonium moieties from QAMS-containing acrylic disks (bromophenol blue assay). (A) Baseline and 3 month aging in deionized water. (B) Aging in the absence of porcine liver esterase in phosphate-buffered saline (PBS) for 2 weeks. Note that the scales of the y-axis in the two charts are different. For each parameter (upper case letters for baseline and lower case letters for 3 months in (A) and upper case letters for no esterase and lower case letters for with esterase in (B)), groups with the same letters are not statistically significant (p > 0.05; note: only groups that are not significantly different are labeled). For comparisons between baseline and 3 months, groups connected with a bar are not statistically significant (p > 0.05).
Fig. 6B shows cumulative leaching of quaternary ammonium moieties from QAMS-free and QAMS-containing acrylic disks after those disks were immersed in porcine liver esterase (PLE)-free and PLE-containing PBS for 14 days. Because there was no leachable N+ from the QAMS-free acrylic, data from the two control subgroups were not included in the statistical analysis. Statistical analysis indicated that while “QAMS concentration” has a statistically significant effect on leaching of N+ species (p < 0.001), the presence of PLE in the aging medium did not significantly influence the amount of N+ species leaching the acrylic resin disks (p = 0.826). The interaction of these two factors was not statistically significant (p = 0.991). Leaching of N+ species was significantly higher from acrylic resin disks containing 6 wt.% QAMS. However, there was no significant difference between acrylic resin disks aged in PBS with or without PLE.
4. Discussion
The results of the present study did not warrant rejection of the hypothesis that biocidal properties of the orthodontic acrylic are retained after 3 months of water-aging. The degree of conversion of MMA to PMMA is never complete; unreacted monomers are left unbound and initially trapped with the polymerized resin [22,23]. Self-cured orthodontic acrylic resins have a higher level of unreacted monomers than heat-polymerized denture base acrylic resin [24]. Water-aging testing of PMMA demonstrated continuous release of unreacted monomer into water for 8 weeks, with a rapid phase of burst release within the first 24 h [25]. QAMS molecules, if not copolymerized with the PMMA network, would also be expected to be released after aging in water or saliva. Thus, it is important to examine the effect of 3 month water-aging on the biocidal activities of QAMS-containing orthodontic acrylic resin.
In the present study, CLSM, CFU counts and XTT assay were used to evaluate the biocidal activities of QAMS-containing orthodontic acrylic resins, before and after 3 months of water-aging. 3-D reconstruction of CLSM images enables the distribution of live/dead bacteria to be identified from different depths of a biofilm, thereby revealing the biocidal effect of an antimicrobial agent as a function of the distance from the substrate surface. Based on the analyses performed, the QAMS-containing orthodontic acrylic resin kills S. mutans, A. naeslundii and C. albicans in a dose-dependent manner and by surface contact. Nevertheless, dead microorganisms are not only confined to the substrate surface (Figs. 2 and 3). Although diffusion of non-covalently bound QAMS from the PMMA substrate may have resulted in killing of microorganisms within the bulk of a biofilm, toxic substances released by dead microorganisms that are in contact with the substrate surface may also have resulted in reduction of the viable biovolume [26].
Fig. 3.
Representative live/dead stained A. naeslundii biofilms grown on the surfaces of 3 month water-aged acrylic disks with varied QAMS concentrations. Green: live bacteria; red: dead bacteria. Left column represents 3-D plots of the 24 µm thick biovolume-of-interest from each biofilm. Right column represents the biomass of live and dead bacteria as a function of biofilm level.
When biofilms are detached from the acrylic resin disks, diluted appropriately and cultured, CFU counts enable us to quantify the total number of viable bacteria or fungi present on the disks. The CFU count results are complementary to the CLSM data, confirming that the reduction in viable microorganisms is dependent upon the concentration of QAMS incorporated into the acrylic resin. The CFU count results also show that the biocidal activities of the QAMS-containing acrylic resins are not significantly reduced after 3 months of water-aging. Compared to other cell metabolism assays, the XTT assay is a simplified procedure with higher sensitivity and a higher dynamic range, and has been used for quantitating the intracellular mitochondrial dehydrogenase activities of biofilms and planktonic bacteria [27]. While the XTT results also show decline in metabolisms of S. mutans, A. naeslundii and C. albicans with increasing QAMS concentrations, there were, in general, increases in the metabolic activities of S. mutans and A. naeslundii after the QAMS-containing resins were subjected to 3 months of water-aging. We speculate that the increases in metabolic activity after water-aging may be partially explained by the toxicity associated with the unreacted MMA monomers within the acrylic resin. Using a slightly different metabolic assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide or MTT assay), the authors have previously shown that PMMA is initially very toxic to mammalian cells, and that its cytotoxicity to mammalian cells is substantially reduced after leaching of the unreacted MMA [28]. Prior to water-aging, the initially greater reduction in microbial metabolism in the QAMS-containing PMMA resins may be interpreted as the combined result of the toxic effect of unreacted MMA monomer and the biocidal effect of QAMS. With the leaching of unreacted MMA monomers in the 3 month water-aged specimens, the “reduced” XTT activity is more likely to be reflective of the effect of the QAMS component on the metabolic activities of the microorganisms, than the effect from MMA leaching. 3-(trimethoxysilyl)propyldimethyl-octadecyl ammonium chloride (SiQAC), the chemical precursor from which QAMS is synthesized, has a broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria, fungi and yeasts [29]. Though the exact antimicrobial mechanism of quaternary ammonium silanes such as SiQAC has yet to be determined, it is suggested that they cause lysis of bacteria by binding to and puncturing their cell wall components. This causes leakage of the cytoplasmic components that ultimately results in cell death [30,31].
Colonization of Candida species on acrylic resin surfaces represents a significant clinical problem, because fungi are known to adhere strongly to acrylic and are resistant to mechanical or chemical removal [32,33]. Unlike bacteria, C. albicans biofilm formation is characterized by initial adhesion of the fungal cells to glycoprotein-coated surface during the first 2 h, followed by yeast-hyphal transition, proliferation and maturation of the biofilm within 48 h [34]. Adhesion of fungal cells to abiotic substrates is mainly mediated by non-specific hydrophobic and electrostatic interactions; microbial attachment is the result of the balance between attraction and repulsion [35]. In the present study, surface adhesion of C. albicans was severely inhibited by the QAMS-containing acrylic resin. It is possible that during copolymerization with PMMA, the QAMS molecules are spatially aligned on the acrylic surface, due to their long octadecyl lipophilic chain, so that they exert an electrostatic repelling effect on the cell wall of C. albicans. This result is similar to a previous study showing significantly reduction of C. albicans adhesion on SiQAC-coated titanium. In that study, the authors suggested that the octadecyl alkyl chain of the SiQAC molecule has the potential to inhibit initial adhesion of C. albicans [36].
It is difficult for antimicrobial testing to distinguish between contact-killing and agent-releasing systems, because those agent-releasing systems also exhibit contact-killing characteristics. This is especially so when the concentration of leached antimicrobial agent is below the minimal inhibitory concentration (MIC) in the top region of the biofilm [37]. Thus, the bromophenol blue assay was performed as a complementary method to demonstrate that QAMS was released minimally, after 3 months of water-aging, from acrylic resins containing less than 4 wt.% of QAMS. The result is supportive of a predominant contact-killing mechanism for the QAMS-containing orthodontic acrylic resin. Bromophenol blue assay is a colorimetric method that has been widely used in the textile industry for detecting leached quaternary ammonium salts from antimicrobial fabrics [38]. Degradation of acrylic resin caused by water-aging over time was believed to accelerate leaching of compounds from the polymer network [39]. The results from the present study suggest that, when an appropriate amount of QAMS is added (≤4 wt.%), QAMS is predominantly copolymerized with the polymethyl methacrylate network, and only a minuscule amount of free QAMS molecules is present within the polymer network after water-aging, which may leach out upon degradation of the acrylic polymer network. As revealed by FTIR analysis in our previous study [16], addition of 6 wt.% QAMS reduced the overall conversion of PMMA resin. The un-polymerized QAMS in acrylic resin with lower degree of cure would leach out dramatically after water-aging. The leached QAMS may contribute to a relatively higher percentage of dead microbial. To further evaluate the effect of leaching upon the degradation of the PMMA network, we examined the amount of leached N+ species with the bromophenol blue assay after challenging the QAMS-containing PMMA with porcine liver esterase for 2 weeks. Our results indicated that there were no significant increases in the amount of leachable N+ species in each of the QAMS concentration tested. The results suggested that the QAMS that did not leach may have formed an interpenetrative network within the polymerized acrylic resin; the Si–O–Si (siloxane) linkage in the QAMS is not susceptible of hydrolysis by esterase. Thus, further work has to be performed to determine if an interpenetrative network exists in the QAMS-containing acrylic resin.
Since most orthodontic appliances have to be worn for a prolonged period of time under repeated masticatory loads, it is important that the QAMS-containing orthodontic acrylic resin possesses comparable mechanical and physical properties with QAMS-free acrylic resin. Although our previous study has shown that acrylic resin copolymerized with QAMS demonstrates improved fracture toughness without adversely affecting the flexural strength and modulus [16], future work should be performed to investigate the effect of water-aging on the mechanical properties of QAMS-containing acrylic resin.
5. Conclusion
In conclusion, when incorporated into PMMA resin, QAMS exhibited a contact-killing effect on S. mutans and A. naeslundii, and an adhesion-inhibition effect on C. albicans on the acrylic surface, all in a dose-dependent manner. Acrylic resin with persistent antimicrobial activities represents a promising method for preventing bacteria- and fungus-induced stomatitis, an infectious disease commonly associated with the wearing of removable orthodontic appliances.
Acknowledgments
This work was supported by grant R01 DE015306-06 from NID-CR (PI. David Pashley), IRRM award from Georgia Health Sciences University (PI. Franklin Tay), and General Research Fund (GRF) HKU 784410 M from The University of Hong Kong (PI. Cynthia Yiu). The authors thank Mrs Michelle Barnes for her secretarial support.
Appendix A. Appendix: Figures with essential colour discrimination
Certain figures in this article, particularly Figs. 1–6, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi:10.1016/j.actbio.2013.02.031.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–954. doi: 10.1177/154405910608501016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 5.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 6.Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. [DOI] [PubMed] [Google Scholar]
- 7.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 8.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 9.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 10.Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, Russell RR. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–3609. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, et al. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans . J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesisand characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong SQ, Niu LN, Kemp LK, Yiu CK, Ryou H, Qi YP, et al. Quaternary ammonium silane-functionalized, methacrylate resin composition with antimicrobial activities and self-repair potential. Acta Biomater. 2012;8:3270–3282. doi: 10.1016/j.actbio.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlström B, Thompson RA, Edebo L. The effect of hydrocarbon chain length, pH, and temperature on the binding and bactericidal effect of amphiphilic betaine esters on Salmonella typhimurium . APMIS. 1999;107:318–324. doi: 10.1111/j.1699-0463.1999.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 16.Gong SQ, Epasinghe J, Rueggeberg FA, Niu LN, Mettenberg D, Yiu CK, et al. An ORMOSIL-containing orthodontic acrylic resin with concomitant improvements in antimicrobial and fracture toughness properties. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0042355. e42355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chávez de Paz LE. Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Appl Environ Microbiol. 2009;75:1734–1739. doi: 10.1128/AEM.02000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund M, Munksgaard EC. Enzymatic degradation of BISGMA/TEGDMA-polymers causing decreased microhardness and greater wear in vitro. Scand J Dent Res. 1990;98:351–355. doi: 10.1111/j.1600-0722.1990.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang MC, Lin LD, Chuang FH, Chan CP, Wang TM, Lee JJ, et al. Carboxylesterase expression in human dental pulp cells: role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012;8:1380–1387. doi: 10.1016/j.actbio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Santerre JP, Shajii L, Tsang H. Biodegradation of commercial dental composites by cholesterol esterase. J Dent Res. 1999;78:1459–1468. doi: 10.1177/00220345990780081201. [DOI] [PubMed] [Google Scholar]
- 21.Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–1719. doi: 10.1016/s0142-9612(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 22.Ruyter IE, Oysaed H. Conversion in denture base polymers. J Biomed Mater Res. 1982;16:741–754. doi: 10.1002/jbm.820160520. [DOI] [PubMed] [Google Scholar]
- 23.Baker S, Brooks SC, Walker DM. The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: an assay for monomer in saliva. J Dent Res. 1988;67:1295–1299. doi: 10.1177/00220345880670101001. [DOI] [PubMed] [Google Scholar]
- 24.Stafford GD, Brooks SC. The loss of residual monomer from acrylic orthodontic resins. Dent Mater. 1985;1:135–138. doi: 10.1016/s0109-5641(85)80005-1. [DOI] [PubMed] [Google Scholar]
- 25.Vallittu PK, Miettinen V, Alakuijala P. Residual monomer content and its release into water from denture base materials. Dent Mater. 1995;11:338–342. doi: 10.1016/0109-5641(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 26.Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerca N, Martins S, Cerca F, Jefferson KK, Pier GB, Oliveira R, et al. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56:331–336. doi: 10.1093/jac/dki217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ames JM, Loushine RJ, Babb BR, Bryan TE, Lockwood PE, Sui M, et al. Contemporary methacrylate resin-based root canal sealers exhibit different degrees of ex vivo cytotoxicity when cured in their self-cured mode. J Endod. 2009;35:225–228. doi: 10.1016/j.joen.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Isquith AJ, Abbott EA, Walters PA. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl Microbiol. 1972;24:859–863. doi: 10.1128/am.24.6.859-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabata N, Nishiguchi M. Antibacterial activity of soluble pyridinium-type polymers. Appl Environ Microbiol. 1988;54:2532–2535. doi: 10.1128/aem.54.10.2532-2535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29:4157–4163. doi: 10.1016/j.biomaterials.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Chandra J, Patel JD, Li J, Zhou G, Mukherjee PK, McCormick TS, et al. Modification of surface properties of biomaterials influences the ability of Candida albicans to form biofilms. Appl Environ Microbiol. 2005;71:8795–8801. doi: 10.1128/AEM.71.12.8795-8801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA. Candida biofilms and oral candidosis: treatment and prevention. Periodontol 2000. 2011;55:250–265. doi: 10.1111/j.1600-0757.2009.00338.x. [DOI] [PubMed] [Google Scholar]
- 34.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coenye T, De Prijck K, Nailis H, Nelis HJ. Prevention of Candida albicans biofilm formation. Open Mycol J. 2011;5:9–20. [Google Scholar]
- 36.Nikawa H, Ishida K, Hamada T, Satoda T, Murayama T, Takemoto T, et al. Immobilization of octadecyl ammonium chloride on the surface of titanium and its effect on microbial colonization in vitro. Dent Mater J. 2005;24:570–582. doi: 10.4012/dmj.24.570. [DOI] [PubMed] [Google Scholar]
- 37.Siedenbiedel F, Tiller JC. Antimicrobial polymers in solution and on surfaces: overview and functional principles. Polymers. 2012;4:46–71. [Google Scholar]
- 38.Song L, Baney RH. Antibacterial evaluation of cotton textile treated by trialkoxysilane compounds with antimicrobial moiety. Text Res J. 2011;81:504–511. [Google Scholar]
- 39.Bettencourt AF, Neves CB, de Almeida MS, Pinheiro LM, Oliveira SA, Lopes LP, et al. Biodegradation of acrylic based resins: a review. Dent Mater. 2010;26:e171–e180. doi: 10.1016/j.dental.2010.01.006. [DOI] [PubMed] [Google Scholar]