Abstract
Objectives
The aim of this study was to examine rapid-rate nonsustained ventricular tachycardia (RR-NSVT) during routine implantable cardioverter-defibrillator (ICD) evaluation in patients with heart failure and its relationship to outcomes.
Background
The clinical implications of RR-NSVT identified during routine ICD interrogation are unclear. In this study, the occurrence of RR-NSVT and its association with ICD shocks and mortality in SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) were examined.
Methods
The 811 patients who received ICDs in SCD-HeFT constituted the study population. The occurrence of RR-NSVT and its association with ICD shocks and mortality in SCD-HeFT were examined.
Results
RR-NSVT was documented on ICD interrogation in 186 of 811 patients (22.9%). The mean duration of RR-NSVT was 26.4 ± 9.1 beats (7.5 ± 2.6 s), with a mean cycle length of 259 ± 32 ms. Polymorphic RR-NSVT accounted for 56% of episodes. Compared with patients without RR-NSVT, those with RR-NSVT were less likely to be taking beta-blockers, statins, or aspirin at enrollment. After adjusting for other known predictors of mortality in SCD-HeFT, RR-NSVT was independently associated with appropriate ICD shocks (hazard ratio: 4.25; 95% confidence interval: 2.94 to 6.14; p < 0.0001), with all-cause mortality (hazard ratio: 2.40; 95% confidence interval: 1.62 to 3.54; p < 0.0001), and with a composite of all-cause mortality and appropriate ICD shocks (hazard ratio: 3.03; 95% confidence interval: 2.21 to 4.15; p < 0.0001).
Conclusions
RR-NSVT identified on routine ICD interrogation should be considered an important clinical event. RR-NSVT during ICD interrogation is associated with appropriate ICD shocks and all-cause mortality. The clinical evaluation of patients with RR-NSVT should include intensification of medical therapy, particularly beta-blockers, or other appropriate clinical interventions. (Sudden Cardiac Death in Heart Failure Trial [SCD-HeFT]; NCT00000609)
Keywords: arrhythmia, heart failure, implantable cardioverter-defibrillator, mortality, ventricular tachycardia
Patients with implantable cardioverter-defibrillators (by both electrophysiologists and implanting cardiologists, often in nurse-directed device clinics or via remote monitoring. Current device interrogations contain an increasingly large amount of data that require review beyond those rhythms that trigger ICD therapy. The significance of identifying rapid-rate nonsustained ventricular tachycardia (RR-NSVT) may be unclear.
Some studies have shown that nonsustained ventricular tachycardia (NSVT) increases mortality (1–3), while others have shown that it has no additional effect on mortality (4,5). These studies have generally used the occurrence of NSVT on ambulatory outpatient monitoring for analysis. However, the prognostic importance of finding RR-NSVT during routine ICD interrogation has not been studied in any large clinical trials. RR-NSVT that meets detection criteria for ICD therapy but terminates before the delivery of ICD therapy may well have different significance than short NSVT episodes identified on outpatient ambulatory monitoring. The purpose of this study was to examine the frequency and characteristics of RR-NSVT detected during ICD interrogation in patients with moderate heart failure (HF) and assess its association with appropriate shocks and mortality.
Methods
The study design, subject demographics, and main study outcomes of SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) have been reported previously (6,7). SCD-HeFT randomized 2,521 subjects in equal proportions to receive single-lead ICDs, amiodarone, or placebo. The median duration of follow-up was 45.5 months. Of the 829 patients randomized to receive ICDs, 17 refused the ICDs after randomization and 1 patient died before receiving the device. Therefore, 811 patients actually received ICDs. Among the 811 patients who received ICDs, there were 163 deaths, 42 among patients with RR-NSVT and 121 among those without RR-NSVT. Subjects enrolled in SCD-HeFT were at least 18 years of age, had chronic stable New York Heart Association class II or III HF for at least 3 months due to ischemic or nonischemic causes, had left ventricular ejection fraction ≤35%, and were on optimal HF medical therapy. Subjects were enrolled from September 16, 1997, to July 18, 2001, with follow-up continuing through October 31, 2003. Vital status was available for 100% of subjects at the end of the follow-up period. SCD-HeFT was approved by the institutional review committee at each participating site, and all subjects provided written informed consent.
The ICDs implanted in SCD-HeFT were single-lead devices (model 7223; Medtronic, Inc., Minneapolis, Minnesota) because there were no pre-trial indications for pacemaker therapy in the SCD-HeFT population. The maximal device output was 30 J for a total of 6 shocks per detected tachyarrhythmia episode.
SCD-HeFT included a pre-specified protocol for ICD programming. This included a single zone of therapy at a detection rate of ≥188 beats/min. The initial detection interval was for 18 of 24 beats, and the redetect interval was for 12 of 16 beats. RR-NSVT for which electrograms would be available were those that also met the detection criteria for ICD shock delivery but terminated before shock delivery or “abortion” of shock delivery. The SCD-HeFT protocol for therapy programming was shock-only mode. No antitachycardia pacing (ATP) was included in the implantation protocol, because the use of ATP was not considered routine for patients without known histories of ventricular tachycardia (VT) at the time the study was conducted. Pre-shock electrogram storage allowed for approximately 10 seconds of data. Post-shock electrograms stored up to 9 beats. The SCD-HeFT protocol for antibradycardia pacing was set to 50 beats/min with hysteresis of 34 beats/min, the minimal allowable rate.
All episodes of RR-NSVT meeting ICD detection criteria were reviewed by the SCD-HeFT ICD core laboratory and examined for morphology, ventricular origin confirmation, and cycle length. The arrhythmia confirmation algorithm in the Medtronic model 7223 would deliver a shock if any 2 of the 4 intervals after charge end were 60 ms over the fibrillation detection interval of 320 ms (≤380 ms). Thus, the VT could have terminated, but for instance, a single premature ventricular contraction after charge end could cause an ICD shock to be delivered.
Sponsorship and oversight of SCD-HeFT were provided by the National Heart, Lung, and Blood Institute. ICDs were contributed to the study by the manufacturer (Medtronic, Inc.). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Definitions
RR-NSVT was defined as any rhythm identified in the ICD log that met the ICD programmed detection criteria but self-terminated. Over the 5 years of the study, individual investigators occasionally implemented programming changes and, therefore, NSVT detected at shorter or longer detection integer counts or lower rates than the protocol-determined criteria (at least 18 beats in duration and at least 188 beats/min) were included in this analysis, if electrograms were available for review to determine a ventricular origin. The determination of polymorphic versus monomorphic depended on an assessment of QRS configuration consistency and cycle length variability on review by the investigators. If QRS configuration was variable from beat to beat and/or across the extent of the rhythm, coupled with a variable cycle length (generally ≥40 ms), the entire episode was considered polymorphic. A monomorphic episode was identified if the QRS configuration was identical or nearly so for all QRS complexes, with generally <40-ms variability in cycle length across the entire episode and QRS configuration distinct from the baseline rhythm QRS configuration.
Statistical analysis
Baseline clinical characteristics are summarized using medians with 25th and 75th percentiles for continuous variables and frequencies and percents for categorical variables. Group comparisons with respect to these characteristics were performed using likelihood ratio chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. The associations of RR-NSVT with the end points of: 1) appropriate shock (i.e., shock for VT or ventricular fibrillation [VF]); 2) total mortality; and 3) the composite of death or appropriate shock were examined using Cox proportional hazards models in which the first occurrence of RR-NSVT during the follow-up period was considered as a time-dependent covariate. In the appropriate shock and composite models, the first appropriate shock was credited to the RR-NSVT group only if it occurred after the first episode of RR-NSVT. Thus, these models specifically addressed the question of whether RR-NSVT is associated with subsequent first appropriate shock (i.e., appropriate shock among patients who had not yet experienced one at the time of their RR-NSVT episode). Cox models were adjusted for baseline prognostic factors identified in the full SCD-HeFT cohort to examine the added prognostic value (if any) of RR-NSVT beyond that of baseline clinical factors. The adjustment variables included age, sex, cause of HF, New York Heart Association class, time since HF diagnosis, left ventricular ejection fraction, distance covered on 6-minute walk, systolic blood pressure, diabetes, use of angiotensin-converting enzyme inhibitors, use of digoxin, use of beta-blockers, mitral regurgitation, renal insufficiency, substance abuse, baseline electrocardiographic intervals, and score on the Duke Activity Status Index (8,9).
Risk relationships are expressed as hazard ratios (HRs) with associated 95% confidence intervals (CIs) derived from the Cox models. A p value <0.05 was considered statistically significant. All analyses were performed using SAS version 8.2 (SAS Institute Inc., Cary, North Carolina).
Results
Frequency and characteristics of RR-NSVT
A total of 681 episodes of RR-NSVT were documented in 186 of the 811 patients who received ICDs (22.9%). The mean length of RR-NSVT was 26.4 ± 9.1 beats (7.5 ± 2.6 s) (Table 1). Of the 681 episodes, 381 (56%) were considered polymorphic and 300 (44%) were identified to be monomorphic (Figs. 1 and 2). The mean cycle length was 259 ± 32 ms. Polymorphic RR-NSVT had a shorter cycle length (243 ± 31 ms) compared with monomorphic RR-NSVT (273 ± 21 ms) (p < 0.0001). If only the final 8 beats of an RR-NSVT episode are considered, 38% of episodes were polymorphic, and 62% were monomorphic; that is, many episodes “stabilized” to monomorphic VT by the time of the final 8 beats.
Table 1.
Characteristics of RR-NSVT (n = 681)
| Characteristic | Mean ± SD | Median (25th, 75th Percentile) | Range |
|---|---|---|---|
| Duration of RR-NSVT episodes (number of beats) | 26.4 ± 9.1 | 24 (20, 31) | 12–51 |
| Duration of RR-NSVT episodes (seconds) | 7.5 ± 2.6 | 7 (6, 10) | 5–20 |
| Cycle length (ms) of all RR-NSVT episodes (n = 681) | 259 ± 32 | 260 (240, 280) | 180–350 |
| Cycle length (ms) of all monomorphic RR-NSVT (n = 300) | 273 ± 21 | 270 (260, 290) | 230–350 |
| Cycle length (ms) of all polymorphic RR-NSVT (n = 381) | 243 ± 31 | 260 (210, 270) | 180–310 |
The difference in cycle length between monomorphic and polymorphic episodes was statistically significant (p < 0.0001).
RR-NSVT = rapid-rate nonsustained ventricular tachycardia.
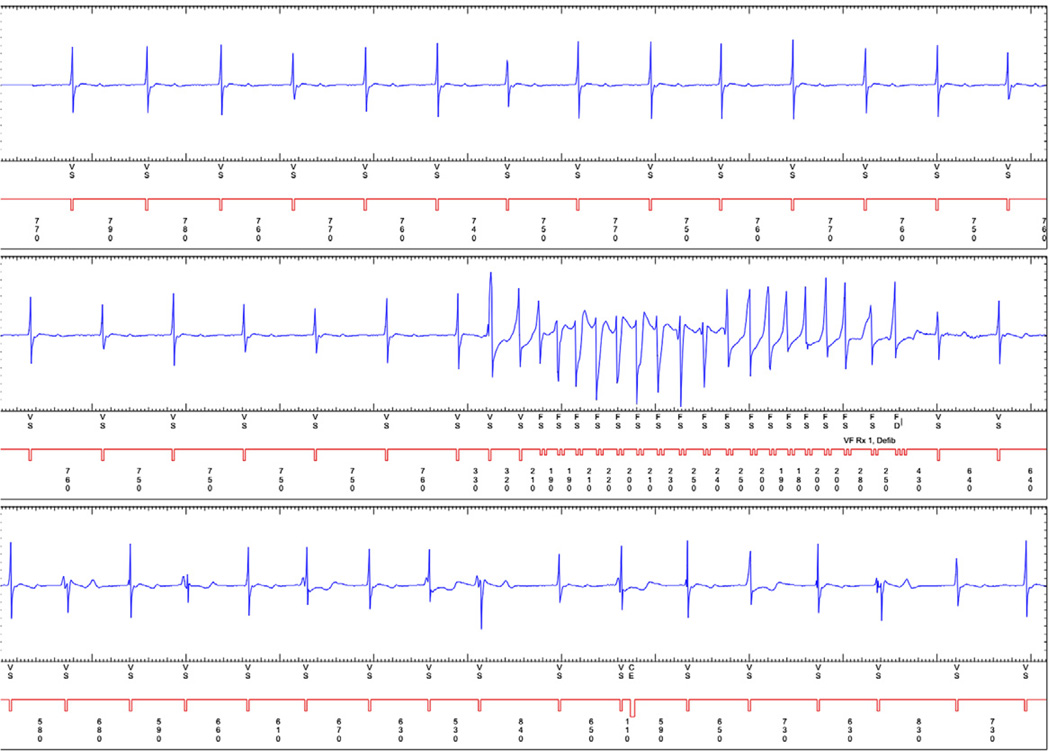
Figure 1. A Representative Example of Polymorphic Nonsustained Ventricular Tachycardia.
An implantable cardioverter-defibrillator electrogram demonstrating polymorphic nonsustained ventricular tachycardia.
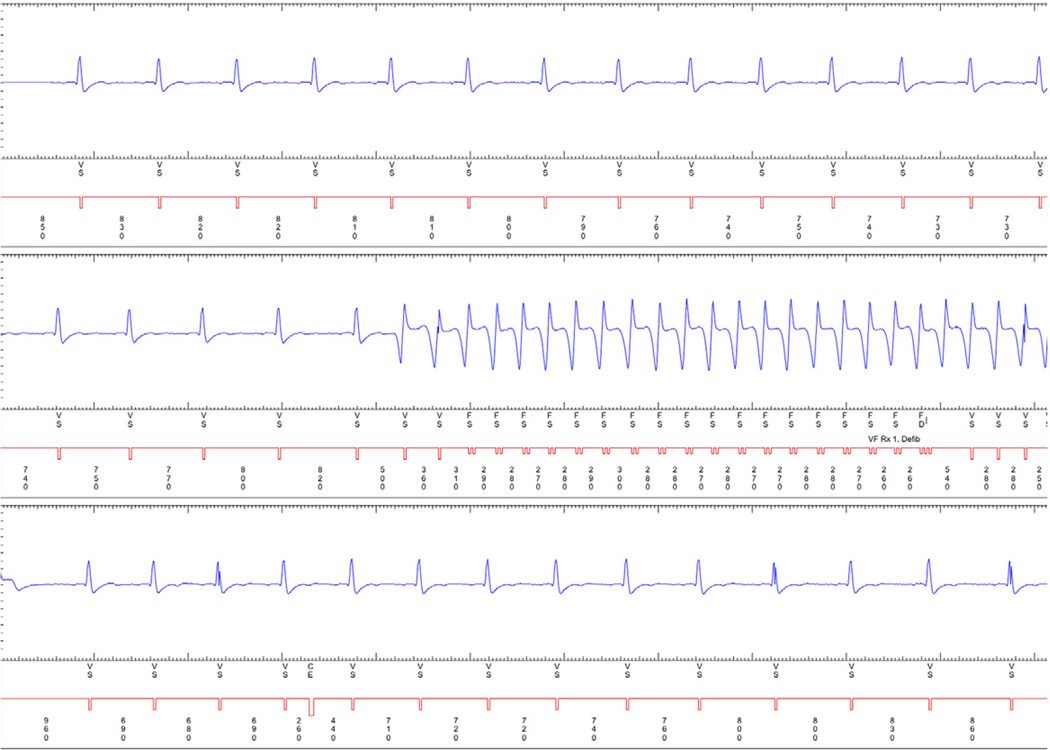
Figure 2. A Representative Example of Monomorphic Nonsustained Ventricular Tachycardia.
An implantable cardioverter-defibrillator electrogram demonstrating monomorphic nonsustained ventricular tachycardia.
Fifty-four of the 681 episodes (7.9%) received ICD shocks despite self-termination of the RR-NSVT. These shocks were considered inappropriate and occurred in 38 of the 186 patients (20.4%) who experienced 1 or more episodes of RR-NSVT (Fig. 3). Thus, in total, 4.7% of the 811 patients who received ICDs in SCD-HeFT received inappropriate shocks for RR-NSVT, or 0.94% per year during the SCD-HeFT follow-up period.
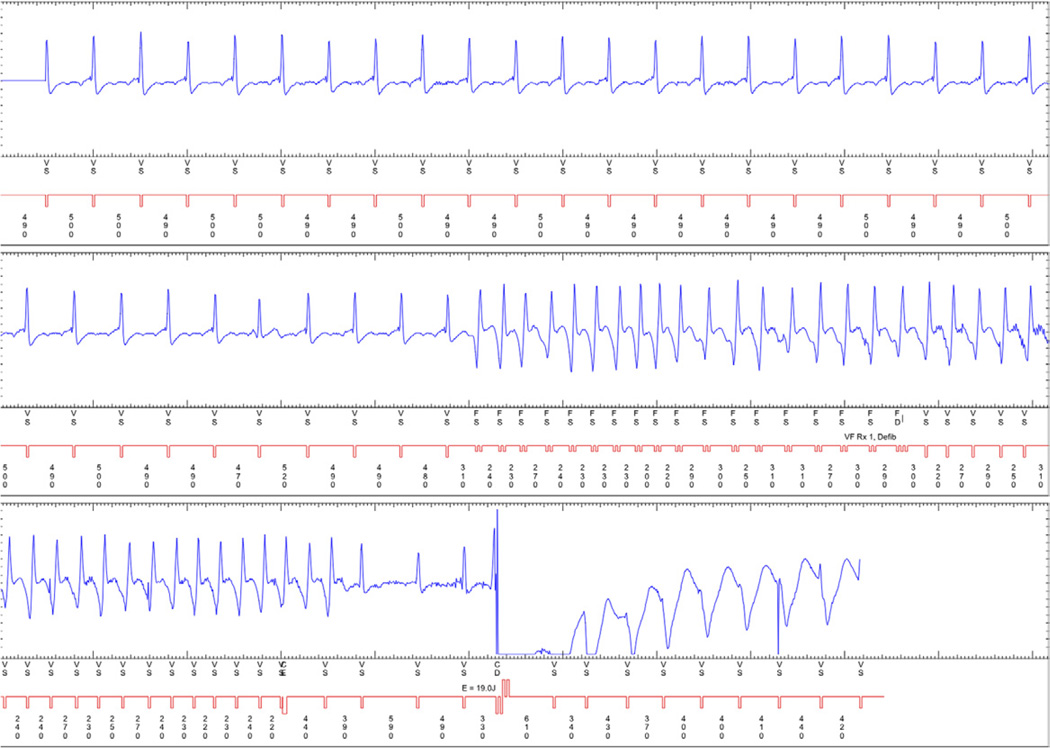
Figure 3. Nonsustained Ventricular Tachycardia Leading to an Inappropriate Shock.
An implantable cardioverter-defibrillator (ICD) electrogram demonstrating nonsustained ventricular tachycardia that self-terminates and is followed by an inappropriate ICD shock.
Clinical characteristics
The baseline clinical characteristics of ICD patients who experienced ≥1 episode of RR-NSVT during the trial are compared with the characteristics of those patients who did not experience any RR-NSVT in Table 2. Significant differences in patients with RR-NSVT compared with those without RR-NSVT included younger age, a higher percentage of men, lower ejection fractions, higher heart rates, more nonischemic causes of HF, less diabetes, and less hyperlipidemia. At baseline, patients with RR-NSVT were significantly more likely to be on an angiotensin-converting enzyme inhibitors or angiotensin receptor blockers but less likely to be on beta-blockers, statins, or aspirin compared with those without RR-NSVT.
Table 2.
Baseline Clinical Characteristics for Patients With RR-NSVT Versus No RR-NSVT
| Baseline Characteristic | RR-NSVT (n = 186) |
No RR-NSVT (n = 625) |
p Value* |
|---|---|---|---|
| Age (yrs) | 58 (50, 67) | 61 (53, 70) | 0.024 |
| Women | 17% (31) | 25% (154) | 0.020 |
| Nonwhite race | 27% (51) | 21% (132) | 0.076 |
| Weight (lb) | 192 (165, 226) | 189 (162, 217) | 0.14 |
| NYHA functional class III | 35% (66) | 31% (191) | 0.21 |
| Ischemic HF etiology | 40% (74) | 55% (346) | 0.001 |
| Ejection fraction (%) | 20 (18, 26) | 25 (20, 30) | 0.001 |
| Diabetes | 23% (42) | 33% (205) | 0.007 |
| Pulmonary disease | 22% (40) | 21% (131) | 0.87 |
| Hyperlipidemia | 42% (78) | 55% (343) | 0.002 |
| Hypertension | 56% (104) | 54% (337) | 0.63 |
| Atrial fibrillation/flutter | 18% (33) | 17% (106) | 0.80 |
| Syncope | 8% (15) | 6% (37) | 0.31 |
| QRS duration ≥120 ms | 41% (77) | 41% (259) | 0.99 |
| Systolic blood pressure (mm Hg) | 118 (106, 132) | 118 (104, 130) | 0.49 |
| Diastolic blood pressure (mm Hg) | 70 (62, 80) | 70 (60, 80) | 0.91 |
| Heart rate (beats/min) | 76 (68, 88) | 73 (64, 84) | 0.008 |
| Serum sodium (mEq/l) | 139 (137, 141) | 139 (137, 141) | 0.52 |
| Serum creatinine (mg/dl) | 1.10 (0.90, 1.30) | 1.10 (1.00, 1.40) | 0.34 |
| ACE inhibitors or ARBs | 98% (182) | 93% (584) | 0.011 |
| Beta-blockers | 59% (110) | 73% (455) | 0.001 |
| Digoxin | 72% (133) | 66% (411) | 0.14 |
| Aspirin | 51% (95) | 60% (372) | 0.041 |
| Statins | 26% (49) | 41% (256) | 0.001 |
| Aldosterone antagonist | 15% (27) | 20% (126) | 0.076 |
Values are median (25th, 75th percentile) or % (n).
Likelihood ratio chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; HF = heart failure; NYHA = New York Heart Association; RR-NSVT = rapid-rate nonsustained ventricular tachycardia.
RR-NSVT and appropriate ICD shocks
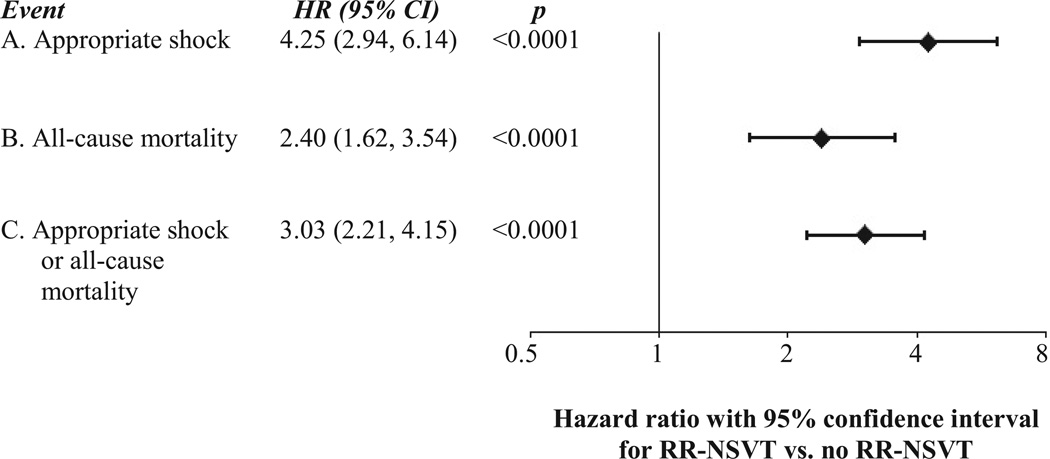
Of the 811 patients with ICDs, 182 patients had 1 or more appropriate ICD shocks for VT or VF with SCD-HeFT protocol programming. Eighty-six of the 182 patients (47.3%) also had 1 or more episodes of RR-NSVT. The time course of first RR-NSVT or first appropriate shock was variable, with 46 of the 86 patients with RR-NSVT (53%) having at least 1 RR-NSVT episode before any appropriate shock and 40 patients (47%) having at least 1 appropriate shock before any RR-NSVT. In a multivariable Cox model adjusted for independent predictors of mortality, RR-NSVT was found to be highly associated with the risk for a subsequent first appropriate shock (HR: 4.25; 95% CI: 2.94 to 6.14; p < 0.0001) (Fig. 4).
Figure 4. Association of RR-NSVT With Appropriate Shocks for VT/VF, All-Cause Mortality and a Composite of Appropriate Shocks Figur and All-Cause Mortality.
The association of RR-NSVT with A) the occurrence of appropriate implantable cardioverter-defibrillator (ICD) shocks; B) all-cause mortality, and C) Appropriate shocks or all-cause mortality. All outcomes are adjusted for baseline prognostic factors identified in the SCD-HeFT trial (age, sex, cause of heart failure, New York Heart Association functional class, time since the diagnosis of heart failure, left ventricular ejection fraction, distance covered on a 6-minute walk, systolic blood pressure, diabetes, use of an ACE-inhibitor, use of digoxin, use of beta blocker, mitral regurgitation, renal insufficiency, history of substance abuse, baseline electrocardiographic intervals, and score on the Duke Activity Status Index8–9).
RR-NSVT = Rapid Rate Nonsustained VT; Appropriate shocks are those ICD shocks delivered for ventricular tachycardia or ventricular fibrillation; CI = confidence interval; HR = hazard ratio; VT = ventricular tachycardia; VF = ventricular fibrillation.
Baseline clinical characteristics of the 86 patients with RR-NSVT who also had appropriate shocks for VT or VF were compared with those of the 100 patients with NSVT who never had appropriate shocks for VT or VF over the course of the study. Patients with RR-NSVT and appropriate shocks were older (p = 0.018) and less likely to be on beta-blockers (p = 0.040) compared with those without appropriate shocks (Table 3).
Table 3.
Baseline Clinical Characteristics for Patients With RR-NSVT With Appropriate ICD Shocks Versus Those With RR-NSVT Without Appropriate ICD Shocks
| Baseline Characteristic | RR-NSVT With APP (n = 86) |
RR-NSVT Without APP (n = 100) |
p Value* |
|---|---|---|---|
| Age (yrs) | 63 (51, 69) | 56 (48, 65) | 0.018 |
| Women | 15% (13) | 18% (18) | 0.60 |
| Nonwhite race | 23% (20) | 31% (31) | 0.24 |
| Weight (lb) | 192 (160, 221) | 193 (167, 227) | 0.55 |
| NYHA functional class III | 41% (35) | 31% (31) | 0.17 |
| Ischemic HF etiology | 47% (40) | 34% (34) | 0.082 |
| Ejection fraction (%) | 20 (18, 25) | 22 (16, 30) | 0.41 |
| Diabetes | 20% (17) | 25% (25) | 0.39 |
| Pulmonary disease | 24% (21) | 19% (19) | 0.37 |
| Hyperlipidemia | 43% (37) | 41% (41) | 0.78 |
| Hypertension | 51% (44) | 60% (60) | 0.23 |
| Atrial fibrillation/flutter | 19% (16) | 17% (17) | 0.78 |
| NSVT (before enrollment) | 38% (33) | 29% (29) | 0.18 |
| Syncope | 10% (9) | 6% (6) | 0.27 |
| QRS duration ≥120 ms | 43% (37) | 40% (40) | 0.68 |
| Systolic blood pressure (mm Hg) | 120 (108, 132) | 115 (106, 131) | 0.38 |
| Diastolic blood pressure (mm Hg) | 70 (63, 80) | 70 (61, 79) | 0.46 |
| Heart rate (beats/min) | 76 (68, 88) | 76 (68, 88) | 0.99 |
| Serum sodium (mEq/l) | 139 (137, 141) | 139 (137, 141) | 0.91 |
| Serum creatinine (mg/dl) | 1.10 (0.96, 1.30) | 1.10 (0.90, 1.30) | 0.84 |
| ACE inhibitors or ARBs | 97% (83) | 99% (99) | 0.24 |
| Beta-blocker | 51% (44) | 66% (66) | 0.040 |
| Digoxin | 76% (65) | 68% (68) | 0.25 |
| Aspirin | 53% (46) | 49% (49) | 0.54 |
| Statins | 26% (22) | 27% (27) | 0.83 |
| Aldosterone antagonist | 10% (9) | 18% (18) | 0.14 |
Values are median (25th, 75th percentile) or % (n).
Likelihood ratio chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
APP = appropriate ICD shock for ventricular tachycardia or ventricular fibrillation; ICD = implantable cardiac defibrillator; NSVT = nonsustained ventricular tachycardia. Other abbreviations as in Table 2.
RR-NSVT and all-cause mortality
In multivariable Cox analyses adjusted for other independent predictors of mortality identified in SCD-HeFT, RR-NSVT was associated with a >2-fold higher risk for death compared with patients without RR-NSVT (HR: 2.40; 95% CI: 1.62 to 3.54; p < 0.0001) and a 3-fold risk for appropriate shock or death (HR: 3.03; 95% CI: 2.21 to 4.15; p < 0.0001) (Fig. 4).
Discussion
We performed the first study investigating the characteristics and prognosis of RR-NSVT detected on routine ICD interrogation in patients with moderate HF with primary prevention ICD therapy. The main result of this study is that long runs of RR-NSVT discovered during routine ICD interrogation are associated with subsequent appropriate ICD shocks and an increase in mortality. Prior studies examining the significance of NSVT have relied on Holter ambulatory electrocardiographic monitoring or telemetric monitoring and have included any NSVT regardless of rate and duration (generally <10 beats) (1–5). Considerable ambiguity regarding the significance of NSVT resulted from these studies, with some showing that NSVT increases mortality (1–3) and others showing that NSVT had no effect on mortality in patients with cardiovascular disease (4,5). Although the clinical implications of NSVT in patients without ICDs have shown conflicting outcomes (1–5), this study has provided new information regarding RR-NSVT identified during routine interrogation of ICDs.
Our study is unique, as the NSVT we studied was that found on routine ICD interrogation meeting criteria to potentially trigger ICD therapy. One advantage of our study is the recording capabilities of an ICD. In contrast, previous studies looking at the prognostic implications of NSVT did so using only Holter monitors or hospital telemetric monitoring (1–5) and thus recorded patients’ heart rhythms only for brief periods of time, unlike an ICD, which continuously records rhythms above the programmed VT or VF rate zone. Our result is particularly important given the increased use of remote ICD monitoring, where the finding of RR-NSVT might be ignored if ICD therapy was not delivered. However, our results suggest that RR-NSVT discovered during routine ICD interrogation should be considered an important clinical event, similar to sustained VT or VF requiring an ICD shock.
It is not surprising that we also identified an association of RR-NSVT occurrence with an increased likelihood of an appropriate shock for VT or VF; however, the temporal relationship between RR-NSVT and sustained VT or VF was variable. RR-NSVT preceded the first occurrence of sustained VT or VF in only about half of the patients who experienced both events. Previous studies have shown that in patients with HF with ICDs implanted for primary prevention, ICD shocks are associated with higher mortality (9,10). Approximately 50% of patients with appropriate ICD shocks also had RR-NSVT. Because of the variable relationship of the occurrence of RR-NSVT and sustained VT or VF triggering appropriate shocks, and the variable time relationship between the occurrence of the 2 rhythms in patients who experienced both, the ability to separate the mortality risks of RR-NSVT and rhythms lasting long enough to result in an appropriate shock was not possible in this analysis.
Another important finding is that RR-NSVT was frequently identified (23% of the patients with ICDs) and was often polymorphic. The modestly long detection interval, combined with a capacitor charge time of about 4 to 15 s, depending on battery age and shock strength, allowed the identification of many self-terminating VT episodes. If the detection time or charge time had been faster, many otherwise self-terminating VT events would have been treated and categorized as “appropriate” shocks for VT or VF.
Similar observations regarding self-terminating VT have previously been noted in an analysis of the Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (Pain-FREE Rx II) trial, in which many more appropriate ICD-treated VT episodes were identified in patients randomized to receive ATP therapy first (a therapy that will be delivered sooner than shock therapy) compared with those randomized to receive only ICD shock therapy (11). In the shock-only treatment arm, 34% of fast VT episodes terminated spontaneously before therapy (11), suggesting that many of the ATP interventions, although successful at terminating the VT, may in fact have been unneeded. A high rate of self-terminating VT was also observed in MIRACLE-ICD (Multicenter InSync ICD Randomized Clinical Evaluation), in which 48% of VF or VT episodes converted spontaneously during the ICD charge-up cycle (12).
The longer detection time used in SCD-HeFT was novel for that era, and this factor, in addition to a single high-heart rate treatment zone (≥188 beats/min), may well have protected many patients from unnecessary shocks. More recently, the use of both longer detection times and higher heart rate treatment zones has received widespread acceptance on the basis of ICD programming strategy studies evaluating this question (13–15). Also, the improved redetection confirmation algorithms of contemporary devices will decrease the unneeded shocks for otherwise self-terminating VT. Thus, relatively long detection times are an important factor in minimizing unneeded ICD therapy, be it shocks or ATP, both of which could be particularly detrimental in polymorphic VT.
A final interesting observation from our study was that patients with RR-NSVT were less likely to be taking beta-blockers or statins at enrollment into the trial than patients without RR-NSVT. Recently, there are data to suggest that statins may be associated with a decrease in sudden cardiac death in patients with HF (16,17). We cannot state from our data analysis whether the absence of beta-blocker or statin therapy in these patients identifies a previously overlooked therapeutic opportunity or instead serves as an indicator of a more ill patient population.
Study limitations
For the majority of patients in this trial, only NSVT ≥188 beats/min (≤320 ms) that met the programmed detection criteria for the ICD could be identified in this study. Thus, only longer and faster episodes of NSVT were documented. We therefore cannot state that similar findings would have been observed with slower and shorter episodes of NSVT. Additionally, the question of whether RR-NSVT effect on mortality is independent of appropriate shocks for longer episodes of VT or VF cannot be clearly separated by this patient cohort and analysis.
Conclusions
The significant correlation between RR-NSVT identified on ICD interrogation with both appropriate ICD shocks for VT or VF and all-cause mortality should prompt physicians to consider long runs of RR-NSVT as important clinical events. Similar to patients with ICD shocks for VT and VF who have been previously identified as being a higher risk patient population (9,10), the clinical evaluation of patients with RR-NSVT on ICD interrogation should be directed at maximizing HF medical management and evaluating patients for remedial conditions, such as ischemia, or considering the need for additional advanced HF therapies.
Acknowledgments
This study was supported by grants U01 HL55766, U01 HL55297, and U01 HL55496 from the National Heart, Lung, and Blood Institute and by Medtronic. Ms. Anderson is a consultant for Boston Scientific Corporation. Dr. Mark has received research funding from Eli Lilly & Company, Medtronic, Gilead, and AstraZeneca. Dr. Lee performs limited consulting for Medtronic. Dr. Bardy has received research funding from St. Jude Medical, is a consultant for and board member of Cameron Health Corporation and has equity and intellectual property rights with the company, and has received research funding from and has intellectual property with Cardiac Science. Dr. Poole has received lecture fees from Biotronik, Boston Scientific Corporation, Medtronic, and St. Jude Medical; has received compensation for scientific advisory board memberships from Boston Scientific Corporation, Cardiac Science, and Cameron Health; and has equity options in Cameron Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations And Acronyms
- ATP
antitachycardia pacing
- CI
confidence interval
- HF
heart failure
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- NSVT
nonsustained ventricular tachycardia
- RR-NSVT
rapid-rate nonsustained ventricular tachycardia
- VF
ventricular fibrillation
- VT
ventricular tachycardia
REFERENCES
- 1.Cheema AN, Sheu K, Parker M, Kadish AH, Goldberger JJ. Nonsustained ventricular tachycardia in the setting of acute myocardial infarction: tachycardia characteristics and their prognostic implications. Circulation. 1998;98:2030–2036. doi: 10.1161/01.cir.98.19.2030. [DOI] [PubMed] [Google Scholar]
- 2.Doval HC, Nul DR, Grancelli HO, et al. Nonsustained ventricular tachycardia in severe heart failure: independent marker of increased mortality due to sudden death: GESICA-GEMA Investigators. Circulation. 1996;94:3198–3203. doi: 10.1161/01.cir.94.12.3198. [DOI] [PubMed] [Google Scholar]
- 3.Pires LA, Lehmann MH, Buston AE, Hafley GE, Lee KL for the Multicenter Unsustained Tachycardia Trial Investigators. Differences in inducibility and prognosis of in-hospital versus out-of-hospital identified nonsustained ventricular tachycardia in patients with coronary artery disease: clinical and trial design implications. J Am Coll Cardiol. 2001;38:1156–1162. doi: 10.1016/s0735-1097(01)01482-6. [DOI] [PubMed] [Google Scholar]
- 4.Singh SN, Fisher SG, Carson PE, Fletcher RD for the Department of Veterans Affairs CHF STAT Investigators. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. J Am Coll Cardiol. 1998;32:942–947. doi: 10.1016/s0735-1097(98)00338-6. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Jalaluddin M, Anderson S, et al. for the PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. Circulation. 2000;101:40–46. doi: 10.1161/01.cir.101.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Bardy GH, Lee KL, Mark DB, Poole JE, Fishbein DP . the SCD-HeFT Investigators. Sudden Cardiac Death-Heart Failure Trial (SCD-HeFT) In: Woosley RL, Singh SN, editors. Arrhythmia Treatment and Therapy. New York, NY: Marcel Dekker; 2000. pp. 323–342. [Google Scholar]
- 8.Hlatsky MA, Boineau RE, Higgenbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 9.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daubert JP, Zareba W, Cannom DS for the MADITII Investigators. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;14:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 11.Wathen MS, DeGroot PJ, Sweeney MO PainFREE Rx IIInvestigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (Pain-FREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 12.Wilkoff BL, Hess M, Young J, Abraham WT. Difference in tachyar-rhythmia detection and implantable cardioverter defibrillator therapy by primary or secondary prevention indication in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2004;9:1002–1009. doi: 10.1046/j.1540-8167.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilkoff BL, Williamson BD, Stern RS, et al. for the PREPARE Investigators. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Gasparini M, Menozzi C, Proclemer A, et al. A simplified biventricular defibrillator with fixed long detection intervals reduces implantable cardioverter defibrillator (ICD) interventions and heart failure hospitalizations in patients with non-ischaemic cardiomyopathy implanted for primary prevention: the RELEVANT [Role of Long Detection Window Programming in Patients With Left Ventricular Dysfunction, Non-Ischemic Etiology in Primary Prevention Treated With a Biventricular ICD] study. Eur Heart J. 2009;22:2758–2767. doi: 10.1093/eurheartj/ehp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AJ, Schuger C, Beck CA for the MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 16.Levantesi G, Scarano M, Marfisi R, et al. Meta-analysis of effect of statin treatment on risk of sudden death. Am J Cardiol. 2007;100:1644–1650. doi: 10.1016/j.amjcard.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Vrtovec B, Okrajsek R, Golicnik A, et al. Atorvastatin therapy may reduce the incidence of sudden cardiac death in patients with advanced chronic heart failure. J Cardiovasc Fail. 2008;2:140–144. doi: 10.1016/j.cardfail.2007.10.013. [DOI] [PubMed] [Google Scholar]