Abstract
Previously, we reported significant bone mineral density (BMD) loss in postmenopausal women after modest weight loss. It remains unclear whether the magnitude of BMD change in response to weight loss is appropriate (i.e., proportional to weight loss) and whether BMD is recovered with weight regain. We now report changes in BMD after a 1-year follow-up. Subjects (n = 23) in this secondary analysis were postmenopausal women randomized to placebo as part of a larger trial. They completed a 6-month exercise-based weight loss program and returned for follow-up at 18 months. Dual-energy X-ray absorptiometry (DXA) was performed at baseline, 6, and 18 months. At baseline, subjects were aged 56.8 ± 5.4 years (mean ± s.d.), 10.0 ± 9.2 years postmenopausal, and BMI was 29.6 ± 4.0 kg/m2. They lost 3.9 ± 3.5 kg during the weight loss intervention. During follow-up, they regained 2.9 ± 3.9 kg. Six months of weight loss resulted in a significant decrease in lumbar spine (LS) (−1.7 ± 3.5%; P = 0.002) and hip (−0.04 ± 3.5%; P = 0.03) BMD that was accompanied by an increase in a biomarker of bone resorption (serum C-terminal telopeptide of type I collagen, CTX: 34 ± 54%; P = 0.08). However, weight regain was not associated with LS (0.05 ± 3.8%; P = 0.15) or hip (−0.6 ± 3.0%; P = 0.81) bone regain or decreased bone resorption (CTX: −3 ± 37%; P = 0.73). The findings suggest that BMD lost during weight reduction may not be fully recovered with weight regain in hormone-deficient, postmenopausal women. Future studies are needed to identify effective strategies to prevent bone loss during periods of weight loss.
INTRODUCTION
Overweight and obese postmenopausal women are at increased risk for multiple comorbidities, including cardiovascular disease, diabetes, and musculoskeletal pain. Weight reduction is commonly recommended to combat these risks. However, weight loss has been associated with a loss of bone mineral density (BMD) in postmenopausal women (1–3) and increased risk for osteoporotic fracture (4,5). The vast majority of women gain back weight after a period of voluntary weight loss (6) but it is unknown whether this weight regain is accompanied by recovery of BMD.
In postmenopausal women, both fat-free mass and fat mass are significant determinants of BMD (7,8). We previously reported significant decreases in BMD following modest exercise-induced weight loss, even though fat-free mass was fully preserved (1). It remains unclear whether weight loss-induced bone loss is an appropriate adaptation to reduced loading forces, or whether systemic factors related to negative energy balance during weight loss trigger excess bone loss. If the latter hypothesis is correct, it suggests that weight regain may not restore BMD.
Previously, we published our findings that sex hormone-deficient women had decreases in BMD in response to modest weight loss when compared with weight-stable controls (1). The purpose of this study was to determine whether weight regain restored BMD. Weight-reduced women from the previous study were followed for an additional 12 months with reassessment of BMD and body composition by dual-energy X-ray absorptiometry (DXA). We hypothesized that there would be no significant increase in BMD in response to weight regain.
METHODS AND PROCEDURES
Study populations
Inclusion and exclusion criteria for the study population have been previously reported (1). Briefly, the inclusion criteria for study participants were: postmenopausal women, aged 50–70 years, no sex hormone therapy or drugs that influence bone metabolism for at least 6 months, no diabetes or cardiovascular disease, normal pap smear and mammogram in the past 12 months, nonsmokers, and overweight or moderately obese. Screening tests included a medical history, physical examination, blood chemistries, 12-lead electrocardiogram, and an exercise stress test. All subjects were confirmed to be euthyroid or on adequate replacement therapy based on a normal ultrasensitive thyroid stimulating hormone level. The Colorado Multiple Institutional Review Board approved the study. All volunteers who underwent screening for the study provided written informed consent to participate.
For the parent study, eligible volunteers were randomized to three treatment arms, which were administered in a double-blinded manner: placebo, raloxifene, or hormone therapy. To separate the effects of weight regain from the effects of drug treatment, only those women assigned to placebo treatment (n = 38) were included in the present analysis. Participants who started bisphosphonate therapy (n = 4) during the study were also excluded from the analysis. For the first 6 months of treatment, subjects participated in a supervised endurance exercise training program (i.e., treadmill walking/running, rowing, cycling, and/or elliptical exercise) to induce a weight loss of 4–5 kg (1). After the 6-month weight loss intervention, women remained on study drug (placebo for women included in this analysis) and were followed for an additional 12 months. During this time they no longer participated in the supervised exercise program. Follow-up DXA scans were performed at 18 months. During the follow-up phase, 15 (39%) participants in the placebo arm were lost to evaluation due to time commitment(3), personal (5), medical (1), relocation (1), or unknown (5) reasons. The remaining 23 women included 19 whites, 3 black/African Americans, and 1 American Indian/Alaska Native.
DXA
Participants had DXA scans performed at baseline, 6, and 18 months using either a Lunar DPX-IQ instrument (Madison, WI) or Hologic Delphi-W instrument (Waltham, MA) because of a programmatic plan at the institution that phased out the use of the Lunar instrument. For each individual, the baseline and all follow-up scans were obtained on the same instrument. Total body, lumbar spine (LS) (L2-L4), and proximal femur (total hip, femoral neck, trochanter, subtrochanteric region) scans were performed at each visit. Body composition outcomes (total mass, fat mass, and fat-free mass) were measured during the total body scans. Lunar extended research analysis software version 4.7c and Hologic software version 11.2 were used for both total and central BMD measures.
Bone turnover markers
Markers of bone formation (bone-specific alkaline phosphatase; Quidel, San Jose, CA) and resorption (C-terminal telopeptide of type I collagen, CTX; NordicBioscience Diagnostics, Herlev, Denmark) were determined from fasted morning serum samples acquired at baseline, 6 months, and 18 months and stored at −80 °C. No exercise was performed for at least 24 h prior to blood sampling. In our laboratory, the intra-assay coefficients of variation for bone-specific alkaline phosphatase and CTX are 7.5 ± 7.1% and 6.2 ± 4.4%, respectively, in samples from older women and men. Because the interassay coefficients of variation are higher (11.5 ± 9.5% and 20.5 ± 11.3%, respectively), all samples for an individual were analyzed in batch.
Aerobic power (VO2 peak)
Aerobic power was directly measured at baseline, 6 months, and 18 months using an individualized treadmill protocol with open-circuit spirometry (ParvoMedics, Sandy, Utah). Subjects warmed up to determine a comfortable walking speed that elicited a heart rate of ~60–70% of age predicted maximum. During the test, this speed was maintained but the treadmill elevation was increased by 2% every 2 min. Heart rate was monitored continuously using a 12-lead electrocardiogram (Quinton Q4500; Quinton Instruments, Seattle, WA) and blood pressure was measured during each exercise stage. VO2peak was attained when subjects met one of the following criteria: (i) heart rate within 10 beats per minute of age predicted maximum, (ii) respiratory quotient ≥1.1, or 3) plateau in VO2.
Statistical methods
The study was exploratory and used the subset of women who had participated in the weight loss program, were in the placebo treatment arm, and had 18-month follow-up data available. The primary objective was to determine the effect of weight change on BMD during the weight loss period (0–6 months) and the weight regain period (6–18 months). The effects of weight change on change in BMD over time were evaluated using maximum likelihood estimates in a repeated measures model to account for within-subject correlation among the repeated measures. For each BMD site, the change in BMD was regressed on the baseline value of BMD, the change in weight, time, and weight change-by-time interaction. The same approach was used to evaluate the effects of weight change and time on bone markers bone-specific alkaline phosphatase and CTX. Changes in body composition were regressed on baseline measures of body composition and time.
To further illustrate the effects of weight loss and gain on changes in BMD, we also compared the subgroups that were above vs. below the median weight change from 0 to 6 months. These are referred to as the HI weight loss subgroup (0–6 month weight change below the group median; ≤−4.5 kg) and the LO weight loss subgroup (0–6 month weight change above the group median; >−4.5 kg). Baseline differences between women who were lost to follow-up and those who completed testing were compared using unpaired, two-tailed, t-tests for continuous variables and χ2 tests for categorical variables.
For all analyses, statistical significance was defined as α ≤0.05. Data are reported as mean ± s.d. unless otherwise stated. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Subjects (n = 23) were aged 56.8 ± 5.4 years, 10.0 ± 9.2 years postmenopausal, with a BMI of 29.6 ± 4.0 kg/m2. Based on the definition of t-score <−1 at LS, total hip, femoral neck, or trochanter, 39% of subjects had low bone mass or osteoporosis (n = 1) at baseline. There were no significant differences in baseline characteristics between subjects included in this analysis and those lost to follow-up (n = 15; data not shown).
Weight loss during the 6-month intervention averaged −3.9 ± 3.5 kg and consisted entirely of fat loss (−3.6 ± 3.1 kg); there was no change in fat-free mass (Table 1). By 18 months, subjects had gained back an average of 2.9 ± 3.9 kg, which was almost entirely fat mass (2.7 ± 3.8 kg). The significant improvement in aerobic power during the exercise intervention followed by a significant decline in fitness by 18 months suggests that the changes in body composition were mediated by increases and decreases in energy expenditure, respectively.
Table 1.
Baseline values, absolute and relative changes in body composition, bone mineral density (BMD), bone markers and aerobic power during the exercise-induced weight loss intervention (0–6 months) and the follow-up period (6– 18 months)
| Baseline values
|
Absolute and relative changes (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0–6 months
|
6–18 months
|
|||||||
| N | Mean ± s.d. | N | Mean ± s.d. | P | N | Mean ± s.d. | P | |
| Body composition (kg) | ||||||||
| Body mass | 23 | 78.7 ± 11.7 | 23 | −3.9 ± 3.5 | <0.001 | 23 | 2.9 ± 3.9 | 0.001 |
| % Change | −5.1 ± 4.7 | 4.0 ± 5.5 | ||||||
| Fat mass | 23 | 34.6 ± 7.7 | 23 | −3.6 ± 3.1 | <0.001 | 23 | 2.7 ± 3.8 | 0.001 |
| % Change | −11.4 ± 10.0 | 10.3 ± 14.5 | ||||||
| Fat-free mass | 23 | 44.1 ± 5.5 | 23 | −0.21 ± 1.2 | 0.54 | 23 | 0.2 ± 2.0 | 0.56 |
| % Change | −0.39 ± 2.9 | 0.35 ± 4.5 | ||||||
| BMD (g/cm2) | ||||||||
| Lumbar spine | 23 | 1.1 ± 0.15 | 23 | −0.02 ± 0.04 | 0.002 | 23 | 0.00 ± 0.04 | 0.15 |
| % Change | −1.7 ± 3.5 | 0.05 ± 3.8 | ||||||
| Hip | 23 | 0.9 ± 0.10 | 23 | 0.00 ± 0.03 | 0.03 | 23 | −0.01 ± 0.03 | 0.81 |
| % Change | −0.04 ± 3.5 | −0.6 ± 3.0 | ||||||
| Femoral neck | 23 | 0.9 ± 0.10 | 23 | −0.01 ± 0.03 | 0.68 | 23 | −0.01 ± 0.04 | 0.99 |
| % Change | −1.0 ± 3.4 | −0.5 ± 4.6 | ||||||
| Trochanter | 23 | 0.7 ± 0.09 | 23 | −0.00 ± 0.03 | 0.14 | 23 | 0.00 ± 0.03 | 0.67 |
| % Change | −0.1 ± 4.5 | 0.3 ± 3.9 | ||||||
| Subtrochanter | 23 | 1.1 ± 0.13 | 23 | 0.02 ± 0.04 | 0.08 | 23 | −0.01 ± 0.04 | 0.63 |
| % Change | 1.4 ± 3.7 | −1.2 ± 3.2 | ||||||
| Bone markers | ||||||||
| CTX (ng/ml) | 21 | 0.6 ± 0.4 | 21 | 0.12 ± 0.22 | 0.08 | 20 | −0.06 ± 0.2 | 0.73 |
| % Change | 34.1 ± 54.2 | −3.2 ± 36.9 | ||||||
| BAP (U/l) | 21 | 29.6 ± 8.5 | 21 | −1.2 ± 6.7 | 0.004 | 20 | 2.5 ± 7.1 | 0.12 |
| % Change | −0.7 ± 22.0 | 10.0 ± 26.8 | ||||||
| Aerobic power | ||||||||
| VO2peak (ml/kg/min) | 21 | 22.0 ± 4.5 | 20 | 1.6 ± 2.4 | 0.01 | 17 | −1.4 ± 2.4 | 0.03 |
| % Change | 8.0 ± 11.4 | −5.3 ± 9.4 | ||||||
| VO2peak (l/min) | 21 | 1.73 ± 0.29 | 20 | 0.04 ± 0.19 | 0.60 | 20 | −0.03 ± 0.16 | 0.51 |
| % Change | 0.02 ± 0.11 | −0.02 ± 0.09 | ||||||
P values are based on a repeated measured model regressing the change in each measure on the change in weight in each interval, conditioning on baseline. Regressions for the 3 body composition measures did not include weight change as a covariate.
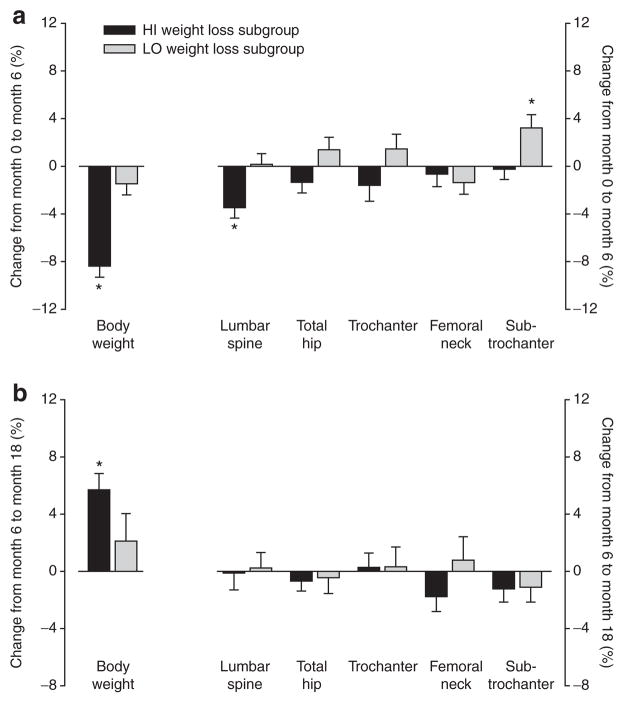
Although the 6-month exercise-induced weight loss intervention resulted in a significant association between weight loss and a decrease in LS and hip BMD, weight regain over the 12-month follow-up period was not associated with significant bone regain in either region (Table 1). There were no significant changes in other bone regions (trochanter, femoral neck, or subtrochanter) during either weight loss or regain (Table 1). When participants were divided into HI and LO weight loss categories based on the median change during the 0- to 6-month intervention, there was a significant decline in BMD in the HI group from 0 to 6 months, but no regain of BMD from 6 to 18 months even though there was a significant regain of body weight (Figure 1). Interestingly, the LO weight loss group demonstrated a significant increase in subtrochanteric BMD from 0 to 6 months.
Figure 1.
Relative changes in body weight and bone mineral density (BMD) (a) during the weight loss intervention (0 to 6 months) and (b) during follow-up (6 to 18 months); black bars represent cases below the median weight change from 0 to 6 months (HI weight loss subjects; n = 12) and gray bars represent cases above the median weight change from 0 to 6 months (LO weight loss subjects; n = 11). *P < 0.05 using a two-group t-test.
There was an increase in bone resorption from baseline to 6 months, as reflected by the relative increase in CTX (Table 1), and this was weakly associated with weight loss (P = 0.08). However, this increased resorption did not reverse during follow-up, as CTX remained unchanged from 6 to 18 months. For bone-specific alkaline phosphatase, a marker of bone formation, there was a decrease from baseline to 6 months and from 6 to 18 months (Table 1), which was associated with weight loss (P = 0.004). The secondary analysis supported the above findings with a relative increase in CTX from 0 to 6 months in the HI weight loss group (41 ± 61%, P = 0.047), but no change in CTX from 6 to 18 months (−7 ± 22%, P = 0.36).
Changes in bone mineral content (BMC) and area for all skeletal regions of interest were also examined (data not shown). There were small but significant increases in trochanter BMC (0.39 ± 0.77 g) and area (0.54 ± 0.82 cm) and decreases in sub-trochanter BMC (−0.27 ± 0.78 g) and area (−0.48 ± 0.50 cm) from month 0 to 6. No other changes in BMC or area during weight loss or gain were significant.
DISCUSSION
The aim of this study was to gain insights into whether the decrease in BMD in response to weight loss in postmenopausal women that we and others have observed (1–3) is an appropriate or inappropriate adaptation to reduced body weight. If it is an appropriate adaptation, it seems plausible that BMD would be recovered with subsequent weight regain. Conversely, if bone mineral loss represents an inappropriate adaptation to weight loss, there may be little, if any, recovery of BMD during weight regain. This was evaluated in the current study by conducting follow-up evaluations of postmenopausal women who had participated in an exercise-induced weight loss intervention in our laboratory (1). The major finding of this follow-up study was that the decreases in LS and hip BMD in response to modest weight loss were not recovered over the subsequent 12 months, despite the fact that significant weight regain occurred. In contrast, there were no significant effects of weight loss or regain on BMD of any subregion in the proximal femur. However, this negative finding must be interpreted cautiously because the study may not have been adequately powered to detect changes. In the larger cohort we reported on previously, weight loss resulted in significant reductions in total hip and trochanter BMD (1). In fact, the BMD loss at the total hip (~1.5% over 6 months) in the HI weight loss subgroup was of a magnitude similar to our prior findings and potentially clinically important.
Because low body weight is a risk factor for osteoporosis (9), it has been suggested that obesity confers protection against osteoporotic fractures. However, it has been estimated that the prevalence of low bone mass among obese women and men is 30–40% (10,11). This was the case in this study, in which the majority of women were overweight or obese before the weight loss intervention, yet nearly 40% had low BMD (t-score <−1). Such observations raise the question of whether low bone mass in overweight and obese adults is due, in part, to repeated weight loss attempts that generate bone loss that is not recovered with weight regain. In support of this, Bacon et al. (10). demonstrated that chronic dieting behavior was a significant predictor of low BMD in obese premenopausal women, and Fogelholm et al. (12). reported that LS and distal radius BMD were lower in women with a history of weight cycling (e.g., repeated weight loss/gain cycles) when compared with non-weight cyclers. In a prospective cohort study of peri- and post-menopausal women, weight loss over 11 years of follow-up was associated with a decline in wrist BMD, but the converse was not true for weight gain (13). Weight loss resulting from either diet or exercise has been found to increase markers of bone remodeling (14–16) and decrease BMD in many (1–3,16–20), but not all (14,21,22), studies. Because weight loss is generally thought to confer multiple health benefits, it is important to understand the mechanisms by which bone loss occurs and whether such loss increases risk for osteoporotic fractures.
Determining whether weight regain is accompanied by BMD regain is one way to examine whether weight loss-induced BMD loss is appropriate or inappropriate. An intervention study of obese, premenopausal women examined changes in bone during 3 months of a very low calorie diet (~14% weight loss) and 33 months of follow-up (~62% weight regain (19)). The significant weight loss-induced decrease in LS BMD was not recovered by the end of the follow-up period, even in the 25% of subjects who regained all the weight lost (19). However, weight regain did result in a significant restoration of trochanter BMD (19). The results of the current study were consistent with these findings, in that the decline in LS BMD during the 6-month weight loss intervention was not restored by nearly complete weight regain over 12 months of follow-up. However, the patterns observed at the trochanter differed between the two studies, because we did not find a significant decrease in BMD after weight loss. In our previous study (1), which included a larger cohort, weight loss did induce a significant decrease in trochanter BMD.
Weight loss was generated through exercise training in the current study, which is typically thought to have beneficial skeletal effects. Indeed, BMD in hip regions tended to increase in the women in the LO weight loss subgroup in response to the intervention (Figure 1a). However, it was not clear why only the increase at the subtrochanteric region was significant. Because the goal of the intervention was to generate weight loss, the exercise program was not specifically designed to be bone-loading. Rather, participants were allowed to perform their preferred mode and intensity of exercise to encourage compliance. It is possible that weight loss-induced decreases in BMD could have been prevented or minimized if the exercise program had focused on bone-loading activities (23). Villareal and colleagues (24) found that BMD was maintained during exercise-induced weight loss but not diet-induced weight loss, although it could not be determined whether this was attributable to the type of exercise. Their study population included women on hormone therapy and men, and it remains unclear whether weight loss-induced bone loss is attenuated in postmenopausal women by estrogen-based hormone therapy (25).
Weight regain in this study may have resulted, in part, from a decrease in physical activity after the supervised exercise program ended. Thus, it is possible that a decrease in exercise counteracted a positive effect of weight regain on BMD. However, an incomplete restoration of BMD with weight regain has been observed by others (2,3). Postmenopausal women who underwent 6 months of diet-induced weight loss followed by 6 months of complete weight regain had a larger decrease in LS BMD at the end of the study than weight-stable controls (−4.8% vs. −2.5%); a similar effect was not observed at the femoral neck (−2.5% vs. −2.1% (2)). Premenopausal women randomized to a 4.5-year lifestyle intervention to prevent menopausal weight gain had larger decreases in hip BMD (−0.20%/year) than women randomized to a control group (−0.03%/year (3)). Importantly, among women in that study who were postmenopausal and not on hormone therapy after another 2 years of follow-up, the annualized change in hip BMD was −1.1% in those who lost weight (≤−3%), but −0.8% and −0.7% in those who maintained or gained weight (≥3%), respectively (3). Similar differences were observed for other regions of the proximal femur, but not the LS. Thus, although there are inconsistencies regarding which skeletal regions are affected, there is growing evidence for a detrimental effect of weight loss on BMD in postmenopausal women and concerns that weight gain does not reverse this process.
The failure to recover BMD despite a recovery of weight suggests that factors other than weight change per se exaggerate the decline in BMD during weight loss. For example, disruptions in calcium absorption during weight loss and fluctuating levels of cortisol, insulin-like growth factor-1 (IGF-1), cytokines, and leptin may contribute to increased bone mobilization and BMD loss (25). When weight loss is induced through energy restriction, it is possible that reduced availability of certain nutrients contributes to bone loss. Increased calcium supplementation during diet-induced weight loss was found to attenuate bone loss in premenopausal women (15), but the same strategy was not effective in postmenopausal women (15). Although data on calcium intake were not available in the present study, it is unlikely that changes in calcium intake contributed to the findings because weight loss was generated through exercise rather than food restriction. It was recently demonstrated that a high-protein intake during diet-induced weight loss attenuated the decreases in BMD at the LS, total hip and distal radius when compared with a normal protein intake (26). It is not known whether a high-protein diet could also attenuate bone loss in response to exercise-induced weight loss. Further research is needed to better understand the potential mediators of bone loss during weight loss.
It has been suggested that changes in the thickness of soft tissue over- and underlying bone, as occur with weight change, result in BMD measurement error (27,28). Theoretically, a decrease in soft tissue thickness could diminish the degree of x-ray attenuation by soft tissue during a DXA scan and be reflected as reduced BMC. This could also lead to improved edge detection, resulting in increased area. Based on this theoretical argument, decreases in BMD in response to weight loss may be the result of measurement error in BMC (decreased) and/or area (increased); opposing errors could occur with weight regain. Two lines of evidence argue against the possibility that the observed changes in BMD reflected measurement error associated with changes in soft tissue thickness: (i) the decreases in BMD in response to weight loss did not reverse with weight regain; and (ii) the theoretical changes in BMC and area did not occur.
It must be acknowledged that the current study was exploratory and not designed or powered a priori to evaluate the effects of weight change on BMD change. Limitations included a small sample size and the absence of a randomly assigned weight-stable group. Because our subjects were 10 years postmenopausal on average, they would be expected to lose ~0.5–1.5% of BMD over a 12-month period (3,29,30). Thus, the stability in BMD during the period of weight regain may reflect some degree of benefit. It is also possible that full recovery of BMD after weight loss takes longer than 1 year. Additional limitations of the present study included the lack of data for calcium and vitamin D intake and measures of physical activity during the period of weight loss and regain. Weight loss-associated changes in BMD might have been influenced by changes in calcium and vitamin D intake. In addition, the response of BMD to weight regain may have been influenced by changes in calcium and vitamin D intake and physical activity.
Further research is needed to identify the underlying mechanisms of weight-loss induced bone loss so that effective strategies can be designed to preserve BMD during weight loss. Because of the multiple purported health benefits of weight loss in overweight and obese adults, it is important to determine whether sustained weight loss has a negative effect on bone strength and fracture risk. Finally, if adults are likely to regain body weight but not BMD after weight loss attempts, focusing on weight maintenance and fitness rather than weight reduction may be a more successful strategy for optimizing both bone and metabolic health. This strategy may be particularly true for postmenopausal women because of the increased risk for osteoporosis.
Acknowledgments
We express our gratitude to the nursing, bionutrition, core laboratory, information systems, and administrative staffs of the clinical Translational Research center and Energy Balance core of the clinical Nutrition Research Unit for their support of the study. We also acknowledge the members of our research group who carried out the day-to-day activities for the project. Finally, we thank the women who volunteered to participate in the study for their time and efforts. This research was supported by awards from the National Institutes of Health, including R01 AG18198, F32 AG05899 (W.S.G.), K01 AG19630 (R.E.V.P.), M01 RR00051 colorado cTSI UL1 RR025780, and P30 dK048520 (Nutrition and Obesity Research center).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab. 2005;90:52–59. doi: 10.1210/jc.2004-0275. [DOI] [PubMed] [Google Scholar]
- 2.Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr. 1994;48:561–566. [PubMed] [Google Scholar]
- 3.Park HA, Lee JS, Kuller LH, Cauley JA. Effects of weight control during the menopausal transition on bone mineral density. J Clin Endocrinol Metab. 2007;92:3809–3815. doi: 10.1210/jc.2007-1040. [DOI] [PubMed] [Google Scholar]
- 4.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:857–863. [PubMed] [Google Scholar]
- 5.Langlois JA, Mussolino ME, Visser M, et al. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12:763–768. doi: 10.1007/s001980170053. [DOI] [PubMed] [Google Scholar]
- 6.Kohrt WM, Ehsani AA, Birge SJ., Jr HRT preserves increases in bone mineral density and reductions in body fat after a supervised exercise program. J Appl Physiol. 1998;84:1506–1512. doi: 10.1152/jappl.1998.84.5.1506. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., 3rd Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 8.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 9.De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 10.Bacon L, Stern JS, Keim NL, Van Loan MD. Low bone mass in premenopausal chronic dieting obese women. Eur J Clin Nutr. 2004;58:966–971. doi: 10.1038/sj.ejcn.1601922. [DOI] [PubMed] [Google Scholar]
- 11.Greco EA, Fornari R, Rossi F, et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010;64:817–820. doi: 10.1111/j.1742-1241.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 12.Fogelholm M, Sievänen H, Heinonen A, et al. Association between weight cycling history and bone mineral density in premenopausal women. Osteoporos Int. 1997;7:354–358. doi: 10.1007/BF01623777. [DOI] [PubMed] [Google Scholar]
- 13.Forsmo S, Langhammer A, Schei B. Past and current weight change and forearm bone loss in middle-aged women: the Nord-Trøndelag Health Study, Norway. Menopause. 2009;16:1197–1204. doi: 10.1097/gme.0b013e3181a6cbb1. [DOI] [PubMed] [Google Scholar]
- 14.Redman LM, Rood J, Anton SD, et al. Pennington Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) Research Team. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci TA, Chowdhury HA, Heymsfield SB, et al. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–1050. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 16.Jensen LB, Quaade F, Sørensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9:459–463. doi: 10.1002/jbmr.5650090404. [DOI] [PubMed] [Google Scholar]
- 17.Jensen LB, Kollerup G, Quaade F, Sørensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord. 1996;20:513–520. [PubMed] [Google Scholar]
- 19.Fogelholm GM, Sievänen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001;12:199–206. doi: 10.1007/s001980170130. [DOI] [PubMed] [Google Scholar]
- 20.Chao D, Espeland MA, Farmer D, et al. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753–759. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 21.Ryan AS, Nicklas BJ, Dennis KE. Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol. 1998;84:1305–1310. doi: 10.1152/jappl.1998.84.4.1305. [DOI] [PubMed] [Google Scholar]
- 22.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95:131–140. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 23.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 25.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sukumar D, Ambia-Sobhan H, Zurfluh R, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized controlled trial. J Bone Miner Res. 2011;26:1339–1348. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svendsen OL, Hendel HW, Gotfredsen A, Pedersen BH, Andersen T. Are soft tissue composition of bone and non-bone pixels in spinal bone mineral measurements by DXA similar? Impact of weight loss. Clin Physiol Funct Imaging. 2002;22:72–77. doi: 10.1046/j.1475-097x.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 28.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41:138–154. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Cauley JA, Robbins J, Chen Z, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald HM, New SA, Campbell MK, Reid DM. Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int. 2005;16:163–171. doi: 10.1007/s00198-004-1657-7. [DOI] [PubMed] [Google Scholar]