Abstract
Animal cells are protected from oxidative damage by an antioxidant network operating as a coordinated system, with strong synergistic interactions. Lifespan studies with whole animals are expensive and laborious, so there has been little investigation of which antioxidant interactions might be useful for life extension. Animals in the phylum Rotifera are particularly promising models for aging studies because they are small (0.1–1 mm), have short, two-week lifespan, display typical patterns of animal aging, and have well characterized, easy to measure phenotypes of aging and senescence. One class of interventions that has consistently produced significant rotifer life extension is antioxidants. Although the mechanism of antioxidant effects on animal aging remains controversial, the ability of some antioxidant supplements to extend rotifer lifespan was unequivocal. We found that exposing rotifers to certain combinations of antioxidant supplements can produce up to about 20% longer lifespan, but that most antioxidants have no effect. We performed life table tests with 20 single antioxidants and none yielded significant rotifer life extension. We tested 60 two-way combinations of selected antioxidants and only seven (12%) produced significant rotifer life extension. None of the 20 three- and four-way antioxidant combinations tested yielded significant rotifer life extension. These observations suggest that dietary exposure of antioxidants can extend rotifer lifespan, but most antioxidants do not. We observed significant rotifer life extension only when antioxidants were paired with trolox, N-acetyl cysteine, l-carnosine, or EUK-8. This illustrates that antioxidant treatments capable of rotifer life extension are patchily distributed in the parameter space, so large regions must be searched to find them. It furthermore underscores the value of the rotifer model to conduct rapid, facile life table experiments with many treatments, which makes such a search feasible. Although some antioxidants extended rotifer lifespan, they likely did so by another mechanism than direct antioxidation.
Keywords: Antioxidant, Rotifera, Lifespan, Aging, ROS, l-Carnosine, N-acetyl cysteine, EUK-8, Vitamin E
Introduction
A powerful strategy for developing drugs capable of slowing aging processes is to employ short-lived animal models to identify promising aging interventions and then to develop quantifiable aging biomarkers to track their effects in humans (Le Couteur et al. 2011). Antioxidants are examples of compounds that can slow aging and sometimes produce life extension in experimental animals (Halliwell 2011), although the mechanisms remain disputed (Gutteridge and Halliwell 2010). Antioxidant research has focused on finding the most powerful antioxidants, increasing bioavailability, and improving stability in vivo (Vertuani et al. 2004), but there has been little investigation of antioxidant interactions and how these might be effectively used to extend lifespan. This is likely because lifespan studies with whole animals are expensive and laborious, so relatively little of the parameter space has been explored. In fact, one of the reasons for contradictory reports on the effects of antioxidants on animal aging is that studies typically explore only a small number of antioxidants with exposures as single compounds, so only a few of the possible outcomes are sampled haphazardly. A more global exploration of parameter space may reveal patterns in antioxidant effects that have yet to be described.
Cells are protected from oxidative damage by a complex network of endogenous antioxidants composed of vitamins, minerals, phytochemicals, lipids, peptides, proteins, and enzymes (Vertuani et al. 2004). It is generally thought that the antioxidant network operates as a coordinated system, so that deficiencies in one component can affect the effectiveness of other components (Catoni et al. 2008). Consequently, it is reasonable to expect that combinations of antioxidants may provide better protection against oxidative damage than individual antioxidants alone and that many antioxidants act synergistically. Examples of such cooperative interactions between carotenoids and tocopherols and between ascorbic acid and vitamin E have been reported (Bohm et al. 1998; Stahl et al. 2000), but how common these interactions are has not been widely investigated.
Comparative biology has a central role in aging studies and new invertebrate models of aging can be especially useful (Austad 2009). Animals in the phylum Rotifera are particularly promising models for aging studies. Rotifers are small (0.1–1 mm in length), with fully developed organs including digestive, reproductive, nervous, and osmoregulatory systems. They are eutelic, with no cell division after embryological development, yet they display typical patterns of aging and senescence. Most rotifers have very short lifespans (10–15 days), are easy to use in life table experiments, and exhibit survivorship curves similar in shape to many other animals. Rotifer phenotypes associated with aging like changes in appearance and vitality are well characterized and easy to measure. Rotifers also are in the Lophotrochozoa, a superphylum containing molluscs, annelids and several smaller invertebrate phyla (Giribet 2008). This phylogenetic distinction is important because the most widely used invertebrate models are Caenorhabditis elegans and Drosophila, both in the Ecdysozoa. Lophotrochozoans may use different genes and pathways to modulate aging, so investigating some model animals with the capacity for experimental manipulation in this group could be especially productive. Rotifers have been used in aging studies for many years, the early research summarized by Enesco (1993). New genetic tools like sequenced genomes (Lee et al. 2011) and the capability of RNAi (Snell et al. 2011) make rotifers very attractive tools for elucidating new gene targets regulating aging and have re-invigorated rotifers as models for aging research.
We have initiated our studies of rotifer aging by searching for interventions that extend rotifer lifespan. One class of interventions that has produced significant rotifer life extension is antioxidant supplements (Enesco 1993). A recent study has underscored this, reporting a threefold life extension in the rotifer Philodina acuticornis treated with an indole-3-propionic acid derivative (Poeggeler et al. 2010). Although the mechanism of antioxidant effects on animal aging remains controversial (e.g., Gutteridge and Halliwell 2010), the ability of antioxidants to extend rotifer lifespan is indisputable. We therefore designed a series of experiments to probe the responsiveness of rotifers to dietary antioxidants and to discover which antioxidants are life-extending, and under what conditions. In this work we exploited the capability of rotifers for facile life table analysis and we screened many well known antioxidants for interactions that extend lifespan in whole animals. We discovered that rotifer exposure to certain combinations of antioxidant supplements can produce up to about 20% longer lifespan, but that most antioxidants have no effect. Most interestingly, antioxidants that had no effect in exposures as single compounds often produced significant rotifer life extension when two antioxidants were dosed in combination. These observations encouraged us to perform a systematic screen of two-, three-, and four-way interactions of several antioxidants, revealing a pattern of life extension previously not described in animal models.
Methods
Source of rotifers
Brachionus manjavacas (Fontaneto et al. 2007), originally called B. plicatilis Russian strain and collected from the Azov Sea region in Russia, was used for these experiments. This species has been propagated continuously in the lab since 1983, with periodic resting egg production, collection, and storage. Rotifers used in the following experiments were hatched from a resting egg batch produced on July 25, 2007.
Rotifer cultures
Resting eggs were hatched in 25 ml of 15 ppt artificial seawater (ASW), incubated overnight (22–24 h) under constant fluorescent illumination (2000 lux) at 25°C. The hatchlings were fed Tetraselmis suecica cultured in F medium (Guillard 1983) in a 560 ml chemostat with 1/4 replacement daily under constant fluorescent illumination (2000 lux) at 25°C. Optimal rotifer culture conditions for brachionid rotifers are well known (Wallace and Snell 2010). These rotifers are the workhorses of experimentation around the world and dozens of labs have grown them under experimental conditions. Moreover, brachionids are the subject of extensive research on culture conditions since they are cultured in commercial aquaculture facilities as larval fish feed. Rotifers also are used in ecotoxicological tests to estimate the toxicity of environmental samples (Dahms et al. 2011). This latter application requires consistent, reproducible responses with minimal food issues. Techniques for providing quantitative doses of algae food are well developed. We controlled algae quality by growing Tetraselmis in a chemostat with constant inflow. This produces algae of very consistent nutritional value (Ferreira et al. 2011). Algae quality can be verified by monitoring rotifer reproductive rate which is quite responsive to subtle changes in food quality. Bacterial contamination is not an issues in lab cultures and contamination by other algae species is readily spotted by daily observations.
Source of chemicals
The following chemicals were purchased from Sigma-Aldrich company: 3-indolepropionic acid (I), pyrroloquinoline quinone (P), (±)-6-hydroxy-2,5,7, 8-tetramethylchromane-2-carboxylic acid (Trolox, T), vitamin B12 (B12), l-carnosine (Cn), quercetin (Q), N-acetyl-l-cysteine (N), beta-carotene (Bc), 5-hydroxy-1,4-naphthoquinone (juglone), N-acetyl-5-methoxytryptamine (melatonin, M), and 5-fluoro-2′-deoxyuridine (FDU). EUK-8 (E) was purchased from Calbiochem. Carboxy-H2DCFDA (CDCFDA) and MitoSOX™ Red (MitoSOX) were purchased from Invitrogen.
Rotifer life cycle
Brachionus manjavacas is a species of monogonont rotifer that reproduces parthenogenetically in low density cultures (Snell et al. 2006). In parthenogenetic reproduction, females produce asexual (amictic) eggs that hatch into amictic daughters that are genetic clones of their mothers. However, at population densities higher than the mictic threshold (Serra et al. 2004), females respond to a chemical signal and begin to reproduce sexually. The mixis threshold is reached when the concentration of the mixis induction protein (MIP), produced and secreted into the surrounding media by the rotifers themselves, reaches a high enough level to trigger mictic reproduction (Kubanek and Snell 2008). Mictic hatchlings produce mictic eggs, which if unfertilized, hatch into haploid males and if fertilized, produce diploid resting eggs. Resting eggs are diapausing embryos resistant to temperature extremes, UV, and desiccation, and enable rotifers to re-initiate population growth once favorable environmental conditions return.
Aging time series
Several 24-well plates were set up at staggered intervals with two hatchlings per well containing 750 µl of T. suecica (1 × 105 cells/ml) with 20 µM 5-fluoro-2′-deoxyuridine (FDU) added to inhibit amictic egg hatching. FDU blocks DNA replication so that dividing cells are killed, but non-dividing cells are unaffected (Mitchell et al. 1979). In rotifers, FDU treated females produce eggs normally, but they fall to the bottom of the well and do not hatch. Because the cells of adult rotifers are post-mitotic (eutely), adult rotifers are little unaffected by FDU. However, tests comparing the effect of FDU on rotifer lifespan have revealed about 20% life extension in rotifers treated with FDU compared to untreated controls. We are following up on this interesting observation and it will be the subject of a subsequent paper. Meanwhile, FDU is useful in life table experiments to simplify the tracking of maternal female survival without the need to remove daily the newborn offspring, so we employed it uniformly in our experiments. Five rotifers at each age (day 0 to 14 and dead rotifers) were imaged at ×100 under bright-field illumination with a Zeiss Imager.Z1 microscope. The most representative image at each time point was used to create the aging time series. The most striking phenotypic changes during aging were noted and used to estimate the age of individual rotifers. These age-related phenotypic changes include foot dragging/increased angle relative to the body, decreased swimming speed, cessation of egg production, and increased opacity of the pseudocoelom. Individuals possessing some of these aged phenotypes could be reliably classified as old.
Juglone challenge/antioxidant rescue
Our search for antioxidants capable of rotifer life extension began with two screening experiments, followed by a large scale, definitive life table experiment. The first screening experiment was a juglone challenge and antioxidant rescue. Juglone (5-hydroxy-1,4-naphthoquinone) is a compound produced by the black walnut tree to inhibit the growth of competitors. The mechanism of action of juglone is to stress cells by producing large amounts of ROS and it has been used to experimentally probe the ROS defenses of animals (DeCastro et al. 2004; Przybysz et al. 2007; Tanaka et al. 2009). We developed the juglone challenge test as a rapid screen to determine which antioxidants have activity in rotifers. Forty unfed hatchlings (0–4 h old) were simultaneously exposed to 0.1 µM juglone (DMSO stock) and a variety of antioxidants (see below). There were ten hatchlings per well in 1 ml, with four replicate wells per treatment in 24-well plates. All B. manjavacas exposed to 0.1 µM juglone for 48 h were killed. However, rotifers could be rescued from this 100% mortality by exposure to certain antioxidants. Our initial screen for antioxidant rescue included single- and double-antioxidant exposures at the following concentrations: 20 µM 3-indolepropionic acid (I) (DMSO stock), 40 µM Trolox (T) (DMSO stock), 20 µM quercetin (Q) (DMSO stock), 100 µM EUK-8 (E) (DMSO stock), 20 µM l-carnosine (Cn) (ASW stock), 20 µM N-acetyl-l-cysteine (N) (ASW stock), and 40 µM β-carotene (Bc) (acetone stock). These concentrations were determined by performing a range-finding test based on rotifer reproductive tests (Snell and Moffat 1992). About 100 treatments were tested, including single antioxidants, and two-, three- or four-way interactions. For each antioxidant treatment, percentages of alive/dead and swimming/non-swimming rotifers were recorded after 48 h exposure. Treatments were compared to an untreated control using Fisher’s Exact Test with a Bonferroni correction. A rotifer was considered swimming if she was swimming freely in the water column rather than lying on the bottom or attached to the sides of the well.
Initial screen of antioxidants for rotifer life extension
The second screening experiment was the initial screen for rotifer life extension and its rationale was to provide further confirmation of the effects of promising antioxidants identified in the juglone challenge. We were interested in big effects, so we examined many treatments with small sample size per treatment in this initial lifespan screen. False positives were greatly reduced by conducting multiple screens. Daily survival was recorded, so that mean lifespans (N = 20) could be compared among treatments using analysis of variance. Experiments were conducted in 24-well plates with 1 ml of ASW containing 6 × 105 T. suecica cells/ml in each well with 20 µM FDU and the appropriate antioxidant(s). Each well contained five hatchlings, with four wells for each single and double antioxidant treatment. Both DMSO and acetone control wells were set up at the highest concentration of DMSO/acetone used in the treatments to confirm no lifespan effects due to the solvent used to dissolve the antioxidants. Each well was supplemented with more algae (100 µl containing 6 × 105 cells/ml) and 20 µM FDU every 7 days after the initial setup. A second and third screening experiment was performed following the same protocol to explore more antioxidant combinations that looked promising based on the juglone challenge and the initial lifespan screens.
Effect of single and combinations of antioxidants on rotifer lifespan
Antioxidants producing the largest effects in the juglone challenge and the initial lifespan screens were followed up in a definitive life table experiment with larger sample size to confirm their effects. Twenty wells with five hatchlings per well were set up for each treatment (E, N, Q, Cn, I) for a total of 100 rotifers per treatment in 24-well plates. Each well contained 1 ml of 6 × 105 T. suecica cells/ml with 20 µM FDU and the appropriate antioxidant at the desired concentration. A DMSO control, with 120 individual’s total, was set up with a DMSO concentration matching the highest concentration found in the treatments. We performed ANOVA on rotifer lifespan to determine whether there were significant well or row effects in our experimental plates. Finding none, we treated all 120 individuals as independent observations. The wells were scored daily and the number of surviving rotifers was recorded for each treatment. A new set of plates was prepared and all surviving animals were transferred to the new plates 1 week after the initial setup. Both sets of plates were identical in terms of algae and antioxidant concentration. The effects of selected two-, three-, and four-way antioxidant combinations on rotifer lifespan were also tested using the same experimental design described for the single antioxidant experiment.
Change in female swimming speed with age
A program designed by Jeff Stirman at Georgia Tech using LabVIEW 2010 software was used to estimate swimming speed (mm/s) of individual rotifers. Video of rotifers swimming at ×20 magnification was captured with a PixeLINK camera attached to a stereomicroscope. Digital files were stored on an iMAC computer and transferred to LabVIEW for analysis. The speeds were converted into mm/s based on measurements from a stage micrometer. Several 6-well plates were set up at staggered intervals to produce rotifers of different ages so that their swimming speeds could be estimated. Three 30s replicate videos were taken of eight to ten rotifers for each age: 0, 1, 3, 4, 6, 8, 10, 12, and 14 days. Each female was placed on a slide painted with ten dots in about 15 µl ASW with a cover slip. Two layers of tape were placed on either end of the slide to support the cover slip and provide space for the rotifers to swim. The average swimming speed (mm/s) was calculated for each treatment using LabVIEW software.
To test the effects of antioxidants on swimming speed at different ages, rotifers were incubated in antioxidants from birth. Each treatment included 40 rotifers (eight wells total in a 24-well plate, five rotifers per well) fed 6 × 105 T. suecica cells/ml with antioxidants and 20 µM FDU added to inhibit egg hatching. On day 10, 30 s videos of ten randomly selected individuals from each treatment were recorded and the average swimming speed was calculated and compared to an untreated control.
ROS estimation using CDCFDA
Intracellular ROS concentrations can be estimated using carboxylated H2DCFDA (carboxy-H2DCFDA, Invitrogen, C400), which has two negative charges at physiological pH to enhance retention of the fluorescent product inside cells. CDCFDA is commonly used to detect the generation of reactive oxygen intermediates in neutrophils and macrophages, where upon cleavage of the acetate groups by intracellular esterases and oxidation, the nonfluorescent CDCFDA is converted to the highly fluorescent 2′,7′-dichlorofluorescein (Tsuchiya et al. 1994). This approach has been used to quantify intracellular ROS concentrations in rotifers (Kim et al. 2011).
Several 12-well plates were set up at staggered intervals to produce rotifers of different ages. Ten rotifers were added to each well with 2 ml ASW containing 6 × 105 T. suecica cells/ml and 20 µM FDU to inhibit egg hatching. At each time point (days 0, 1, 2, 4, 6, 8, 10, and 12), 18 rotifers were isolated in ASW for 60 min to clear their guts of algae, rinsed, and then incubated in 0.5 ml of ASW with 1 µl CDCFDA (2.5 mg/ml DMSO stock) in a 24-well plate. The rotifers were incubated with CDCFDA for 60 min in the dark, rinsed in ASW, anesthetized with 0.5 ml of club soda, and fixed with 10 µl of 20% formalin. Prepared slides were imaged at ×100 with a GFP filter set at 1.5 s exposures using a Zeiss Imager.Z1 microscope. The pixel intensity at each age was estimated using ImageJ software by defining the region of interest as the whole rotifer, measuring pixel intensity in the background of an identical region, and subtracting the background. The pixel intensity difference was then normalized by dividing by the area of each rotifer, and the mean was calculated for each age. Comparison of CDCFDA intensity among rotifers of different ages was done by analysis of variance followed by Dunnett’s test to compare treatments to control.
Mitochondria superoxide estimation using MitoSOX
The production of superoxide by mitochondria can be visualized by fluorescence microscopy using MitoS-OX™ Red (Invitrogen, M36008). MitoSOX permeates live cells where it selectively targets mitochondria and is rapidly oxidized by superoxide but not other ROS or reactive nitrogen species. The oxidized product is highly fluorescent upon binding to nucleic acid.
Several 6-well plates were set up at staggered intervals to image rotifers of different ages. Fifteen rotifers were added to each well with 5 ml T. suecica (6 × 105 cells/ml) and 20 µM FDU to inhibit egg hatching. At each time point (days 0, 4, 8), ten rotifers were rinsed in ASW, cleared of algae for 1 h, and then incubated in 0.5 ml of ASW with 2 µl MitoSOX (5 mg/ml DMSO stock) in a 24-well plate. The rotifers were incubated for 30 min in the dark, transferred to a rinse well containing ASW only and were then anesthetized with club soda and fixed with 20% formalin. Prepared slides were viewed at ×100 with an Alexa568 filter set at 1 s exposure using a Zeiss Imager.Z1 microscope. The pixel intensity was determined for rotifers at each time point using ImageJ software as described above.
Statistical analysis
Fisher’s exact test was calculated using the program simple interactive statistical analysis (SISA, http://www.quantitativeskills.com/sisa/). Mean lifespans were compared by one-way analysis of variance followed by Dunnett’s test to compare treatments to control using the statistical package JMP 8 (SAS Institute). Survival curves were compared using the JMP 8 reliability and survival analysis, with a Wilcoxson’s test calculated to compare survival curves of control and treatments. Swimming speed and CDCFDA and MitoSOX staining intensities were compared by ANOVA with Dunnett’s test to compare treatments to control.
Results
An example of a B. manjavacas survivorship curve and fecundity schedule under control conditions is presented in Fig. 1a. Under these experimental conditions (22°C, ad libitum food), animals began reproducing after a short juvenile period of 2 days. Reproduction peaked at age 5 days at about six offspring per female per day and then began a slow decline until it finally ceased at age 15 days after a female had produced an average of 24.9 offspring. In these conditions rotifer reproduction was exclusively parthenogenetic and populations were entirely female. Survival through the juvenile period was excellent, with the first mortality occurring on day 6. Mortality gradually increased with age, yielding a mean lifespan of 12.3 days, median of 13 days, and maximum lifespan (95% mortality) of 23 days. It should be noted that there was a long post-reproductive period from 15 to 29 days, which was about as long as the 13 day reproductive period.
Fig. 1.
a Typical survival and fecundity curves for B. manjavacas under control conditions. b Photomicrographs of females of various ages, illustrating changes in morphology associated with aging
Aging phenotypes over the B. manjavacas lifespan are illustrated in Fig. 1b. New hatchlings are born with a length of 270 µm and grow 1.5 fold to 420 µm as they mature into adults. This increase in size is due to water uptake and cytoplasm synthesis, but not cell division since rotifers are eutelic. Egg production is a prominent feature of females because eggs are carried at their posterior until they hatch. At peak reproduction females may carry 4–8 eggs. Egg production in most females is greatly diminished after day 8 and they enter a long, slow decline in function. Besides the absence of eggs, other characteristics of aged phenotypes include slower swimming speed, foot dragging at an increasing angle to the body, body morphology changes from more cylindrical to more pear-shaped, and increased opacity of tissues in the pseudocoelom. With this suite of traits, one can observe a female and predict fairly accurately her state of aging.
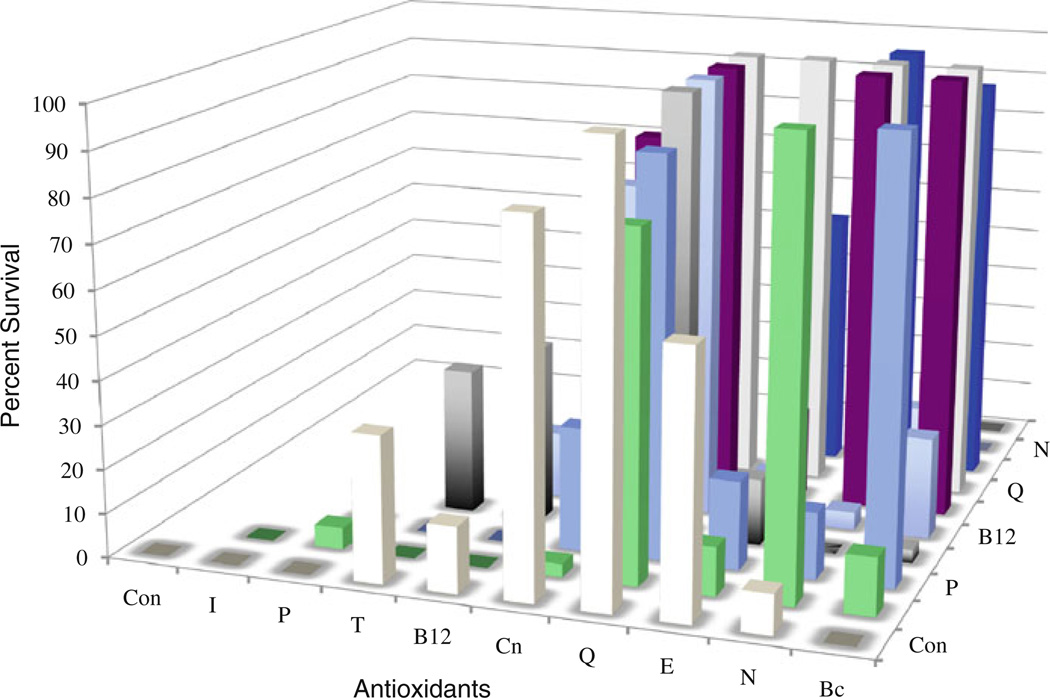
The effects of antioxidants on survival was surveyed by challenging B. manjavacas with a 48 h exposure to 0.1 µM juglone (Fig. 2). In controls mortality was 100%; however, if there was a simultaneous exposure to certain antioxidants, mortality was greatly reduced. Exposure to the single antioxidants quercetin, l-carnosine, and EUK-8 produced survival of more than 50%, and trolox about 30%. Some antioxidants that did not rescue rotifers as single compounds, yielded good survival when paired with another antioxidant. N-acetyl cysteine alone rescued only 10% of rotifers exposed to juglone; however, when paired with indolepropionic acid, l-carnosine, quercetin, or EUK-8, survival exceeded 90%. Similarly, beta-carotene alone rescued none of the rotifers from the toxic effects of juglone, but when paired with pyrroloquinoline quinone, l-carnosine, quercetin, or EUK-8, survival exceeded 90%. The juglone challenge therefore was a useful first screen because it allowed us to rapidly determine which antioxidants were likely to have strong effects in rotifers and which strongly interacted with other antioxidants.
Fig. 2.
Effects of exposure to 0.1 µM juglone on rotifer survival after 48 h. Antioxidant abbreviations: con control, I indole-3-propionic acid, P pyrroloquinoline quinone, T trolox, B12 vitamin B12, Cn l-carnosine, Q quercetin, E EUK-8, N N-acetyl cysteine, Bc beta carotene
Promising antioxidants identified in the juglone challenge were screened for their ability to extend B. manjavacas lifespan in small scale life table experiments (Fig. 3). Data are the means of three independent experiments, each treatment with N = 20 rotifers, examining the effects of single antioxidants and their two-way interactions. Because each experiment had a separate control, data are means normalized to their respective controls. No single antioxidant produced rotifer life extension relative to the untreated controls. In contrast, a few two-way interactions yielded lifespans 10–15% longer than controls. These include trolox and N-acetyl cysteine, trolox and beta-carotene, and N-acetyl cysteine and quercetin. A few other two-way interactions gave hints of positive effects on lifespan, including trolox and l-carnosine, trolox and quercetin, and EUK-8 and indole-3-propionic acid. We used observations from these screening experiments to identify antioxidant treatments worthy of following up in life table experiments with larger sample sizes.
Fig. 3.
Effects of antioxidants on rotifer lifespan normalized to the control. Antioxidant abbreviations: con control, I indole-3-propionic acid, T trolox, Cn l-carnosine, Q quercetin, E EUK-8, N N-acetyl cysteine, Bc beta carotene
We used results from the juglone challenge and initial lifespan screens to design large-scale life table experiments to confirm that exposure to some antioxidant combinations significantly extends rotifer lifespan. These large-scale life table experiments followed survival in populations of 100–120 rotifers for each treatment (Fig. 4a–d) and strengthened confidence in the antioxidant interactions identified in the juglone and initial lifespan screens. When rotifers were exposed to the antioxidants E, N, Q, Cn, or I as single compounds, no significant life extension was observed (Fig. 4a). Although trolox was not included in this experiment, results from the small-scale life table experiments confirmed that trolox alone also does not extend rotifer lifespan.
Fig. 4.
Large scale screen of antioxidants for rotifer life extension. a Effects of single antioxidants on B. manjavacas lifespan. b–d Effects of two-, three- or four-way combinations of antioxidants on rotifer lifespan. Antioxidant abbreviations: con control, I indole-3-propionic acid, T trolox, Cn l-carnosine, Q quercetin, E EUK-8, N N-acetyl cysteine, Bc beta carotene
A large-scale life table experiment to test a variety of antioxidants paired with N-acetyl cysteine produced one treatment, N–Cn, that extended rotifer mean lifespan by 14.5% over the control (10.7 days) (Fig. 4b). Comparison of control and N–Cn survivorship curves using Wilcoxson’s test yielded a significant χ2 value of 5.89 with P = 0.015. Adding the antioxidants Q and E to the N–Cn treatment did not further increase lifespan; rather, it slightly diminished mean lifespan to 11.9 days, rendering it not significantly different from control. A second life table experiment testing several antioxidants paired with quercetin or l-carnosine yielded only a single treatment with significant life extension (Fig. 4c). In this experiment, mean lifespan in the control was 15.3 days and the Cn–E treatment was 16.8 days, a 10% life extension. Comparison of control and Cn–E survivorship curves using Wilcoxson’s test yielded a significant χ2 value of 6.95 with P = 0.0083. Again, adding the antioxidants Q and I to the Cn–E treatment did not improve, but instead diminished the life extension effect. A third life table experiment investigated the effect of several antioxidants paired with trolox (Fig. 4d). In this experiment several treatments produced rotifer life extension over the mean control lifespan of 12.5 days. These include T–Bc (10% life extension), T–Cn (13%), T–N (16%), T–E (14%), and E–I (14%). Wilcoxson’s test comparing control and antioxidant treatment survivorship curves was significant for T–Bc (P = 0.032), T–Cn (P = 0.0059), T–N (P = 0.0021), T–E (P = 0.0013), and E–I (P = 0.0017).
In total, we performed life table tests with 20 single antioxidants and none yielded significant rotifer life extension. We tested 60 two-way combinations of selected antioxidants and only seven (12%) produced significant rotifer life extension. None of the 20 three-and four-way antioxidant combinations tested yielded significant rotifer life extension. These observations suggest that dietary exposure of antioxidants can extend rotifer lifespan, but most antioxidants do not, and those that do, do so when paired with trolox, N-acetyl cysteine, l-carnosine, or EUK-8. Our results also emphasize that extensive regions of parameter space must be searched to find the few antioxidant treatments capable of rotifer life extension.
In addition to lifespan extension, we are interested in interventions capable of extending healthspan. Planktonic rotifers like B. manjavacas are suspension-feeding herbivores that swim continuously to ingest their microalgae food. Swimming speed is therefore a good measure of their vitality and it follows characteristic changes over the lifespan (Fig. 5). When B. manjavacas females hatch, they swim about 1 mm/s for the first day of their lives. As they increase in size and become reproductive, their swimming speed increases until it peaks at 2.5 mm/s at age 4 days. As rotifers age, their swimming speed slowly declines until day 8 when it levels off at about 1 mm/s. This rate is maintained until the very oldest age classes when swimming slows so much that the rotifers fall to the bottom of the well and die. We examined the ability of antioxidants to modify this progression in swimming speed by exposing rotifers to a variety of antioxidants for 10 days, and then measuring swimming speed and comparing it to untreated controls. We found only one antioxidant combination capable of slowing the decline of swimming speed with age (Fig. 5 inset). The N–Cn exposed rotifers swam 34% faster than controls at age 10 days.
Fig. 5.
Effect of aging on B. manjavacas swimming speed (mm/s). Inset histogram—effect of various antioxidants on rotifer swimming speed at age 10 days. Asterisk designates significant difference from control (P < 0.05). Antioxidant abbreviations: con control, I indole-3-propionic acid, Cn l-carnosine, Q quercetin, E EUK-8, N N-acetyl cysteine. Vertical lines indicate standard error
When animals are treated with antioxidants, one possible outcome is that their load of reactive oxygen species (ROS) may be diminished. This would occur if the mechanism of antioxidant life extension was due to direct antioxidation effects. We estimated the concentration of ROS in individual rotifer tissues using the fluorochromes CDCFDA and MitoSOX (Fig. 6a, b). An example of the pattern of fluorescence in rotifers is shown in the inset images. The pattern of change in fluorescence with rotifer age was similar for both CDCFDA and MitoSOX. The highest fluorescence intensities were observed in rotifers just hatched from resting eggs. One day old rotifers had lower values and older age classes (2–12 days) all had similar values. We did not observe increased fluorescence in older age classes, suggesting that there is no increase in ROS load with aging. Treating rotifers with pairs of antioxidants and quantifying fluorescence intensity at age 10 days did not produce a significant reduction in ROS load (data not shown).
Fig. 6.
Changes in ROS and superoxide concentrations within rotifers with aging. a CDCFDA. b MitoSOX. Vertical lines indicate standard error
Discussion
Reviews of antioxidant effects have reported contradictory results, with some claiming significant life extension and some not (e.g. Halliwell 2011). Our results show that a wide variety of outcomes is possible depending on which region of parameter space is investigated. None of the 20 single antioxidants in our experiments extended rotifer lifespan. Moreover, only 12% of antioxidant pairs yielded significant life extension, especially when paired with trolox, N-acetyl cysteine, l-carnosine, or EUK-8. This illustrates that there are antioxidant treatments capable of rotifer life extension, but they are patchily distributed in the parameter space, so large regions must be searched to find them. This also underscores the value of the rotifer model to conduct rapid, facile life table experiments with many treatments, which makes such a search feasible.
Many cellular processes are regulated by multiple, low affinity, low specificity interactions that provide for flexibility and redundancy (Schrattenholz and Soskic 2008). Aging also is likely regulated by similar processes, mediated through a complex tangle of overlapping pathways. Such a system is prone to strong interactions among its elements, and there is some evidence that the endogenous antioxidant network of cells meets these expectations (Vertuani et al. 2004). Interactions among specific antioxidant supplements that we tested were critical in determining whether they were able to extend rotifer lifespan. We observed rotifer life extension of about 10–20% in our dietary antioxidant experiments, but only from seven (12%) of the two-way combinations of particular antioxidants.
Animals have evolved defenses against ROS damage, and the balance between oxidative stress and concentration of available antioxidants has a major influence on lifespan. Important elements of antioxidant defenses include scavenging enzymes such as catalase, glutathione peroxidase, and superoxide dismutase (SOD) (Guarente and Kenyon 2000; Gems and Doonan 2009). The expression of these enzymes in rotifers during oxidative stress has been described (Kaneko et al. 2011). These authors investigated the interaction between caloric restriction (CR) and oxidative stress in the closely related congeneric rotifer B. plicatilis. Feeding rotifers every other day (50% CR) extended lifespan by about 50%. They induced oxidative stress by exposing rotifers to 10 mM of the herbicide paraquat and found that the CR rotifers lived 31% longer than normally fed rotifers. They further showed that one mechanism by which CR conferred resistance to oxidative stress is by increasing the expression of the enzymes catalase and Mn SOD. These results suggest that regulating oxidative damage is an important feature of rotifer aging, and that treatments that enhance the antioxidant capacity of rotifers can extend lifespan.
Exposure of Brachionus sp. to H2O2 served as a stimulus for increased ROS production so that rotifer oxidative stress defenses could be investigated (Rhee et al. 2011). These authors showed that 24 h after exposure to 0.1 mM H2O2, hsp20 gene expression was up-regulated about threefold. They also observed a two to three fold increase in glutathione peroxidase, glutathione reductase, and glutathione S-transferase activity 12 h after exposure to 0.2 mM H2O2. These observations further characterize the type of oxidative stress defenses that brachionid rotifers mobilize in response to an oxidative stressor like H2O2.
Poeggeler et al. (2010) argued that the failure of many antioxidant interventions is due to the generation of pro-oxidant intermediates in vivo and/or the poor bioavailability of antioxidant molecules. They described an amide derivative of indole-3-propionic acid that lacks pro-oxidant properties and has good bioavailability. Exposure of the bdelloid rotifer P. acuticornis to 20–30 µM of this antioxidant extended lifespan up to 300%, perhaps a record life extension for any animal model without genetic manipulation. The length of treated rotifers was 47% longer than controls and they produced 3.4-fold more offspring per female over a 3.6-fold longer reproductive period. In contrast, our experiments with the monogonont rotifer B. manjavacas, exposure to 20 µM indole-3-propionic acid alone did not extend lifespan. However, significant life extension (14%) was observed in treatments of E–I combined.
We found that exposure to combinations of four antioxidants is efficacious in extending rotifer lifespan. This is the first report of the effects of l-carnosine, N-acetyl cysteine, and EUK-8 on rotifers, but trolox (vitamin E) has a long history in rotifer research (discussed below). l-Carnosine is a dipeptide comprised of lysine and methionine. Besides its antioxidant activity, it is involved with fatty acid transport and has been described as a growth factor in mealworms (Martin et al. 1976). N-acetyl cysteine is an acetylated derivative of the amino acid l-cysteine. It is used in humans as a mucolytic drug for treating a variety of respiratory conditions producing excessive or thick mucous. It is also a precursor for biosynthesis of the important cellular antioxidant glutathione (tripeptide of cysteine, glycine and glutamate). EUK-8 has oxyradical scavenging activity that mimics the enzymes SOD and catalase (Gonzalez et al. 1995). Trolox is the trade name for 6-hydroxy-2,5,7, 8-tetramethylchroman-2-carboxylic acid, a water-soluble derivative of vitamin E produced by the Hoffman-LaRoche company. Like vitamin E, it can be used as an antioxidant by cells to reduce oxidative damage. All four of these antioxidants play multiple roles in cells, so it is unknown whether the effects we observed on rotifer lifespan are the result of direct antioxidant activity or other mechanisms. As an example, vitamin E is known to play an important signaling role in the predatory rotifer Asplanchna sieboldi, regulating the switch from asexual to sexual reproduction (Gilbert and Thompson 1968; Birky and Gilbert 1972). However, this is not the case for brachionid rotifers which are herbivores that consume a diet of microalgae rich in tocopherols (Gilbert 1980). The switch to sexual reproduction in brachionids is regulated by quorum sensing of a rotifer-secreted protein (Snell et al. 2006, Kubanek and Snell 2008). We are only beginning to characterize the signals that regulate the rotifer stress response and life history, and the role of antioxidants remains unexplored. Signaling by stress-responsive genes probably plays an important role in aging, but this is just now being characterized in worm and fly models (Kenyon 2010).
Vitamin E has long been investigated for its effects on rotifer aging. Sawada and Enesco (1984) reported that exposure of the rotifer Asplanchna brightwelli to 25 µg/ml (58 µM) of the antioxidant vitamin E extended lifespan by 21% by lengthening the pre-reproductive and reproductive periods. Litton (1987) extended these findings, reporting that exposure to α-tocopherol extended lifespan by about 10% in four other rotifer species. He further observed that ten other synthetic antioxidants had no effect on rotifer life-span. A few synthetic antioxidants (thiazolidine-4-carboxylic acid and 3,3′-thiodipropionic acid) have been reported to extend rotifer lifespan by up to 10% (Bozovic and Enesco 1986; Sawada et al. 1990). Sawada and Carlson (1985) demonstrated that oxidative damage in the form of lipid peroxides accumulates with age in A. brightwelli, and Sawada and Carlson (1990) showed that there is increased superoxide radical levels in rotifer membranes with age. Both of these effects could be reduced by exposure to vitamin E. These observations suggest that exposure to vitamin E has antioxidant activity in rotifers and emphasizes the importance of the accumulation of oxidative damage in rotifer aging. This is in contrast to Pun et al. (2010) who concluded that if antioxidant supplements lengthened C. elegans lifespan, they did not do it through a direct antioxidant mechanism. Gems and Doonan (2009) also concluded that increased antioxidant activity may be necessary, but it is not sufficient to slow aging in worms.
The mechanisms by which antioxidants affect animal lifespan remain controversial. Antioxidants typically are thought to delay aging by slowing the accumulation of unrepaired oxidative damage to essential macromolecules, eventually causing the failure of maintenance systems and ultimately death (Harman 1956; Halliwell 2006; Hayflick 2007; Rattan 2008). According to this theory of aging, reduction of oxidative damage should slow age-related deterioration and extend lifespan (Pun et al. 2010). Even though the oxidative damage theory provides a clear rationale for using antioxidant supplements as an intervention to extend lifespan and healthspan (Calabrese et al. 2010), after 50 years of research results have often been disappointing (Gems and Doonan 2009; Gruber et al. 2009; Halliwell 2011). Moreover, some believe that the oxidative theory of aging is dead (Perez et al. 2009), but others are uncertain that the theory can ever be fully refuted and suggest revision (Gems and Doonan 2009). Antioxidant molecules may act through mechanisms different from antioxidation (Mattson 2008), an example being quercetin which is an antioxidant and a SIRT1 agonist (Howitz et al. 2003). No matter how antioxidants extend lifespan in animal models, they are interesting molecules for aging studies.
Although we have found that combinations of certain antioxidants can extend rotifer lifespan, our results do not support the oxidative damage theory of aging. Exposure of rotifers to antioxidants did not reduce their ROS load as measured by CDCFDA and MitoSOX in vivo fluorescence. Likewise, exposure to only one antioxidant combination (N–Cn) was able to significantly alter the age-related decline in rotifer swimming speed. It is therefore likely that antioxidants affect rotifers through some other mechanism than antioxidation. This is not surprising considering the well established signalling role of vitamin E in regulating the rotifer life cycle described above.
In this work we have used a rotifer model to investigate interactions among well known antioxidant supplements for their ability to extend animal lifespan. Because of the facility of rotifer life table analysis, we explored a much larger region of parameter space than previously examined. We discovered the importance of interactions among certain antioxidant supplements and that these are key to their ability for life extension. This is yet another example of how discoveries with invertebrate models can identify promising life extending interventions for testing in mammals.
Acknowledgments
The authors acknowledge with gratitude the support of the National Institute of Aging, grant R01 AG037960-02 for this study. The authors also express our appreciation for the expert technical assistance provided by Jarrett Smith, Katherine White, and Lauren O’Keefe.
References
- Austad SN. Is there a role for new invertebrate models for aging research? J Gerontol A Biol Sci Med Sci. 2009;64A:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW, Jr, Gilbert JJ. Vitamin E as an extrinsic and intrinsic signal controlling development in the rotifer Asplanchna: uptake, transmission and localization of [3H]α-tocopherol. J Embryol Exp Morphol. 1972;27:103–120. [PubMed] [Google Scholar]
- Bohm F, Edge R, Mcgarvey DJ, Truscott TG. β-Carotene with vitamins E and C offers synergistic cell protection against NOx. FEBS Lett. 1998;436:387–389. doi: 10.1016/s0014-5793(98)01173-9. [DOI] [PubMed] [Google Scholar]
- Bozovic V, Enesco HE. Effects of antioxidants on rotifer lifespan and activity. Age. 1986;9:41–45. [Google Scholar]
- Calabrese V, Cornelius C, Trovato-Salinaro A, Cambria MT, Locascio MS, Di Rienzo L, Condorelli LF, Mancuso C, De Lorenzo A, Calabrese EJ. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16:877–883. doi: 10.2174/138161210790883615. [DOI] [PubMed] [Google Scholar]
- Catoni C, Peters A, Schaefer HM. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim Behav. 2008;76:1107–1119. [Google Scholar]
- Dahms HU, Hagiwara A, Lee JS. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol. 2011;101:1–12. doi: 10.1016/j.aquatox.2010.09.006. [DOI] [PubMed] [Google Scholar]
- De Castro E, De Castro SH, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Rad Biol Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Enesco HE. Rotifers in aging research: use of rotifers to test various theories of aging. Hydrobiologia. 1993;255(256):59–70. [Google Scholar]
- Ferreira M, Seixas P, Coutinho P, Fabregas J, Otero A. Effect of the nutritional status of semi-continuous microalgal cultures on the productivity and biochemical composition of Brachionus plicatilis . Mar Biotechnol. 2011;13:1075–1085. doi: 10.1007/s10126-011-9370-y. [DOI] [PubMed] [Google Scholar]
- Fontaneto D, Giordani I, Melone G, Serra M. Disentangling the morphological stasis in two rotifer species of the Brachionus plicatilis species complex. Hydrobiologia. 2007;583:297–307. [Google Scholar]
- Gems D, Doonan R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- Gilbert JJ. Female polymorphism and sexual reproduction in the rotifer Asplanchna: evolution of their relationship and control by dietary tocopherol. Am Nat. 1980;116:409–431. [Google Scholar]
- Gilbert JJ, Thompson GA., Jr Alpha-tocopherol control of sexuality and polymorphism in the rotifer Asplanchna . Science. 1968;159:734–736. doi: 10.1126/science.159.3816.734. [DOI] [PubMed] [Google Scholar]
- Giribet G. Assembling the lophotrochozoan (=spiralian) tree of life. Phil Trans R Soc B. 2008;363:1513–1522. doi: 10.1098/rstb.2007.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J Pharmacol Exp Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- Gruber J, Ng LF, Poovathingal SK, Halliwell B. Deceptively simple but simply deceptive—Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett. 2009;583:3377–3387. doi: 10.1016/j.febslet.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon CI. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Berg CJ Jr, editor. Culture of marine invertebrates. Stroudsburg: Hutchinson Ross; 1983. [Google Scholar]
- Gutteridge JMC, Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants—Quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Biological ageing is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Funct Ecol. 2011;25:209–216. [Google Scholar]
- Kenyon CI. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kim RO, Rhee JS, Won EJ, Lee KW, Kang CM, Lee YM, Lee JS. Ultraviolet B retards growth, induces oxidative stress, and modulates DNA repair-related gene and heat shock protein gene expression in the monogonont rotifer, Brachionus sp. Aquat Toxicol. 2011;101:529–539. doi: 10.1016/j.aquatox.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Kubanek J, Snell TW. Quorum sensing in rotifers. In: Winans SC, Bassler BL, editors. Chemical communication among microbes. Washington, DC: ASM Press; 2008. pp. 453–461. [Google Scholar]
- Le Couteur DG, McLachlan A, Quinn RJ, Simpson S, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A BioI Sci Med Sci. 2011 doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim RO, Rhee JS, Han J, Hwang DS, Choi BS, Lee CJ, Yoon YD, Lim JS, Lee YM, et al. Sequence analysis of genomic DNA (680 Mb) by GS-FLX-titanium sequencer in the monogonont rotifer, Brachionus ibericus . Hydrobiologia. 2011;662:65–75. [Google Scholar]
- Litton JR. Specificity of the α-tocopherol (vitamin E) effect on lifespan and fecundity of bdelloid rotifers. Hydrobiologia. 1987;147:135–139. [Google Scholar]
- Martin RD, Rivers JPW, Cowgill UM. Culturing mealworms as food for animals in captivity. Int Zoo Yearb. 1976;16:63–70. [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Aging Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DH, Stiles JW, Santelli J, Sanadi DR. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol. 1979;34:28–36. doi: 10.1093/geronj/34.1.28. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggeler B, Sambamurti K, Siedlak SL, Perry G, Smith MA, Papolla MA. A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PLoS One. 2010;5:e10206. doi: 10.1371/journal.pone.0010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybysz AJ, Choe KP, Strange K, Roberts LJ. Ageing inhibits the adaptive response of Caenorhabditis elegans to the reactive oxygen species-generating compound juglone. Free Rad Biol Med. 2007;43:158. [Google Scholar]
- Pun PBL, Gruber J, Tang SW, Schaffer S, Ong RLS, Fong S, Ng LF, Cheah I, Halliwell B. Ageing in nematodes: Do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology. 2010;11:17–30. doi: 10.1007/s10522-009-9223-5. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Kim RO, Choi HG, Lee J, Lee YM, Lee JS. Molecular and biochemical modulation of heat shock protein 20 (Hsp20) gene by temperature stress and hydrogen peroxide (H2O2) in the monogonont rotifer, Brachionus sp. Comp Biochem Physiol. 2011;154:19–27. doi: 10.1016/j.cbpc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Sawada M, Carlson JC. Association of lipid peroxidation during luteal regression in the rat and natural aging in the rotifer. Exp Gerontol. 1985;20:179–186. doi: 10.1016/0531-5565(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Sawada M, Carlson JC. Biochemical changes associated with the mechanism controlling superoxide radical formation in the aging rotifer. J Cellular Biochem. 1990;44:153–165. doi: 10.1002/jcb.240440304. [DOI] [PubMed] [Google Scholar]
- Sawada M, Enesco HE. Vitamin E extends lifespan of the short-lived rotifer Asplanchna brightwelli . Exp Gerontol. 1984;19:179–183. doi: 10.1016/0531-5565(84)90036-6. [DOI] [PubMed] [Google Scholar]
- Sawada M, Carlson JC, Enesco HE. The effects of UV radiation and antioxidants on lifespan and lipid peroxidation in the rotifer Asplanchna brightwelli . Arch Gerontol Geriatr. 1990;10:27–36. doi: 10.1016/0167-4943(90)90041-4. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Soskic Y. What does systems biology mean for drug development? Curr Med Chem. 2008;15:1520–1528. doi: 10.2174/092986708784638843. [DOI] [PubMed] [Google Scholar]
- Serra M, Snell TW, King CE. The timing of sex in cyclical parthenogenetic rotifers. In: Moya A, Font E, editors. Evolution: from molecules to ecosystems. New York: Oxford University Press; 2004. [Google Scholar]
- Snell TW, Moffat BD. A 2-d life cycle test with the rotifer Brachionus calyciflorus . Environ Toxicol Chem. 1992;11:1249–1257. [Google Scholar]
- Snell TW, Kubanek JM, Carter WE, Payne AB, Kim J, Hicks M, Stelzer CP. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera) Mar Biol. 2006;149:763–773. [Google Scholar]
- Snell TW, Shearer TL, Smith HA. Exposure to dsRNA elicits RNA interference in Brachionus manjavacas (Rotifera) Mar Biotechnol. 2011;13:264–274. doi: 10.1007/s10126-010-9295-x. [DOI] [PubMed] [Google Scholar]
- Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light induced erythema in humans. Am J Clin Nutr. 2000;71:795–798. doi: 10.1093/ajcn/71.3.795. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Hashimoto Y, Nakao S, Yoshinaga T. Effect of juglone on the survival time of two Brachionus species (Rotifera): species-specific tolerance against oxidative stress. Fish Sci. 2009;75:191–194. [Google Scholar]
- Tsuchiya M, Suematsu M, Suzuki H. In vivo visualization of oxygen radical-dependent photoemission. Methods Enzymol. 1994;233:128–140. doi: 10.1016/s0076-6879(94)33015-8. [DOI] [PubMed] [Google Scholar]
- Vertuani S, Angusti A, Manfredini S. The antioxidants and pro-antioxidants network: an overview. Curr Pharm Des. 2004;10:1677–1694. doi: 10.2174/1381612043384655. [DOI] [PubMed] [Google Scholar]
- Wallace RL, Snell TW. Rotifera. In: Thorp JH, Covich AP, editors. Ecology and systematics of North American freshwater invertebrates. 3rd edn. NY: Academic Press; 2010. [Google Scholar]