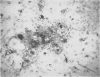
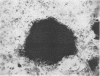
Abstract
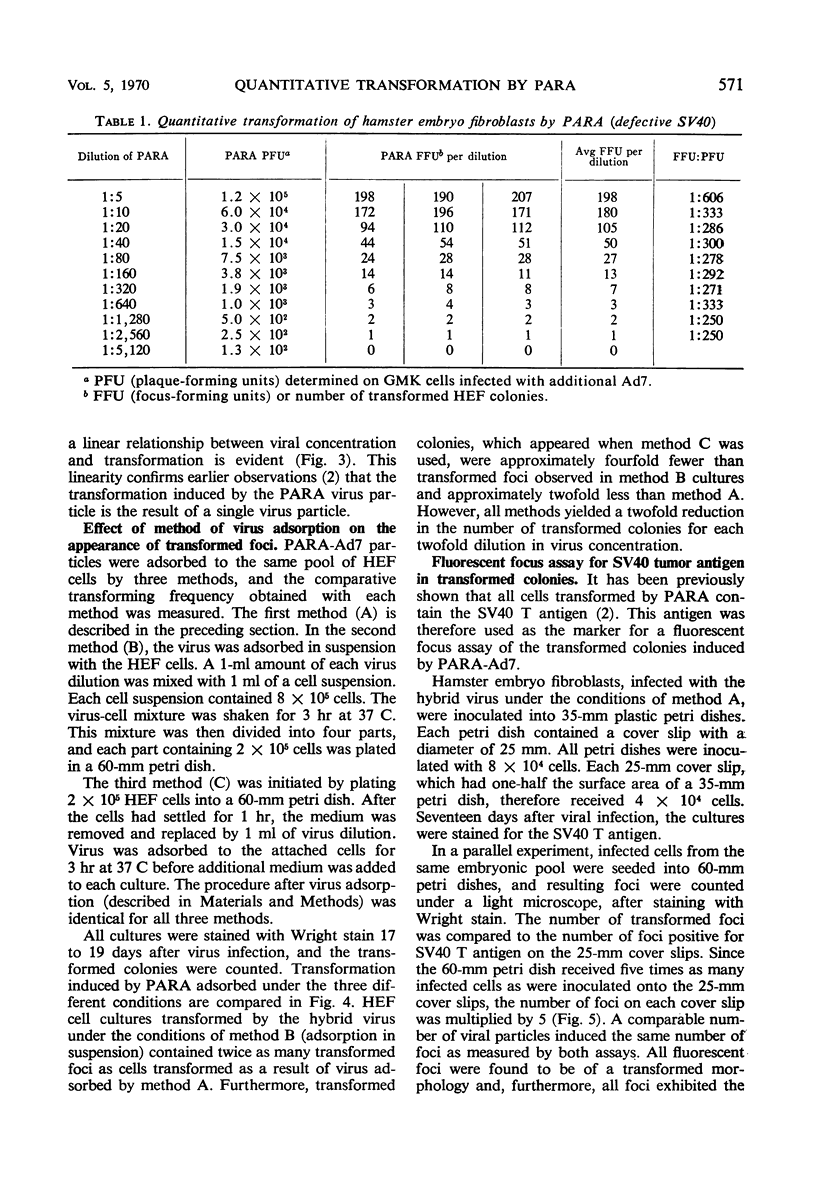
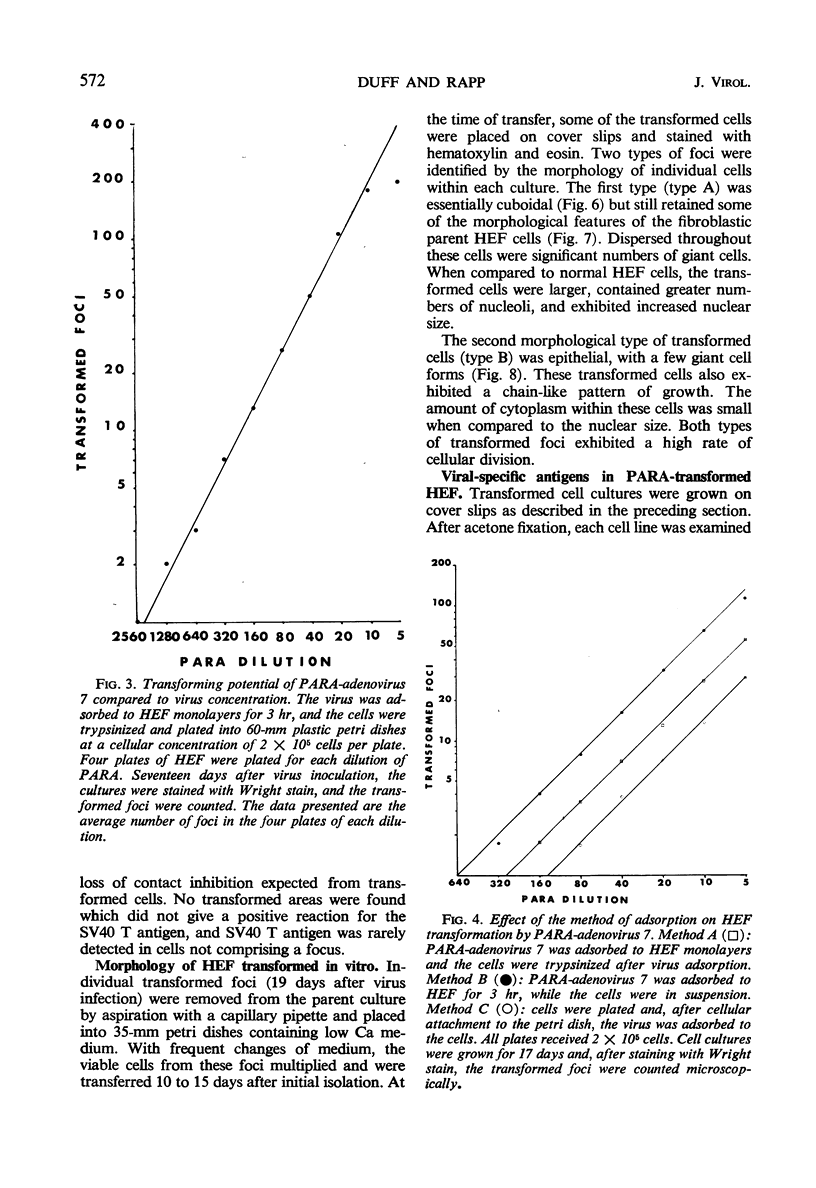
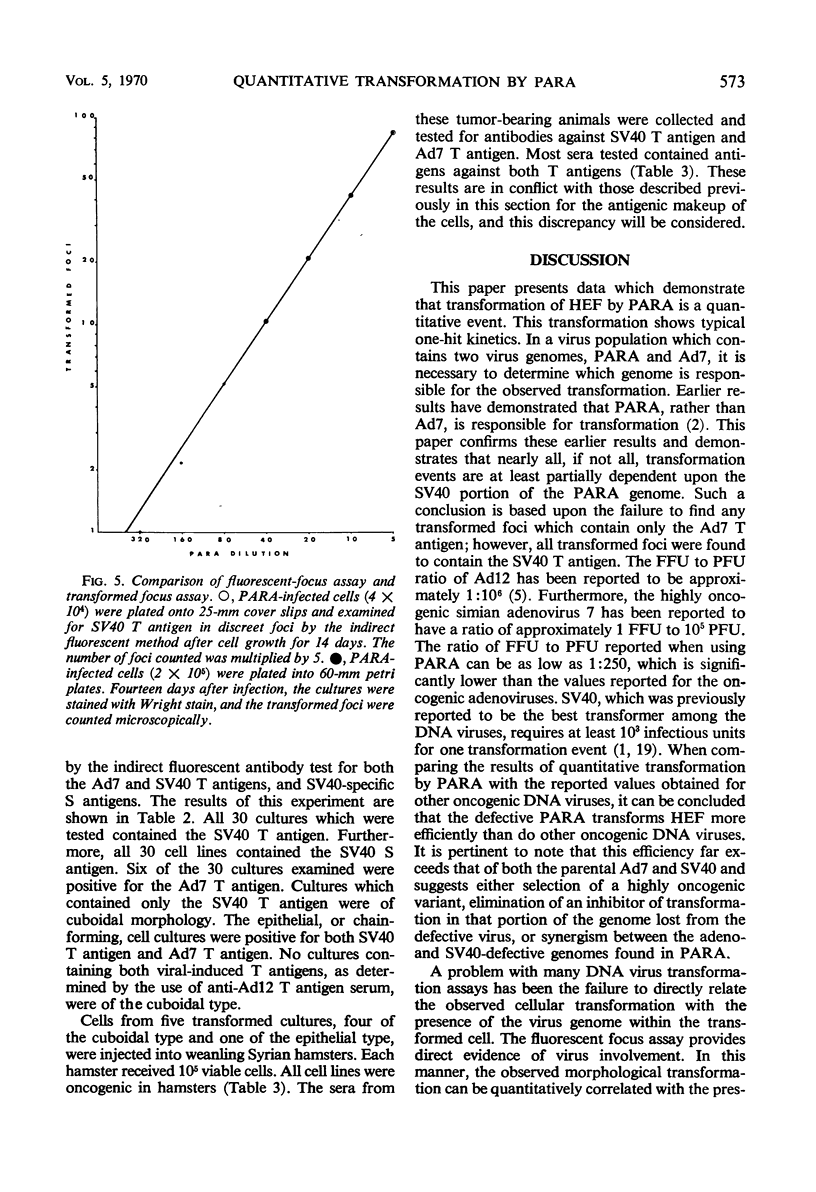
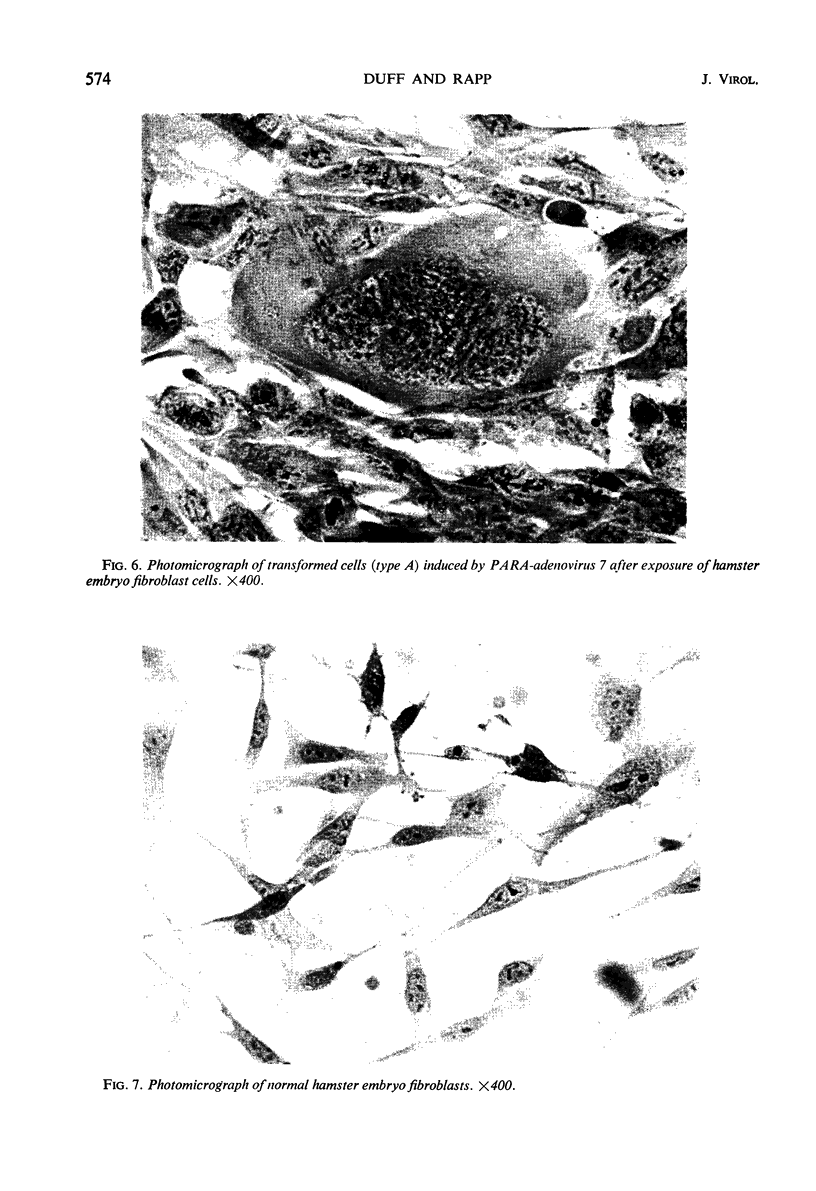
An in vitro method for the quantitative measurement of transformation in hamster embryo fibroblasts by the PARA [defective simian virus 40 (SV40)]-adenovirus 7 hybrid has been developed. Transformation by PARA particles followed one-hit kinetics with a ratio of 1 focus-forming unit per 250 plaque-forming units. The method of viral adsorption had a direct effect upon the total number of foci which developed but not on the quantitative aspects of this assay. A fluorescent-focus assay was developed which provided a direct correlation of the observed morphological transformation and the presence of the PARA genome. This fluorescent-focus assay utilized detection of the SV40 tumor antigen, which was present in all foci transformed by PARA. Single foci induced by PARA were isolated and grown into cell lines. Two types of foci were observed and isolated; the first contained cells having a cuboidal or SV40-type morphology, and the second consisted of epithelial or adenovirus-type transformed cells. Both types contained the SV40 tumor and SV40 surface antigens as determined by the indirect fluorescence technique; however, only the epithelial cells contained the adenovirus 7 tumor antigen. All five cell lines which were injected into weanling Syrian hamsters were found to be oncogenic. These cell lines induced antibodies to both SV40 and adenovirus 7 tumor antigens in tumor-bearing animals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. H., Todaro G. J. In vitro transformation of hamster and human cells with the adeno 7-SV 40 hybrid virus. Proc Natl Acad Sci U S A. 1965 Aug;54(2):374–381. doi: 10.1073/pnas.54.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- Black P. H., White B. J. In vitro transformation by the adenovirus-SV40 hybrid viruses. II. Characteristics of the transformation of hamster cells by the adeno 2-, adeno 3-, and adeno 12-SV40 viruses. J Exp Med. 1967 Apr 1;125(4):629–646. doi: 10.1084/jem.125.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto B. C. Adenovirus transformation of hamster embryo cells. J Virol. 1968 Apr;2(4):376–383. doi: 10.1128/jvi.2.4.376-383.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., CHANOCK R. M., RUBIN B. A., CASEY M. J. INDUCTION BY ADENOVIRUS TYPE 7 OF TUMORS IN HAMSTERS HAVING THE ANTIGENIC CHARACTERISTICS OF SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1333–1340. doi: 10.1073/pnas.52.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., BUTEL J. S., KITAHARA T. THE INCORPORATION OF SV40 MATERIAL INTO ADENOVIRUS 7 AS MEASURED BY INTRANUCLEAR SYNTHESIS OF SV40 TUMOR ANTIGEN. Proc Natl Acad Sci U S A. 1964 Dec;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., Malmgren R. A., Kirschstein R. L. Induction of neoplasia in vitro in hamster kidney tissue by adenovirus 7-SV40 "hybrid" strain (LLE46). Proc Soc Exp Biol Med. 1966 Feb;121(2):486–489. doi: 10.3181/00379727-121-30811. [DOI] [PubMed] [Google Scholar]
- Rapp F., Jerkofsky M., Melnick J. L., Levy B. Variation in the oncogenic potential of human adenoviruses carrying a defective SV40 genome (PARA). J Exp Med. 1968 Jan 1;127(1):77–90. doi: 10.1084/jem.127.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Koprowski H., Defendi V. The genetic heterogeneity of simian virus 40. Proc Natl Acad Sci U S A. 1967 Aug;58(2):599–606. doi: 10.1073/pnas.58.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell K., Maryak J., Schmidt M. Adenovirus transformation of hamster embryo cells. 3. Maintenance conditions. Arch Gesamte Virusforsch. 1968;24(4):352–360. doi: 10.1007/BF01243076. [DOI] [PubMed] [Google Scholar]
- Schell K., Maryak J., Young J., Schmidt M. Adenovirus transformation of hamster embryo cells. II. Inoculation conditions. Arch Gesamte Virusforsch. 1968;24(4):342–351. doi: 10.1007/BF01243075. [DOI] [PubMed] [Google Scholar]
- Schell K., Schmidt M. Adenovirus transformation of hamster embryo cells. I. Assay conditions. Arch Gesamte Virusforsch. 1968;24(4):332–341. doi: 10.1007/BF01243074. [DOI] [PubMed] [Google Scholar]
- TEVETHIA S. S., KATZ M., RAPP F. NEW SURFACE ANTIGEN IN CELLS TRANSFORMED BY SIMIAN PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1965 Jul;119:896–901. doi: 10.3181/00379727-119-30330. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Green H. High frequency of SV40 transformation of mouse cell line 3T3. Virology. 1966 Apr;28(4):756–759. doi: 10.1016/0042-6822(66)90261-3. [DOI] [PubMed] [Google Scholar]