Abstract
Germline proliferation in Caenorhabditis elegans is emerging as a compelling model system for understanding the molecular basis for the developmental and physiological control of cell proliferation. This review covers the discovery and implications of the role of the insulin/IGF-like signaling pathway in germline proliferation during germline development. This pathway plays a host of important roles in C. elegans biology. Its role in germline proliferation is important to generate the proper adult stem/progenitor population and to ensure optimal fecundity. Moreover, in this role, it is restricted to reproductive (as opposed to dauer) larval stages and impinges on the G2 of the cell cycle. Two putative insulin ligands are especially important for the germline role but do not mediate signaling in other tissues. A picture is emerging of a complex web of developmentally and temporally restricted, ligand- and tissue-specific responses to insulin signaling. Avenues for future studies include the regulation of specific insulin-like ligands and the mechanisms for tissue-specific responses to them.
I. Germline Proliferation in C. elegans: A Model for Developmental, Physiological, and Environmental Control of Cell Proliferation
Germline proliferation in Caenorhabditis elegans is a powerful model for understanding the control of cell proliferation and stem cell biology in general (see reviews by Hansen and Schedl, 2006; Joshi et al., 2010; Kimble and Crittenden, 2005, 2007). Unlike somatic cells in C. elegans, the germ line is not under cell lineage constraints and therefore shares features with tissue growth in other organisms. Beyond cell cycle regulation itself, this model is particularly amenable to exploring molecular mechanisms that underlie changes in proliferation rate and in the proliferation/differentiation balance in response to developmental, physiological, and environmental cues (Korta and Hubbard, 2010). Under replete laboratory conditions, C. elegans develop to adulthood in ~3 days and produce prodigious broods of ~300 self-progeny over the subsequent 3–5 days. In poor environmental conditions, they can reversibly arrest their developmental program at several stages prior to reproduction and thereby delay reproduction and improve their chances of reproducing in a more favorable environment. Once they have committed to a reproductive mode of development, they can further modulate the proliferation of the germ line. Several well-conserved genetic and cellular mechanisms alter germline proliferation and hence reproductive capacity. Among these, we focus here on the insulin signaling pathway, an important modulator of germline proliferation.
II. C. elegans Germline Development
C. elegans hermaphrodite germline development was described by Hirsh et al. (1976) and has been extensively reviewed since (see WormBook: http://www.wormbook.org/toc_germline.html for recent reviews). The reader is directed to those reviews as only a brief description is provided here.
The germ line develops over the course of four larval stages (L1–L4) and continues to proliferate in the adult. C. elegans exist as either males or hermaphrodites. The latter are modified females that produce and store sperm late in the last larval stage before undergoing a switch to produce exclusively oocytes as adults. Male C. elegans produce only sperm and reproduce by mating with hermaphrodites. Unlike many other species, the number of sperm is limiting for brood size. Hence, hermaphrodites in the absence of males will produce broods based on the number of self-made sperm whereas mated hermaphrodites can produce additional progeny.
The hermaphrodite germ line proliferates over the course of development, ultimately establishing a pool of “cells” that remain undifferentiated and divide to give rise to gametes. The germ cell nuclei are surrounded by their own cytoplasm but retain an opening to a central core of cytoplasm. Thus, while they are technically syncytial, they behave as individual cells. For example, adjacent germ nuclei do not divide in synchrony, suggesting that cyclin components are not shared between them. Hence, the field refers to them as germ “cells” for simplicity.
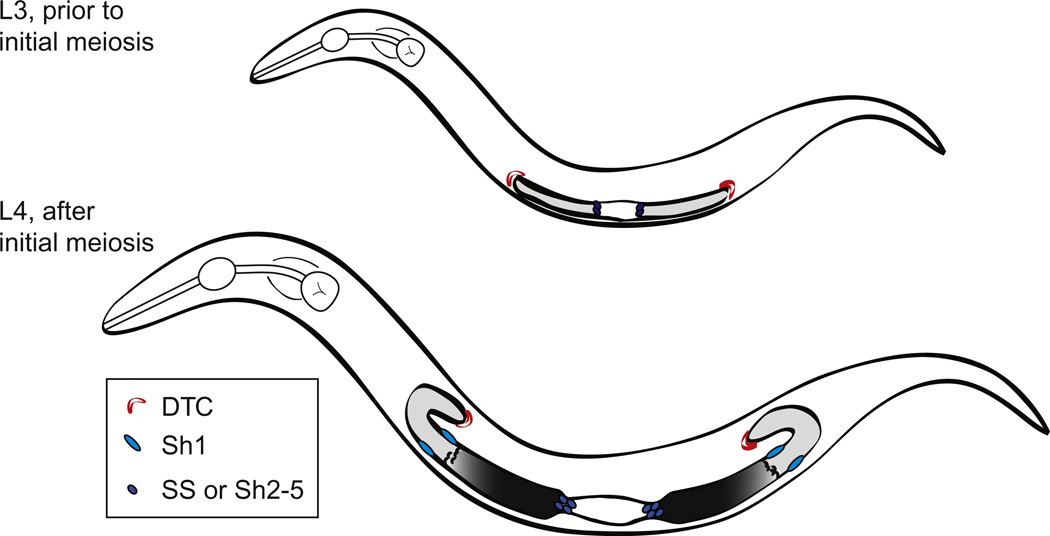
The development of the germ line takes place during the entire larval period of four larval stages and into the adult stage. During early larval stages (L1, L2, and early L3), all germ cells appear undifferentiated (Fig. 3.1). Also during this time, the somatic gonad rearranges such that germ cells are separated into two gonad “arms.” Each arm is blind-ended, capped by a distal tip cell (DTC) at the distal end, and open to a common uterus proximally. In the mid-L3 stage, the proximal-most cells reach a distance of 13 cell diameters from the distal tip and germ cells beyond this distance show the first overt signs of meiotic entry (a change from round to crescent-shaped chromatin morphology visible with DAPI or positive staining for anti-HIM-3, an early meiotic fate marker). This zone of early differentiation (leptotene and zygotene of prophase of meiosis I) is called the “transition zone.” This first appearance of transition nuclei, an event we call “initial meiosis,” sets up the pattern of proliferation and differentiation in the germ line. Once initial meiosis occurs, cells distal to the border are undifferentiated (“proliferative” or “mitotic” zone) and those proximal to it are differentiated (in meiosis and gametogenesis). Although definitive stem cell markers are not yet available in this system, the proliferative zone acts as a stem cell pool and the cells therein can be considered “progenitor” cells in the sense that they are either stem cells or the “transit amplifying” proliferative progeny of stem cells. The proliferative zone continues to grow from its first establishment in the L3 such that by the early adult there are ~200 nuclei in the zone and the transition zone begins at a distance of ~20–22 cell diameters from the distal tip.
Figure 3.1.
Cartoon depictions of L3 and early L4 stage C. elegans hermaphrodites. Bilobed gonad is indicated with proliferative germ cells in light gray, meiotic cells dark gray to black. Crescent-shaped nuclei characteristic of the transition zone are depicted. White central portion indicates position of future spermathecae and uterine cells. Distal tip cells are shown in red and distal and proximal sheath cells in lighter and darker blue, respectively.
In the late L4, sperm are produced, followed by a switch to oocyte production in adulthood. Mature sperm are pushed into the spermatheca by the first ovulating oocyte where they subsequently reside. Oocytes are fertilized one by one in an assembly like fashion and zygotes initiate development in uterus. Sperm can also be introduced by mating with males that exclusively produce sperm.
III. The C. elegans Germ line “Proliferation Versus Differentiation” Decision Is Mediated from the Soma to the Germ line by a Conserved Notch Signaling Pathway
The role of the Notch pathway in C. elegans germline development and its downstream effectors in the germ line have been extensively reviewed elsewhere (Byrd and Kimble, 2009; Hansen and Schedl, 2006; Kimble and Crittenden, 2007). Briefly, the somatic DTC is required to maintain the proliferative zone under wild-type conditions in all stages of development. The DTC produces two DSL family ligands: LAG-2 is expressed from the earliest stages, and then APX-1 is expressed in addition starting in the L3 (Nadarajan et al., 2009). These ligands signal to the Notch family receptor GLP-1 in the germ line. Interference with DTC signaling by killing the DTC or by mutations that prevent Notch signaling cause all proliferative germ cells to differentiate. Moreover, Notch signaling is required continuously. That is, manipulations that interfere with this activity any time during the life of the worm will cause all germ cells to differentiate. Interestingly, reducing (but not removing) Notch signaling shortens the distance between the DTC and the transition zone, but does not change the rate of cell proliferation, as measured by mitotic index (Michaelson et al., 2010). In contrast, elevating Notch activity causes germ cells to remain undifferentiated further from the distal end, thereby expanding the proliferative zone. Elevation of Notch activity also elevates the mitotic index in germ cells beyond the first few cell diameters from the DTC (Maciejowski et al., 2006). The need for Notch signaling for the proliferative fate can be bypassed by interfering with the activity of redundant germline-autonomous downstream factors, including two pathways named for their components GLD-1 and GLD-2 that normally promote the meiotic fate and/or interfere with proliferation (see reviews referenced above). In the wild type, however, regardless of environmental conditions, the germ line requires signaling from the DTC to maintain the undifferentiated state and hence the proliferative zone.
IV. Evidence for Notch-Independent Soma-Germline Signaling Mechanisms That Modulate Germline Proliferation
We discovered genes that are required for robust larval germline proliferation but that do not act in the DTC by analysis of mutants from genetic screens that identified “pro” genes (Killian and Hubbard, 2004; Voutev et al., 2006). For pro-1, the activity was pinpointed to the sheath lineage of the somatic gonad (Fig. 3.1). In addition, time-course analysis of cell–cell interactions and a series of cell-ablation experiments narrowed down the proliferation-promoting activity of the sheath to the distal-most pair of sheath cells (Sh1 cells). Together, our results support a model in which the distal Sh1 cells support or promote robust larval germline proliferation. We therefore became interested in factors that would act in concert with or parallel to Notch signaling to modulate the rate or extent of proliferation, especially in the larval germ line when the proliferative zone is growing to establish the adult progenitor/stem cell pool.
Two immediate questions emerged from these discoveries: (1) what is the molecular basis for the germline proliferation-promoting activity of the distal Sh1 sheath and (2) what mechanisms account for the rapid proliferation of the germ line in larval stages relative to adult stages? The most straightforward genetic approach to address these questions in C. elegans would be to identify mutants that reduce but do not eliminate germline proliferation. Such approaches are suboptimal in this circumstance, however, since many housekeeping genes are required for germline proliferation. Instead, we sought a means to identify genes that were not required for germline proliferation per se but were required for robust proliferation of the larval germ line.
V. A Counter-Intuitive Assay to Indentify Potential Notch-Independent Mechanisms That Promote the Expansion of the Larval Germline Progenitor Pool
We used a somewhat counterintuitive strategy to identify genes that would promote larval germline proliferation but the loss of which not interfere with proliferation in general. In short, we knew that reducing larval proliferation (either by pro gene mutation or by ablation of distal sheath cells) had the secondary effect of delaying initial meiosis. This is due to the expandable nature of the gonad as it grows during larval stages. The distance between the DTC and the gonad center is a function of two processes: a DTC-intrinsic migration program that is at least partially dependent on gon-1 and hlh-12/mig-24, and on the number of germ cells. Importantly, if too-few germ cells are generated in larval stages, the DTC does not move centripetally at the correct rate. As a result, initial meiosis is delayed since Notch signaling exerts its influence over the entire population of germ cells that are now “stuck” within its range of influence. This scenario eventually results in a heterochronic disjunct between the germ line and the somatic gonad in which germ line development is delayed while somatic gonad development continues normally (Fig. 3.2; Killian and Hubbard, 2005; McGovern et al., 2009).
Figure 3.2.
Latent niche concept and related screening strategy. See text for details.
We further discovered a bizarre but opportune twist of development that provided the necessary assay. Certain somatic gonadal cells that are born adjacent to the proximal germ line express DSL family ligands that activate Notch receptor (McGovern et al., 2009).Under normal conditions, these cells are born and express these ligands after initial meiosis has occurred in the germ line such that differentiated germ cells act as a barrier preventing inappropriate signaling to proliferative germ cells that express GLP-1/Notch on their surface. If, however, initial meiosis is delayed for any reason, including failure of robust larval germline proliferation, undifferentiated germ cells are inappropriately juxtaposed to ligand-producing proximal somatic gonad cells. Undifferentiated germ cells express the GLP-1/Notch receptor and therefore respond to the new source of ligands and form a proximally located ectopic “tumor” of undifferentiated germ cells. Thus, counter-intuitively, interfering with genes that are required for timely and robust germline proliferation in larval stages delays initial meiosis and can cause the formation of a proximal germline tumor later in development.
This scenario provided a convenient screening strategy. The etiology of the proximal tumor phenotype suggested that we could identify key control genes while ensuring that germ cells were still capable of proliferation, allowing us to identify genes required for robust larval germline proliferation—that is, key developmental or physiological regulators— while avoiding genes required for all aspects of cell proliferation. We reasoned that if we combined two independent mechanisms to slightly delay meiotic entry without causing tumor formation, one germline-autonomous and one potentially sheath-autonomous, they might together cause a severe delay in meiotic entry that would cause tumor formation. To test this hypothesis, we started with a mutant background that causes a temperature-dependent delay in initial meiosis, glp-1(ar202). In this mutant, initial meiosis is severely delayed at the restrictive temperature of 25 °C and proximal tumors form. At the lower permissive temperature of 15 °C, however, initial meiosis is only slightly delayed and tumors do not form since meiotic entry occurs prior to the birth of the proximal sheath cells and the inappropriate cell–cell interaction does not occur. When we combined this mutant background with laser-surgical removal of the distal sheath cells, however, proximal tumor formation occurs at the permissive of 15 °C (Fig. 3.2; Killian and Hubbard, 2005). Thus, if we interfere with the activity of genes that, like the distal sheath cells, are required for robust larval germline proliferation, they too should cause proximal tumor formation in this mutant background at the permissive temperature. In genetic parlance, such mutations would be termed enhancers of the proximal tumor phenotype (Pro) of glp-1(ar202), or enhancers of Pro. Because this screening strategy requires germ cell proliferation in the proximal tumor as a read-out, it was unlikely to identify genes required for cell proliferation per se.
VI. Identification of the Insulin/IGF-Like Receptor (IIR) Pathway Role in Germline Proliferation
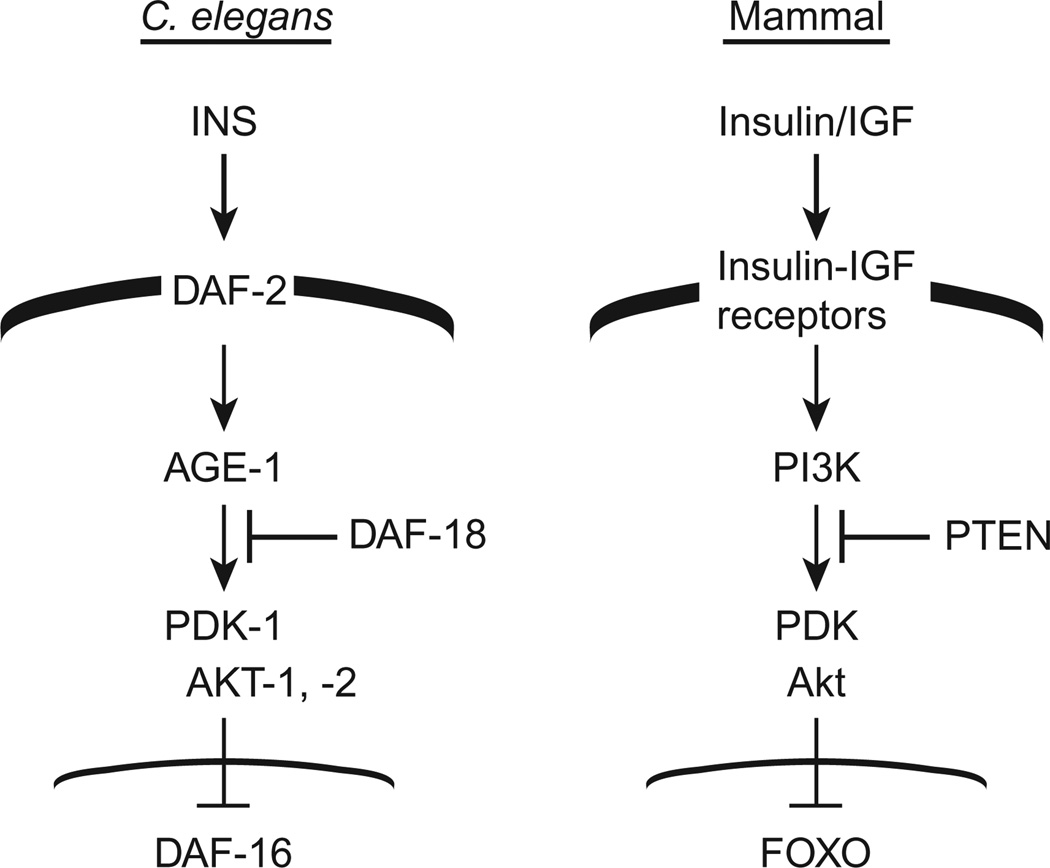
Using this strategy, we tested mutations in genes representing several candidate signaling pathways looking for mutations that would cause a tumor to form in the sensitized glp-1(ar202) genetic background at the permissive temperature. Reducing the function of daf-2, the gene encoding the sole insulin/IGF-like receptor (IIR) ortholog in C. elegans (Fig. 3.3) markedly elevated the percentage of worms that formed a proximal tumor at the permissive temperature. As summarized below, subsequent experiments confirmed that the number of larval cells in the proliferative zone was decreased in the mutants alone (Fig. 3.4).
Figure 3.3.
Insulin/IGF signaling pathways in C. elegans and mammals. Corresponding proteins or classes of proteins are indicated. Broad black line indicates the cell membrane and thin line indicates the nuclear membrane.
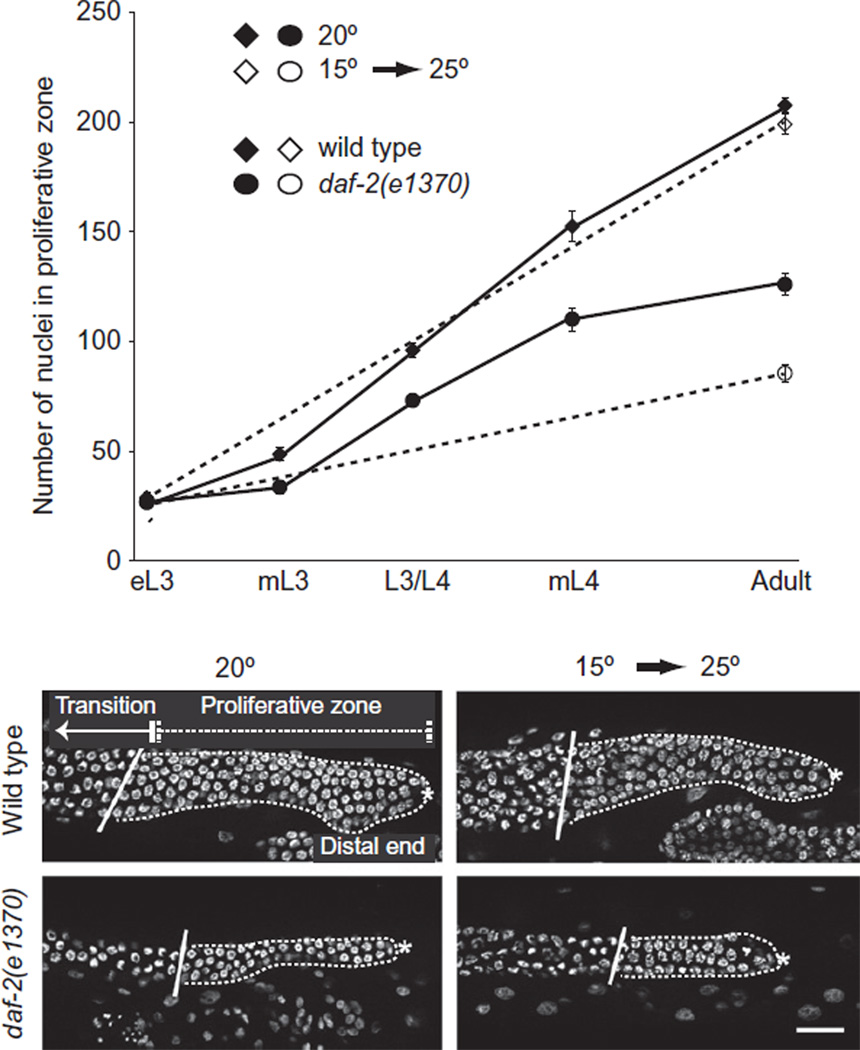
Figure 3.4.
Influence of DAF-2 activity on the proliferative zone. Top: number of nuclei in the proliferative zone in gonad arms of animals of the indicated ages in wildtype and in a daf-2 mutant under two temperature conditions, continuous growth at 20 °C or a shift from 15 to 25 °C. Bottom: images of distal germline nuclei of young adult wild-type and daf-2 mutants. Positions of the proliferative and transition zones are indicated. Asterisk marks the distal end in all panels. Scale bar indicates 20 µm. Figure modified from (Michaelson et al., 2010).
VII. IIR Signaling in C. elegans
The roles of the conserved IIR pathway in C. elegans have been extensively reviewed elsewhere (Fielenbach and Antebi, 2008; Kenyon, 2011, 2010; Kleemann and Murphy, 2009; Landis and Murphy, 2010). Briefly, the daf-2 gene was initially discovered and named for its role in the dauer decision. Dauer is a special pre-reproductive larval stage that worms enter if they encounter poor environmental conditions. The decision to enter dauer occurs in the late L1 and is largely dependent on an assessment of population density and food availability. Certain mutations in daf-2 cause worms to enter dauer constitutively, regardless of the environmental conditions. DAF-2 has also been well studied for its role in modulation of lifespan. While it is best characterized for its roles in dauer formation and lifespan, insulin signaling in worms also affects reproductive timing, metabolism, response to toxins, hypoxia, immunity, and L1 diapause. In each case, IIR signaling acts through a highly conserved PI3K-dependent pathway and negatively regulates the activity of a FOXO transcription factor DAF-16. The relationship between IIR signaling and FOXO was discovered in C. elegans. Though daf-2/IR and daf-16/FOXO exhibit both cell autonomous and nonautonomous activity, both are required primarily in neurons for dauer control while intestinal activity of daf-16/ FOXO accounts for most of the lifespan phenotype (Apfeld and Kenyon, 1998; Libina et al., 2003).
VIII. Insulin Signaling Promotes the Larval Germline Cell Division Cycle
Our analysis of daf-2/IIR mutants required that we avoid early larval arrest and dauer arrest phenotypes (Michaelson et al., 2010). Therefore, we carried out analyses using temperature-sensitive mutations. We examined the germ line under both semipermissive conditions (20 °C, when few animals enter dauer) and after a shift to the restrictive temperature after the dauer decision (L3 shift to 25 °C). We found that the number of adult germ cell progenitors was significantly reduced in the daf-2/IIR mutants and that the reduction occurred during the time that the population of these cells normally expands (Fig. 3.4). Moreover, we found that this phenotype could be completely suppressed by simultaneously reducing the activity of daf-18/PTEN or daf-16/FOXO, indicating that the canonical pathway is used in this context. We examined cell cycle parameters in the larval germ line and established that reducing daf-2 activity reduced the proportion of cells in M-phase and in S-phase. Moreover, measurements of the DNA content of larval germ nuclei indicated a shift to the 4N content, suggesting a delay in the G2 of the cell cycle. Finally, by tissue-specific restriction of gene activity, transgene expression and mosaic analysis, we determined that the receptor pathway and its downstream components are largely required in the germ line to promote robust larval proliferation. Taken together, our studies support the model that the DAF-2/IIR signaling cascade is reused after the dauer decision within the germ line to promote progression through the G2.
IX. C. elegans Insulins
Despite the high degree of conservation between IIR signaling in many organisms, some features of the C. elegans pathway are noteworthy. First, although there appears to be only one gene encoding an IIR (daf-2), at least 40 genes encode putative ligands, some of which behave as antagonists, and some as agonists. Interestingly, while few of the putative insulin peptides have been characterized, they appear to largely mediate different activities of the insulin receptor. For example, the ligands ins-1, ins-6, ins-7, and daf-28 are important and dynamically regulated for the daf-2 role in the dauer decision (Cornils et al., 2011). Recent studies suggest a widely dynamic use of these ligands during many stages of development (Baugh et al., 2011)
To identify insulin-like ligands that might be important for promoting larval germline proliferation, we used the same general screening strategy described above, targeting each ligand gene individually by RNAi (Michaelson et al., 2010). Given the distinct possibility of redundancy among the ligand-encoding genes, we relied on the extreme sensitivity of the genetic assay to help identify weak effects. We identified two ligand genes, ins-3 and ins-33 that, when knocked down individually, caused a phenotype similar to that of daf-2 at the semipermissive temperature. Reporters for these two genes are expressed in head neurons, and head and uterine cells, respectively, and their RNAi phenotypes are dependent on the activity of rrf-1, a gene required for somatic, but not germline RNAi. Therefore, they are required in the soma to influence germline proliferation. Interestingly, reduction of activity of each gene alone yields the same phenotype as reducing both together. This intriguing result is hard to reconcile with a simple ligand–receptor interaction model and future studies will be required to determine precisely how these ligands are working. One hypothesis is that a physical interaction is required between them; an alternate hypothesis is that a ligand–receptor relay exists, possibly including an alternate receptor. Indeed, biochemical and functional assays for tissue-specific aspects of ligand–receptor interaction would advance the field.
X. Many Target Tissues for IIR Signaling
In a curious parallel to the distribution of ligands associated with distinct phenotypes, the activity of the FOXO transcription factor daf-16 also appears to be required more stringently in some tissue types versus others, depending on the phenotype. For example, for dauer it is required primarily in neurons and for lifespan primarily in the intestine (Kwon et al., 2010; Libina et al., 2003). For the role of the IIR signaling pathway in promoting larval germline proliferation, the target appears to be the germ line itself. We found that replacing daf-16/FOXO in neurons or intestine did not rescue the daf-16 mutant phenotype (in this case, suppression of daf-2 mutant defects) though replacing it in the muscle had a mild effect. However, replacing it in the germ line had the strongest effect. Despite being tagged with GFP, the transgene used for these assays was not visible in germ cell nuclei, so we could not assess the change in localization of daf-16 upon removal of daf-2 in the germ line. Moreover, the transgene was very difficult to generate and has, indeed, been lost to germline silencing. Newer methods that allow for single-copy insertions should facilitate the generation of a more stable transgene for future studies.
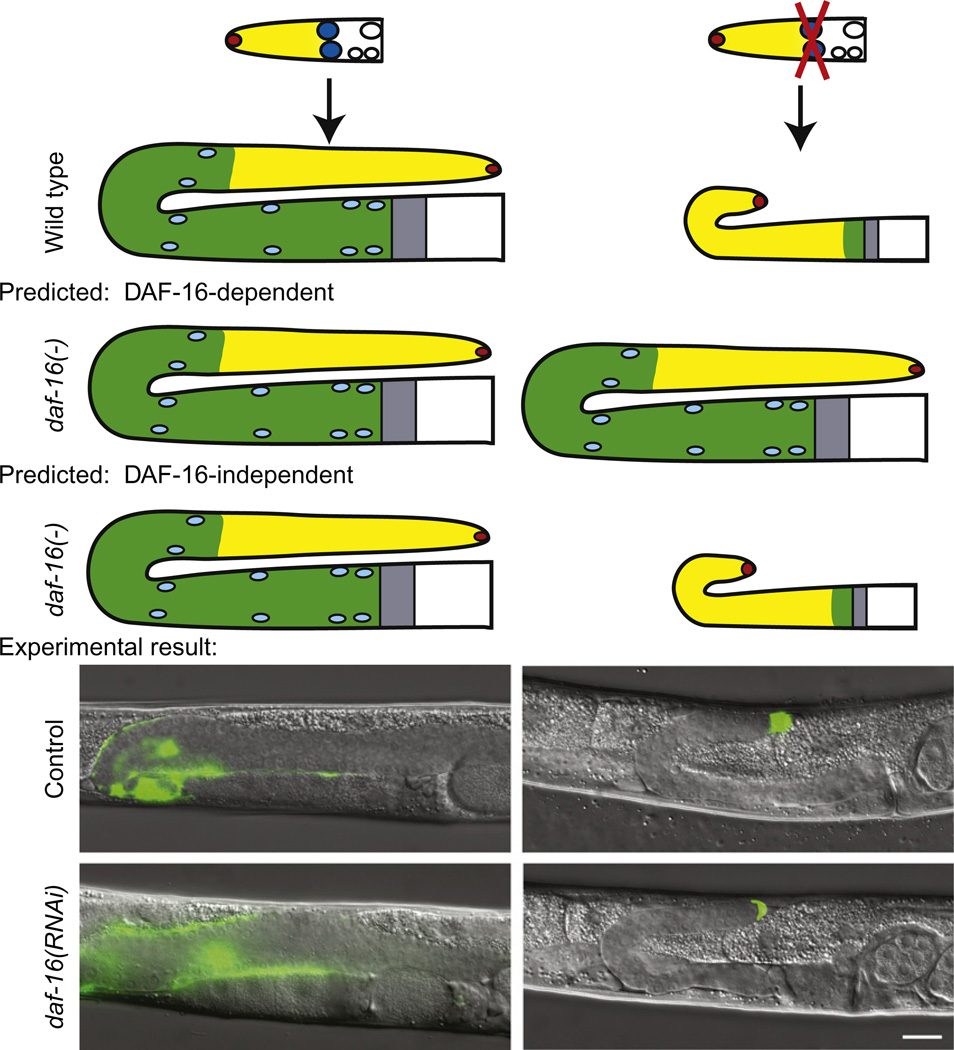
This result that daf-16 activity is required in the germ line for the larval germline proliferation phenotype was important for several reasons. First, it gave us the site of action of the receptor pathway and thereby provides a target tissue for approaches to identify downstream targets. Second, it helped distinguish between the activity of insulin signaling in the dauer decision and in reproductive larval stages. During the dauer stage, the germ line does not proliferate well (Narbonne and Roy, 2006). Therefore, in addition to establishing that the effect of daf-2 on the germ line could occur after the dauer decision by temperature shifts, we established that the receptor pathway is required in a different tissue. Second, it gave us a way to determine whether the distal sheath cells exert their proliferation-promoting function by way of the insulin pathway. For this experiment, we performed the sheath ablation under conditions that fully reversed the daf-2 dauer and germline proliferation defects using potent, independently verified daf-16(RNAi) and asked whether the sheath ablation still had any effect. This experiment is reported in Michaelson et al. (2010) and is summarized in Fig. 3.5. The results clearly indicated that the IIR signaling pathway cannot be the sole mediator of the sheath effect since depleting daf-16 did not prevent the deleterious effects of the sheath ablation on the proliferative zone.
Figure 3.5.
Experimental strategy to test dependence of sheath cell-ablation phenotype on daf-16 activity. Left: unablated conditions. Right: ablation conditions. See text for details. Bottom: Sheath cells and distal tip cell are marked with GFP. Scale bar indicates 20 µm. Modified from Michaelson et al. (2010).
Given that we had identified ligands that appeared to mediate the effects of daf-2 on the germ line but not on other phenotypes, we investigated the effects of the “larval germline phenotype” ligands, ins-3 and ins-33 on daf-16 activity (Michaelson et al., 2010). We knew that the germline proliferation defect resulting from reducing ins-3 or ins-33 activity could be completely suppressed by loss of daf-16/FOXO. We therefore wondered whether reducing the activity of these ligands would modulate the localization of DAF-16 in all tissues. For these assays, we used the intestine, where nuclear localization of a daf-16::GFP in response to loss of daf-2 activity is well-documented. Our results were striking: depleting ins-3 and ins-33 is not equivalent to reducing daf-2 activity as measured by nuclear localization of daf-16 or target gene activation in the intestine. That is, while daf-2 depletion caused nuclear localization of DAF-16 and target gene activation in the intestine, depletion of ins-3 or ins-33 did not. This result provides further evidence for complexity between ligand-receptor interaction in the context of specific target tissues.
XI. Other Germline Roles for the IIR Pathway
Insulin signaling also influences the germ line in earlier stages of development, most notably in the L1 and during dauer. In L1 larvae that hatch in the absence of food both somatic and germline development are arrested in a daf-18/PTEN dependent manner (Baugh and Sternberg, 2006; Fukuyama et al., 2006). In contrast to somatic cell arrest, however, germline cell cycle arrest occurs in the G2, and is not dependent on daf-16. In addition, germline proliferation is kept in check during dauer by pathways that regulate the dauer decision (IIR and TGFβ signaling) and this repression of proliferation requires daf-18/PTEN and the AMPK orthologs aak-1 and aak-2 (Narbonne and Roy, 2006).
XII. IIR Role in Larval Germline Proliferation: A Reproductive Timing and Lifespan Connection?
Insulin signaling influences other aspects of worm life history that potentially intersect with germline proliferation: reproductive timing and lifespan. Our studies to date suggest that these roles for the insulin pathway are separable from its role in promoting larval germline proliferation. Reproductive timing refers to the age and span of time over which C. elegans produce progeny. Dillin et al. (2002) described a daf-2 mutant phenotype in which progeny production spreads over approximately twice the time-frame of adulthood compared to wild-type animals. With respect to aging, loss of IIR pathway activity extends lifespan. Another way to extend lifespan is to eliminate germ cells or curtail germ cell proliferation. However, this manipulation leads to an additive effect on lifespan extension, suggesting separate mechanisms (Mukhopadhyay and Tissenbaum, 2007). Interestingly, Dillin et al. (2002) reported that the reproductive timing phenotype is dependent on activity of the IIR pathway prior to adulthood even though progeny are produced exclusively in adulthood, while the lifespan phenotype is sensitive only in the adult period. While the degree of larval germ cell proliferation could conceivably delay and extend reproductive timing, we found that ins-3 and ins-33 do not influence reproductive timing. We also found that the role of IIR signaling in promoting a more rapid cell cycle is limited to the larval stages, not the adult stage. Together these data do not support a causal link between the role of IIR in larval germline proliferation and its role in reproductive timing or lifespan regulation.
XIII. A Current Model and Future Directions
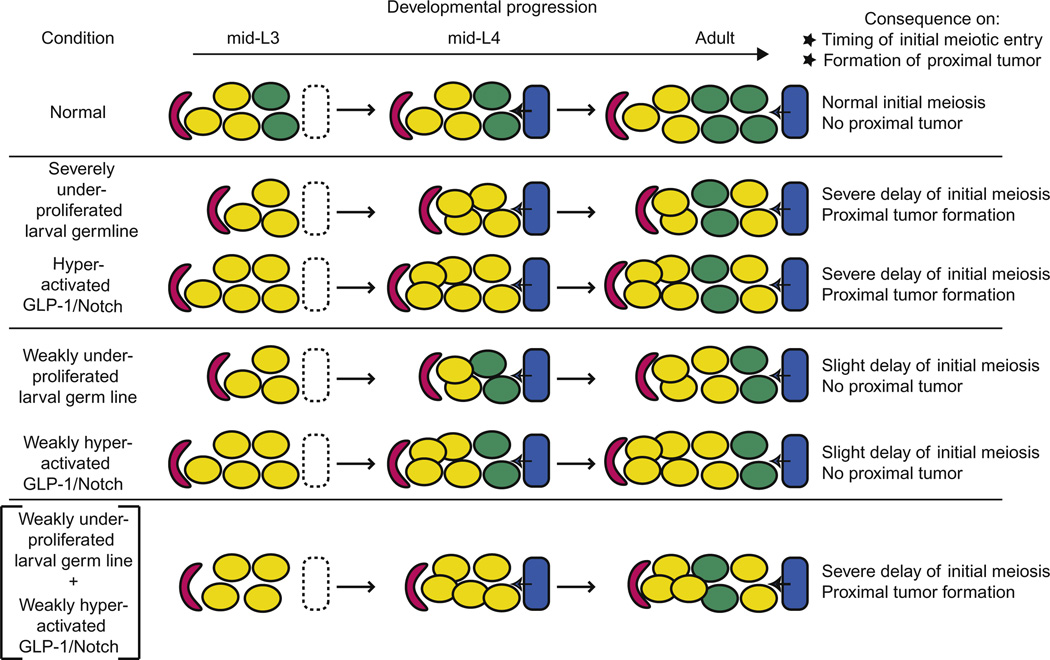
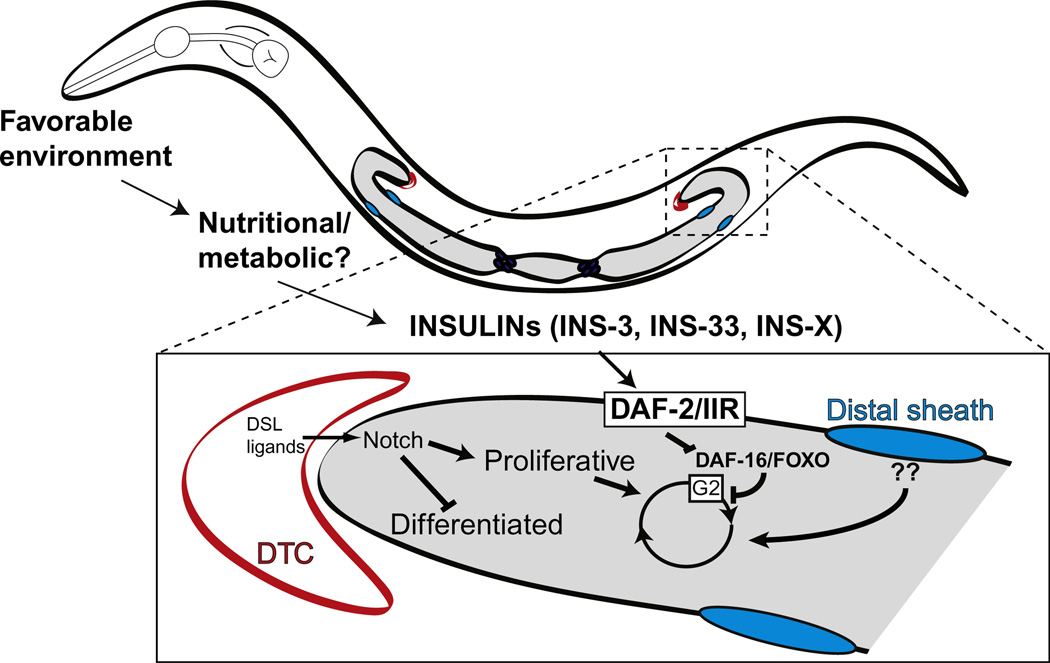
Figure 3.6 depicts a model for the control of larval germline proliferation in which Notch signaling is required primarily for the mitosis/meiosis cell fate decision and independent inputs from the distal sheath and insulin ligands help amplify the size of the germline progenitor pool during larval stages.
Figure 3.6.
Model for the control of larval germline proliferation in response to GLP-1/Notch, DAF-2/IIR, and distal sheath signals.
Nutrition impacts many facets of reproduction and cell proliferation in animals (Pinelli and Tagliabue, 2007). Moreover, the insulin pathway mediates nutrition-sensitive signaling in a variety of contexts in animal development and disease. Our findings suggest that C. elegans germline proliferation is a useful model to investigate the mechanistic connection between nutrition and the control of cell proliferation. Further, despite the evolutionary distance between C. elegans and mammals, many of the signaling pathways that control cell proliferation are conserved. Therefore, worm studies offer the possibility of broadly applicable results. Future studies may also provide insights into the nutritional control of cell proliferation in humans with implications for fertility, cancer, and stem cell biology.
There are many interesting questions remaining to be addressed in this system. At the organismal level, how is diet or metabolic signaling linked to the insulin pathway? Our model for insulin signaling in the germline parallels that seen in Drosophila, suggesting that this role may be highly conserved across species. In particular, there is conservation of the features that multiple inputs that impinge on cell cycle, and that IIR signaling acts on the G2. In Drosophila, the work of the Drummond-Barbosa lab (Drummond-Barbosa, 2008) has connected this role to diet, particularly to protein levels. In certain mouse cancer models, short-term caloric restriction reduces tumor growth in an insulin/IGF-PI3K signaling-dependent manner (Kalaany and Sabatini, 2009). The molecular mechanisms by which nutritionally sensitive molecular pathways control germ cell proliferation are best studied in a whole-organism context. C. elegans is a compelling and facile model for these studies.
Additional mechanistic questions include: How is ligand activity regulated such that different responses occur in different tissues? Do INS-3 and INS-33 bind DAF-2 at the germline membrane? How is it that depleting ins-3 and ins-33 activity causes identical phenotypes that are not further enhanced by loss of both genes? Since loss of these ligands does not cause as strong a phenotype as loss of receptor activity, what other ligands are involved? What are downstream targets of daf-16/FOXO in the germ line?
These and many other related questions will provide much work for the future and will likely contribute to our understanding of conserved connections between insulin/IGF signaling and cell proliferation.
ACKNOWLEDGMENTS
Support for this work is from NIH R01 GM061706. Thanks to members of my laboratory, especially David Michaelson and Dorota Korta, for discussion and comments on the manuscript.
REFERENCES
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Baugh LR, Kurhanewicz N, Sternberg PW. Sensitive and precise quantification of insulin-like mRNA expression in Caenorhabditis elegans. PLoS One. 2011;6:e18086. doi: 10.1371/journal.pone.0018086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DT, Kimble J. Scratching the niche that controls Caenorhabditis elegans germline stem cells. Semin. Cell Dev. Biol. 2009;20(9):1–7. doi: 10.1016/j.semcdb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornils A, Gloeck M, Chen Z, Zhang Y, Alcedo J. Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development. 2011;138:1183–1193. doi: 10.1242/dev.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D. Stem cells, their niches and the systemic environment: An aging network. Genetics. 2008;180:1787–1797. doi: 10.1534/genetics.108.098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Antebi A. Celegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Rougvie AE, Rothman JH. Celegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 2006;16:773–779. doi: 10.1016/j.cub.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Hansen D, Schedl T. The regulatory network controlling the proliferationmeiotic entry decision in the Caenorhabditis elegans germ line. Curr. Top. Dev. Biol. 2006;76:185–215. doi: 10.1016/S0070-2153(06)76006-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Joshi PM, Riddle MR, Djabrayan NJV, Rothman JH. Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 2010;239:1539–1554. doi: 10.1002/dvdy.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The first long-lived mutants: Discovery of the insulin/IGF-1 pathway for ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJA. Celegans pro-1 activity is required for soma/ germline interactions that influence proliferation and differentiation in the germ line. Development. 2004;131:1267–1278. doi: 10.1242/dev.01002. [DOI] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJA. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev. Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Germline proliferation and its control. WormBook. 2005:1–14. doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kleemann GA, Murphy CT. The endocrine regulation of aging in Caenorhabditis elegans. Mol. Cell. Endocrinol. 2009;299:51–57. doi: 10.1016/j.mce.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Korta DZ, Hubbard EJA. Soma-germline interactions that influence germline proliferation in Caenorhabditis elegans. Dev. Dyn. 2010;239:1449–1459. doi: 10.1002/dvdy.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JN, Murphy CT. Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev. Dyn. 2010;239:1405–1412. doi: 10.1002/dvdy.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Maciejowski J, Ugel N, Mishra B, Isopi M, Hubbard EJA. Quantitative analysis of germline mitosis in adult C. elegans. Dev. Biol. 2006;292:142–151. doi: 10.1016/j.ydbio.2005.12.046. [DOI] [PubMed] [Google Scholar]
- McGovern M, Voutev R, Maciejowski J, Corsi AK, Hubbard EJA. A "latent niche" mechanism for tumor initiation. Proc. Natl. Acad. Sci. USA. 2009;106:11617–11622. doi: 10.1073/pnas.0903768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D, Korta DZ, Capua Y, Hubbard EJA. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Tissenbaum HA. Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 2007;17:65–71. doi: 10.1016/j.tcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Nadarajan S, Govindan JA, McGovern M, Hubbard EJA, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–2234. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development. 2006;133:611–619. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- Pinelli G, Tagliabue A. Nutrition and fertility. Minerva Gastroenterol. Dietol. 2007;53:375–382. [PubMed] [Google Scholar]
- Voutev R, Killian DJ, Ahn JH, Hubbard EJA. Alterations in ribosome biogenesis cause specific defects in C. elegans hermaphrodite gonadogenesis. Dev. Biol. 2006;298:45–58. doi: 10.1016/j.ydbio.2006.06.011. [DOI] [PubMed] [Google Scholar]