Abstract
Concurrent measures of event-related potentials (ERPs) and skin conductance responses were obtained in an auditory oddball task consisting of rare target, rare non-signal unique novel and frequent standard tones. Twelve right-handed male social drinkers participated in all four cells of the balanced placebo design in which effects of beverage and instructions as to the beverage content (expectancy) were independently manipulated. The beverage contained either juice only, or vodka mixed with juice in the ratio that successfully disguised the taste of alcohol and raised average peak blood alcohol level to 0.045%. ERPs were sensitive to adverse effects of mild inebriation, whereas behavioral measures were not affected. Alcohol ingestion reliably increased N2 amplitude and reduced the Late Positive Complex (LPC). A large, fronto-central P3a (280 ms latency) was recorded to novel sounds in placebo condition, but only on the trials that also evoked electrodermal orienting responses. Both novel and target stimuli evoked a posterior P3b (340 ms) which was independent of orienting. Alcohol selectively attenuated the P3a to novel sounds on trials with autonomic arousal. This evidence confirms the previously suggested distinction between the subcomponents of the LPC: P3a may be a central index of orienting to novel, task-irrelevant but potentially significant stimuli and is an important component of the arousal system. P3b does not have a clear relationship with arousal and may embody voluntary cognitive processing of rare task-related stimuli. Overall, these results indicate that alcohol affects multiple brain systems concerned with arousal, attentional processes and cognitive-autonomic integration.
Keywords: alcohol, auditory oddball, arousal, P3a, skin conductance, novelty
INTRODUCTION
On a behavioral level, alcohol intoxication affects a variety of perceptual, cognitive and motor functions. Its effects on physiological measures of attention are evident at quite low doses (Jääskeläinen et al., 1999). Event-related potentials (ERPs) recorded on the scalp have been shown to be a sensitive measure of all aspects of alcohol-induced dysfunction such as acute and chronic use, tolerance, withdrawal and permanent dysfunction (Porjesz and Begleiter, 1987). Evidence from studies comparing ERPs of alcoholic and non-alcoholic subjects unequivocally suggests amplitude attenuation and sometimes increase in latency (see Porjesz and Begleiter, 1985; 1987; 1996 for reviews) of late components in alcoholic patients after several weeks of abstinence. In most studies, either auditory or visual oddball paradigms were employed allowing for assessment of ERP responses to signal, novel and non-signal stimuli.
Late endogenous potentials across sensory modalities seem to be affected by chronic alcohol use under different experimental and motivational conditions. Such diffuse and task-nonspecific impairment may indicate widespread detrimental effects of chronic alcohol use on different functional systems in the brain. Moreover, the effects on endogeneous potentials (P3) appear to be irreversible after long abstinence (Porjesz and Begleiter 1987, 1996; Keenan et al., 1997), in contrast to evidence of amplitude recovery of earlier potentials (Salamy et al., 1980). Based on converging evidence obtained from high-risk individuals, it has been suggested that a genetic susceptibility to alcohol dependence may be manifested by smaller P3 amplitudes even prior to alcohol abuse (Begleiter and Porjesz, 1999; Monteiro and Schuckit, 1988).
Evidence from studies employing healthy, social drinkers in placebo and alcohol conditions confirms general findings of studies with alcoholic patients abstaining from alcohol (Campbell and Lowick, 1987; Pfefferbaum et al., 1980; Porjesz and Begleiter, 1983; Jääskeläinen et al., 1999). Acute administration of alcohol results in amplitude depression of late endogenous potentials that seem to be inversely related to the alcohol dose (Teo & Ferguson, 1986) and related to the BAL on both ascending and descending limbs of the BAL curve (Salamy and Williams, 1973).
The Late Positive Complex (LPC), or P3, a large positive potential recorded in most cognitive tasks is not a unitary component but a composite of deflections differing in task and subject-state correlates, latency, topography on the scalp and generating structures. Squires et al. (1975) were among the first to outline two different subcomponents of LPC observed during an auditory oddball experiment. Their subjects were asked to either ignore while reading or count changes in pitch of rare tones presented on the background of frequent tones. Infrequent stimuli differing in pitch evoked a fronto-centrally distributed P3a wave in the “ignore” condition. A posteriorly distributed P3b deflection had a longer latency and was evoked in the “count” condition by the rare tones. Similarly, Courchesne et al. (1975) demonstrated that novel, unfamiliar, rarely occurring visual stimuli evoked anteriorly distributed P3a component whereas the repeating signal stimuli produced a posteriorly prominent P3b component. Evidence from lesion studies (Knight, 1984; 1997, Knight and Scabini,1998) and intracranial recordings (McCarthy and Wood, 1987; Alain et al., 1989; Baudena et al., 1995; Halgren et al., 1995a,b) indicates that a multifocal fronto-parieto-cingular limbic and cortical network is engaged during the novelty P3a potential, suggesting its importance in indexing supramodal orienting to novelty. The P3b is generated to attended, infrequent task-relevant stimuli and may index cognitive closure of a stimulus event processing (Halgren, 1990) that matches the existing memory templates (Knight et al., 1995).
The concept of novelty detection as a matching process with a neuronal model of previously occurring stimuli is the basis of the Orienting Reflex (OR) theory (Sokolov, 1963; 1975; Maltzman, 1990). An OR is evoked by potentially significant stimuli and is manifested when a mismatch is detected between the existing neuronal model and the parameters of the incoming stimulus. The OR is a global term for the large number of organismic changes including inhibition of ongoing activity, autonomic changes, postural adjustments and an increase in sensitivity of sensory organs (Lynn, 1966), that prepare the organism to cognitively integrate and respond to such stimuli (Halgren and Marinkovic, 1995). A large body of literature on OR in humans has been developed on the basis of the peripheral measures of autonomic activity (e.g. electrodermal responses, vascular dilation and constriction, pupillary response, heart rate). However, it is quite conceivable that the mechanisms subserving orienting behavior will be better understood on the basis of the central, rather than slow, non-specific peripheral responses. Indeed, various ERP components have been suggested as likely central analogues of the peripherally measured ORs: N1 (Kenemans et al., 1989), N2 and mismatch negativity (Näätänen and Gaillard, 1983), O-wave (Loveless, 1979; Rohrbaugh, 1984). Based on its sensitivity to the same variables as the autonomic OR measures, the P3 has been most often proposed as an ERP analogue of the peripherally measured OR (Roth, 1983; Donchin et al., 1984).
Direct investigations of the relationship between the peripheral autonomic responses and central ERPs have not been common. The relative paucity of studies employing both peripheral and central measures of higher brain functioning is partially due to differences in theoretical traditions that have selectively “adopted” one or the other physiological measure. Autonomic measures are commonly used by psychophysiologists in the Pavlovian tradition, whereas the ERPs are the measure of choice for the majority of cognitive neurophysiologists. In addition, paradigm differences have impeded integration of the two lines of research (e.g., ERPs are averaged across many trials presented with short ISIs, on the order of 1 sec; the autonomic ORs do not require averaging and are evoked by stimuli presented with long ISIs (>10 sec) in order to account for their long latency). Nonetheless, attempts to integrate the two lines of research are important and come as a natural consequence of common research questions and increasingly shared methodology (Friedman et al., 1973; Becker and Shapiro, 1980; Woestenburg et al., 1983; Simons et al., 1987; Lyytinen et al., 1992; please see Donchin et al., 1984; Loveless, 1979; Näätänen and Gaillard, 1983; Rohrbaugh, 1984 for reviews).
In the present study, concurrent recording of the peripheral autonomic (i.e. electrodermal) activity and ERPs allowed a direct comparison of the two physiological systems. Response latency differences between the two systems were successfully circumvented by minimal paradigm adaptations permitting optimal recording of both peripheral and central measures (Barry et al., 1993; Lyytinen et al., 1992). Employment of an auditory oddball paradigm with novel, deviant sounds in addition to standard frequent and rare target tones permitted an investigation of the differences between the LPC subcomponents. The following hypotheses can be tested with this approach: Two distinct LPC subcomponents (P3a and P3b) can be distinguished based on differences in eliciting conditions, their topographical and temporal attributes. The P3a has predominantly frontal distribution and precedes the P3b component. It is also hypothesized that the concurrent recordings can reveal the association between the centrally measured P3a and peripherally measured SCR, inasmuch they both index the orienting to novelty. Finally, it is hypothesized that the two LPC components can be further distinguished by their differential susceptibility to alcohol challenge.
It has been suggested (Rohsenow and Marlatt, 1981) that in addition to its pharmacological effects, alcohol may influence behavior through the culturally derived beliefs (“expectancies”) about its effects. Results of a meta-analysis of studies manipulating the pharmacological vs. instructional (“expectancy”) effects of alcohol (Hull and Bond, 1986) suggest that some social behaviors and alcohol consumption are affected by the instruction-induced expectancies. Measures of higher brain functions as indexed by ERPs have not yet been investigated within this context to our knowledge. In this study, balanced placebo design was utilized to differentiate effects of a low-to-moderate dose of alcohol from those of beverage instructions on physiological and behavioral responses in a tone discrimination task. Based on previous studies (Hull and Bond, 1986), it is hypothesized that the physiological measures are predominantly affected by alcohol intoxication. In agreement with previous findings, it is expected that the ERPs will be the most sensitive measure of alcohol effects. The moderately low dose will primarily attenuate late endogenous positivity (P3), with less prominent effects on the earlier deflections.
The results reported here are a portion of a comprehensive study investigating effects of alcohol on physiological indices of higher cognitive functions. Results from other tasks have been partially reported elsewhere (Marinkovic et al., 2000).
METHOD
Subjects
Twelve normal, non-smoking Caucasian males successfully completed all four sessions of the balanced placebo experiment, yielding total of 48 recording sessions. They were all native English speakers between 21 and 28 years of age (mean = 23.6) and were right-handed (Oldfield, 1971). They reported no medical, neurological, drug or alcohol abuse problems. None was ever arrested or in treatment for a drug or alcohol related offense. Their answers on the Alcohol Use Questionnaire, adapted from Mills et al. (1983) for probing quantity and frequency of alcohol use, indicated that they drank alcohol, mostly beer, occasionally (2.5 times per week on average) and in low to moderate amounts (3.4 drinks per occasion). The Michigan Alcoholism Screening Test (MAST; Selzer, 1971) indicated no alcoholism-related symptoms and the subjects reported no family history of alcoholism. In addition, their responses on the Childhood Hyperactivity Questionnaire (HK/MBD; Tarter et al., 1977), Eysenck Personality Questionnaire (EPQ; Eysenck and Eysenck, 1975) and Socialization Scale (SSQ) of the California Psychological Inventory (Gough, 1960) were within normal range. Prospective subjects were recruited from an advertisement in the campus newspaper and from another study. They were initially screened by telephone for their age, past and present alcohol, tobacco and drug use and family history of alcoholism. In order to become familiarized with the laboratory setting and experimental procedure all subjects first participated in a brief introductory recording session. No drinks were administered at that time. Twelve individuals successfully completed all four sessions of the experiment out of fifteen subjects that participated. Signed statements of consent approved by the relevant human subject protection review boards were obtained from all participants. They were monetarily reimbursed for their participation. This study required participation across multiple sessions. Consequently, females were not employed since their alcohol absorption and metabolism are altered by the menstrual cycle and oral contraceptives (Zeiner and Kegg, 1981).
Design
A within-subject balanced placebo design was utilized in an attempt to assess the effects of the beverage content itself, independently from instructions as to the beverage content (expectancy) and their interaction. The two factors (beverage and instructions) were fully crossed yielding four experimental conditions. Each subject participated in the four sessions in a random order and underwent the same procedure each time except for the consumed beverage and information concerning the alcohol content. Thus, each person participated in two alcohol and two placebo sessions. The participants were informed in writing that they would consume alcohol or a placebo in each session and that the information given to them regarding the beverage content may be inaccurate.
In order to minimize potential variability in alcohol metabolism and circadian effects all the experimental sessions were scheduled to start between 3 and 4 p.m. at least two days apart. Participants were asked to refrain from alcohol for 24 hours prior to the recording session and to abstain from food for 3 hours before the beginning of the experiment.
In the beginning of each session the subjects were informed about the drink content and a tray with the appropriate cues (e.g. a vodka bottle) was brought in. The participants finished their drinks in 10–15 minutes and were fitted with the recording electrodes. Estimates of the subjects’ blood alcohol levels (BALs) were obtained at several points during the experiment approximately every 20 min with an Alco-sensor III breathalyzer (Intoximeters, Inc.). Subjects reclined comfortably in an armchair in an electrically shielded room and used a hand-held microswitch to indicate their responses. In addition to this task, the subjects participated in a verbal memory task and they also rated their mood changes. At the end of each experimental session the subjects filled out a questionnaire about the task difficulty, the beverage content, self-perceived level and latency-to-maximum of intoxication etc.
Beverage administration
Smirnoff 80 proof vodka (40% alcohol by volume) was administered at a dosage of 0.4 g of 100% ethanol per kg of body weight and was mixed with a juice cocktail consisting of chilled Oceanspray grapefruit juice and Dole Pineapple-orange-guava frozen concentrate. Vodka and juice were mixed in 1:5.5 ratio, as it had been determined in a pilot study prior to the experiment that this mixture satisfactorily disguises the taste of alcohol.
In order to maximize the successful implementation of the “expectancy” manipulation the beverage administration procedure was adapted from Rohsenow and Marlatt (1981). In conditions when subjects were instructed that they were going to consume juice, the beverage (which did or did not contain vodka) was premixed and served in a glass pitcher and was poured into a cup in view of the subject. When subjects were told that they were going to consume alcohol, a premeasured amount of either vodka (in given alcohol/told alcohol condition) or water (in given placebo/told alcohol condition) was poured from a Smirnoff vodka bottle into the pitcher with juice. The drink was mixed in subjects’ view and was consumed out of a wineglass. In addition, a small piece of vodka-saturated gauze, which had been placed in the cap of the bottle unbeknownst to subjects, provided strong olfactory cues in the placebo deception condition. Relative novelty of the taste and lack of experience with this mixed drink on the part of the subjects seem to have contributed to the disguise of alcohol. Care was taken that the beverage containing alcohol did not differ from placebo in volume or taste.
Task
An auditory oddball paradigm with three stimulus types was used. Subjects were requested to count and press a button in response to rarely occurring signal tones embedded in a series of frequently occurring standard tones and occasional non-signal novel sounds requiring no response. Auditory stimuli were presented via speakers at a comfortable volume. Each of the two consecutively presented blocks consisted of 256 stimuli presented for 50 ms, 10 ms rise and fall times with the interstimulus interval of 1.9 s in a semi-random order. Frequent tones (140 Hz sawtooth) comprised 75% of the delivered stimuli whereas signal tones (600 Hz sinewave) and unusual non-signal novel sounds were presented on 12.5% of the trials each. The pitch of frequent and signal tones was counterbalanced across the two stimulus blocks. Novel sounds differed in pitch and harmonics but had the same amplitude envelope as the pure tones serving as frequents and signals.
Recording
EEG was recorded with a lycra fitted electrode cap (Electro-Cap International, Inc.) using 13 scalp sites that included Fz, Cz, Pz, F3, F4, F7, F8, C3, C4, P3, P4, T5, T6 of the 10–20 International system. The reference electrode was placed on the tip of the nose and the right earlobe served as ground. The electrooculogram (EOG) was recorded with bipolarly referred electrodes placed at the outer canthus of the right eye and just above the nasion. The electrode impedance was kept below 5 kOhms. The EEG and EOG were recorded with a Grass 16-channel polygraph with DC amplifiers set at .8 sec time constant and with a bandpass of 0.05 to 75 Hz (1/2 amplitude). The signal was digitized at a rate of 200 Hz with 12-bit accuracy and stored on an IBM-PC compatible computer for off-line analysis.
Skin conductance orienting responses (SCRs) were recorded from Beckman Ag-AgCl electrodes filled with K-Y jelly placed with adhesive collars (1 cm in diameter) on the volar surfaces of the first and third fingertips of one hand. Electrodermal responses were recorded from either left or right hand in an order counterbalanced across sessions. Subjects indicated their behavioral responses by means of a microswitch with the other hand. The SCRs were recorded with Coulbourn Instruments Skin Conductance amplifier through a constant 0.5 volt bridge circuit and digitized at 20 Hz.
Fig. 1 provides a schematic description of a typical segment of a recording sequence. In order to allow for optimal recording of both peripheral SCRs and central ERP measures, each recording epoch spanned two stimuli: either a rare (target or novel) stimulus and a following frequent tone, or two successive frequent tones. The data were recorded for 100 ms before the stimulus onset and continued for 3600 ms for both EEG and SCR signals.
Fig 1.
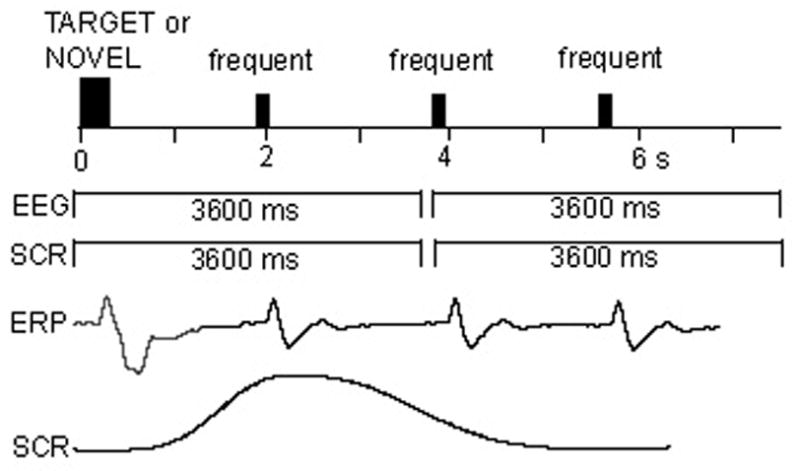
A schematic description of a typical two-trial recording segment. Each recording epoch started 100 ms before the stimulus onset and continued for 3600 ms for both SCR and ERP signals, including two tones: either a rare (target or novel) and a following frequent tone, or two successive frequent tones. Examples of ERP and SCR responses are also presented.
Data Analysis
Behavioral, electrodermal and event-related potential responses were analyzed with repeated measures ANOVAs (Woodward et al., 1990) with factors: beverage (alcohol, placebo), instructions (told alcohol, told placebo), stimulus type (frequent, target, novel), and, in the case of ERPs, electrode sites (13 sites). Huynh-Feldt procedure was used as a protection against the sphericity assumption violations in the repeated measures ANOVA (Huynh and Feldt, 1980). In cases when simple main effects or interactions were investigated, Tukey’s post hoc procedure for obtaining family-wise probability values was employed and the adjusted P-values are reported. Details of the analyses pertaining to each response system are given below.
RESULTS
As indicated by Fig. 2, average BAL measured 0.043 % (SD = 0.009) immediately before the task which started 51 minutes after the beverage was offered to the subjects on average. The maximum BAL value of 0.045 % (SD = 0.01) was measured after the completion of the task, indicating that the peak BAL was probably attained some time during the task performance on average. The subjects rated the difficulty of the auditory discrimination task as very easy (mean = 1.38, SD = 0.52) on a 1–5 Likert scale. Their ratings were not affected by the beverage nor instructions as to the beverage content. In addition, the ratings of the task difficulty did not change across the four sessions.
Fig 2.
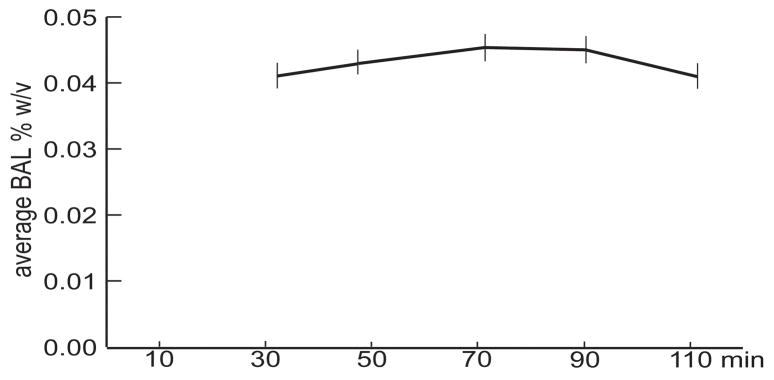
Mean (±SE) blood alcohol level (BAL) expressed as % w/v as a function of time after the beverage was offered to the subjects.
Performance
The overall performance accuracy exceeded 99 % (mean = 99.58, SD = 1.01) for all stimulus types and no effects of the type of beverage or instructions on the performance accuracy were noted. Similarly, reaction times (mean = 601.5 ms, SD = 133.3) were unaffected by any of the factors.
Electrodermal activity
SCRs were measured on each trial with a semi-automatic computer algorithm and were defined as a maximum response (in μS) within a 0.5 to 3.5 s response window. Only the SCRs occurring on trials with correct responses were included in the analyses. Due to equipment failure on three out of 48 sessions, complete data sets were available for nine subjects across all four experimental conditions. Trial examination and analyses were performed in a manner blind to experimental condition or trial type. In order to ameliorate their skewed distribution, the SCRs were transformed with a square root function for the analyses.
Neither beverage nor instructions had significant effects on the SCRs. The main effect of stimulus type, F2/16 = 16.9, P < 0.0001, was examined with Tukey pairwise comparisons that revealed significant differences between frequent and target, F1/8 = 21.0, P < 0.01 and frequent and novel tones, F1/8 = 16.7, P < 0.01. Thus, average SCRs evoked by frequently appearing tones were smaller (mean = 0.12, SD = 0.12 √μS) than the SCRs evoked by target (mean = 0.23, SD = 0.16 √μS) and novel tones (mean = 0.19, SD = 0.15 √μS). Equiprobable targets and novels did not differ significantly with respect to the average amplitude of the SCRs they evoked. Phasic responses to frequent, target and novel tones were observed on 5 %, 33 % and 20 % of all trials respectively.
Event-related potentials
Separate average waveforms were obtained for frequent, rare target and rare novel tones for all electrode sites and conditions. Only trials with correct responses that were clear of artifacts were included in the averages. ERPs obtained in this task were quantified with an automatic algorithm that measured average voltages within the following latency windows in order to estimate their corresponding ERP components: N1 (110–170 ms), N2 (160–250 ms), P3a (250–300 ms), P3b (300–450 ms) and Slow Wave (450–600 ms). In addition, single-point peak amplitude and latency measures of N1 and LPC deflections were successfully obtained by an automatic algorithm. All measures were expressed in microvolts (amplitudes) and milliseconds (latencies) with respect to a baseline period of 100 ms before stimulus onset.
Grand average waveforms in Fig. 3 were obtained by averaging ERPs on frequent, target and novel trials separately for placebo and alcohol conditions. Visual inspection of these waveforms suggests that the pattern is the same for both beverage conditions, with a prominent LPC attenuation in alcohol condition. The earliest component, N1, peaks at about 125 ms after tone onset and has the largest amplitude in fronto-central midline and dorsolateral sites. It is much smaller parietally and it seems to invert in polarity in temporal sites. Frequent tones evoke the smallest N1, which is followed by a P2 at about 230 ms. In contrast, rare stimuli seem to evoke an N2. It does not have a well-formed peak but is visible to novel sounds and appears as a “bump” superimposed on a positivity slope ending in the Late Positive Complex (LPC) evoked by rare stimuli. In frontal and central sites a P3 bifurcation into P3a, 280 ms latency, and P3b, 340 ms latency, is apparent with potentials evoked by novel stimuli being larger fronto-centrally. The LPC is followed by a Slow Wave, which is larger to target tones in posterior sites.
Fig. 3.
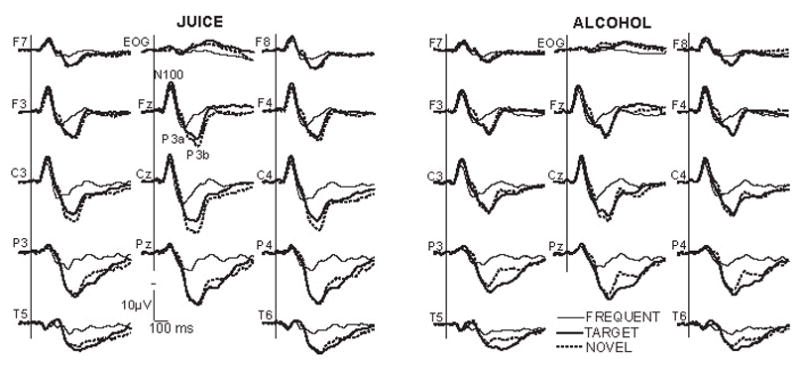
Grand average ERP waveforms elicited by frequent, rare target and rare non-signal novel stimuli. Separate averages are presented for placebo and alcohol conditions. Stimulus onset at the vertical bar. Negative is up.
As seen in Fig. 4, no effects of beverage or instructions on the average amplitude of N1 were observed. There was a main effect of stimulus type, F2/22 = 3.64, P < 0.05, with the target, novel and frequent tones evoking the average amplitudes of −4.1 μV (SD = 3.06), −3.3 μV (SD = 2.7) and −2.6μV (SD = 1.18) respectively (Fig. 3). A significant stimulus type x sites interaction, F24/264 = 4.48, P < 0.005, was due to the largest differences at frontal and central electrode sites.
Fig. 4.
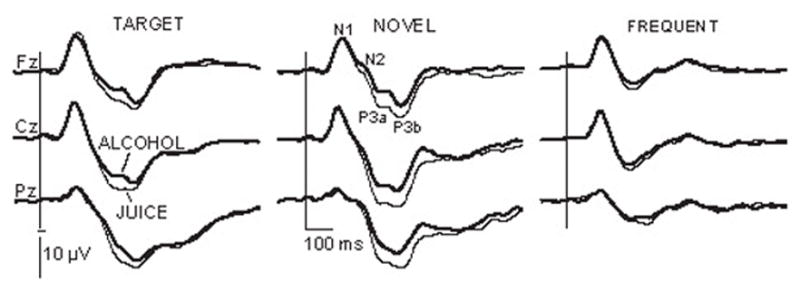
Effects of alcohol intoxication on grand average ERP waveforms to target, novel and frequent stimuli. Negative is up.
The earliest effect of alcohol on the ERPs was observed at the N2 latency range (160–250 ms) resulting in a more negative average ERP amplitude under alcohol (mean = 1.1, SD = 2.0 μV) as compared to juice (mean = 1.8, SD = 2.58 μV), F1/11= 4.9, P < 0.05. This alcohol-induced difference was the greatest over frontal electrodes F1/11= 13.8, P < 0.05, and did not reach post hoc significance criteria at central, parietal and temporal sites. Stimulus type factor interacted with electrode sites, F24/264 = 3.32, P < 0.01, due to the largest N2 deflection evoked by novel sounds in frontal region. Indeed, post hoc analyses resulted in significant differences between frequent and novel, F1/11 = 12.38, P < 0.05, and target and novel tone waveforms, F1/11 = 15.2, P < 0.05.
Similar results obtained in a short time window termed “P3a” (250–300 ms), visible as a bifurcation of the LPC fronto-centrally in Fig. 3 and 4. A main effect of beverage (Fig. 4), F1/11= 6.4, P < 0.05, indicated attenuation of this early positivity in alcohol condition (mean = 5.1μV, SD = 1.95) as compared to placebo (mean = 7.2 μV, SD = 3.19). An interaction between the factors of beverage and electrode sites, F12/132= 5.96, P < 0.0001, suggested that the alcohol effect was the largest in frontal areas. Only rare tones evoked a LPC, resulting in the main effect of stimulus type, F2/22 = 14.1, P < 0.0001.
Within the P3b latency window (300–450 ms), a beverage x sites interaction, F12/132= 3.6, P < 0.05, indicated that this late positive potential was attenuated by alcohol especially in centro-parietal regions. No effects of beverage or instructions were observed for the ERPs in the latency range of the subsequent positivity, here termed Slow Wave (450–600 ms) that was evoked by rare target and novel stimuli over central and posterior scalp.
In addition to the aforementioned average amplitude measures, an automatic algorithm successfully measured single-point peaks of the N1 and LPC deflections for target and novel tones only for all individual subjects’ waveforms. These peak amplitude measures of the two deflections replicated the effects obtained from average amplitude analyses. None of the measures were affected by the expectancy as to the beverage content.
Latency measures
The overall peak latency of the N1 deflection was 1.5 ms slower in alcohol conditions (mean = 127.2 ms, SD = 8.49) as compared to juice (mean = 125.7 ms, SD = 8.65). Even though this lag was very short and might be physiologically irrelevant, it was very consistent as it resulted in the main effect of beverage, F1/11= 4.9, P < 0.05. In addition, the main effect of stimulus type, F2/22 = 6.2, P < 0.02, was due to latency differences between N1 deflections evoked by frequent (mean = 121.7 ms, SD = 6.1), target (mean = 127.2 ms, SD = 10.5) and novel tones (mean = 130.6 ms, SD = 12.2). No reliable effects of beverage on the LPC latency (mean = 336 ms, SD = 24.7) were observed.
Analysis of the ERPs based on trials with (SCR+) and without (SCR−) arousal Data analysis
Concurrent recording of the peripheral autonomic (i.e. electrodermal) activity and central ERPs during this task allowed a comparison of the two physiological systems. In order to investigate the manner in which the alcohol-induced changes in the activity of one system compared to changes in the other, the SCRs were used as a grouping criterion during ERP averaging (Lyytinen et al., 1992). Each subject’s ERP data were averaged into SCR+ and SCR− waveforms for the target and novel stimulus types separately, depending on whether there was a measurable SCR on a particular trial or not. Only the ERPs evoked by target and novel stimuli were analyzed due to very few skin conductance responses to frequently occurring tones. The SCR+ average contained only those ERP trials on which a phasic SCR was evoked. Similarly, SCR− average was formed by including only those ERP trials on which no phasic SCR was recorded. In some subjects the SCRs were more prevalent in the beginning of the task with a tendency to habituate towards the end. In order to avoid including predominantly early trials into SCR+ average and late trials into SCR− average, care was taken to counterbalance the two averaged waveforms with respect to the ordinal position of the trials forming them. Only those SCR− trials whose ordinal position was the closest to the SCR+ trials were included in the SCR− average. Concurrent averages of electrodermal data were obtained for the same trials as the ERPs. On average, the SCR+ and SCR− ERP waveforms were based on 17.1 and 18.7 trials, respectively. Each subject’s waveforms were quantified by obtaining average amplitude measures within time-windows corresponding to N1 (110–150 ms post stimulus), P3a (250–300 ms) and P3b (320–380 ms) deflections. These average amplitude measures were expressed as μV with respect to a baseline period of 100 ms before stimulus onset. All trials on which eyeblinks, artifacts or incorrect responses were observed were excluded from the analyses. Satisfactory averages for both target and novel tones from each of the beverage conditions were obtained from seven subjects permitting a fully crossed analysis with the factors of stimulus type (target, novel), beverage (alcohol, juice), electrodermal responsivity (SCR+ and SCR−) and electrode sites (regional averages of the frontal, central, parietal and temporal sites). The data were collapsed across the beverage instructions factor, as no effects were observed in any of the analyses presented above.
Results
As shown in Fig. 5, the earliest peak evoked by both target and novel stimuli is a negative deflection (N1), followed by a bifurcated positivity: the earlier peak, P3a (280 ms latency) is especially large to novel tones on SCR+ trials in placebo condition. The later peak, P3b (340 ms latency) was evoked by both tones, it was more prominent over posterior sites and it did not have such a clear correlation with engagement of the autonomic system.
Fig. 5.
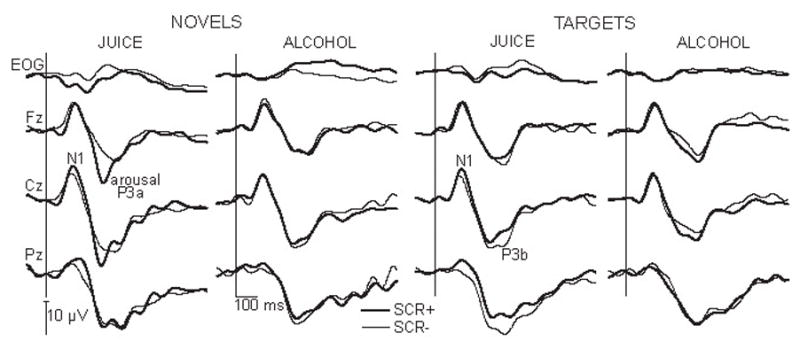
Average ERP waveforms to novel and target stimuli in placebo and alcohol conditions. Waveforms based on trials with (SCR+) and without (SCR−) skin conductance responses indicating arousal are superimposed.
Within the N1 latency window (110–150 ms) the interaction between the stimulus type, beverage and electrode sites, F3/18 = 11.56, P < 0.001 and a marginally significant interaction of the stimulus, SCR and electrode sites factors, F3/18 = 2.7, P < 0.08 suggested that the average potentials on SCR+ trials for both target and novel stimuli tended to be larger than the SCR− in the placebo condition, as it can be observed at Cz in Fig. 5. Although they are suggestive of an early component of the orienting to rare tones, these results do not reach post hoc significance, they need confirmation and will not be discussed further.
The N1 is followed by a P3a deflection (280 ms latency). A bifurcated pattern of the late positive complex evoked by both types of rare tones is suggested by a grand average (Fig. 3), as the LPC with two peaks is observed. However, employing an SCR measure as an averaging criterion clearly exposes the high correlation between the P3a deflection and the phasic SCR appearing a couple of seconds later. A large P3a was evoked by novel sounds only on those trials that also evoked a measurable SCR and only in the placebo condition. The P3a peak has the largest amplitude at fronto-central midline sites, although it is present at all recorded sites. In contrast, no frontal P3a peak is seen on novel trials on which no SCRs were evoked. In addition, a rather mild alcohol intoxication seems to disrupt the central and autonomic coupling, as the arousal-related P3a did not differ from the P3a observed on SCR− novel trials.
Analysis of the frontal P3a amplitude (Fig. 6) showed that alcohol significantly attenuated the P3a deflection, as revealed by a strong effect of beverage, F1/6 = 17.4, P < 0.01. The average amplitude was significantly larger in the placebo (mean = 11.8 μV, SD = 2.8) than alcohol condition (mean = 8.1 μV, SD = 2.0). The factor of beverage interacted significantly with the factors of stimulus type and electrode sites, F 3/18 = 4.6, P < 0.01, as well as factors of SCR and electrode sites, F3/18 = 3.17 P < 0.05). These interactions were primarily due to the larger SCR+ in juice condition to novel sounds over frontal sites. Using the autonomic responses as a criterion in segregating the central ERP responses was a good way to bring out the sensitivity of the P3a to novel, task irrelevant stimuli, suggesting it as a central index of orienting to novelty. In placebo condition, the average frontal P3a evoked by novel tones on SCR+ trials was 9.4 μV larger than the P3a potential evoked on those trials on which the autonomic system was not engaged, F1/6 = 7.2, P < 0.5. The mean on SCR+ trials was 14.9 μV (95% confidence interval = 9.1, 20.7), whereas the mean on SCR− trials was 5.5 μV (95% confidence interval = 1.4, 9.5). This effect was specific to novelty rather than low frequency of occurrence, as the target tones did not evoke any such difference, F 1/6 = 0.2, P > 0.5. Furthermore, alcohol consumption abolished this large arousal-related P3a to novel tones over frontal area, F1/6 = 10.9, P < 0.05. Whereas novel sounds evoked large P3a responses on SCR+ trials when juice was imbibed, there was no such P3a deflection in the alcohol condition. In contrast to novel tones, the target tones did not evoke any P3a that was reliably related to arousal in any of the conditions.
Fig. 6.
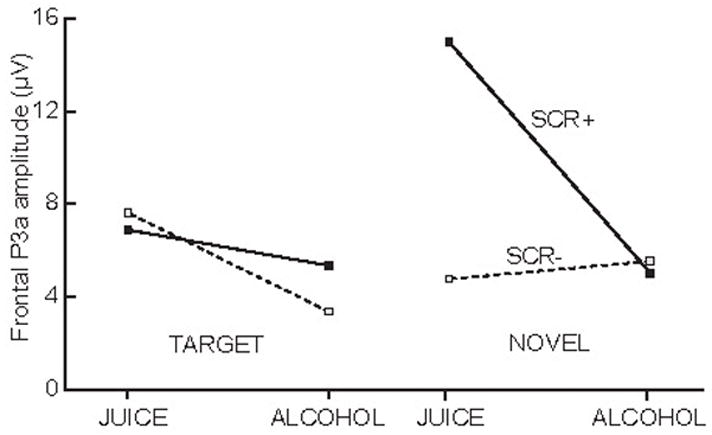
Average amplitude of frontal P3a (250–300 ms latency) based on trials with (SCR+) or without (SCR−) autonomic arousal as a function of stimulus type and beverage. Measured at Fz.
Skin conductance average waveforms obtained on the same trials as the ERPs are presented in Fig. 7 for both novel and target stimuli in placebo and alcohol conditions. Unlike dramatic alcohol-induced differences in ERPs indexed by disappearance of P3a deflection, no such differences can be observed for the skin conductance measure. The average SCRs did not differ significantly as a function of the tone type nor the beverage. On trials on which virtually indistinguishable peripheral SCRs are evoked by novel and target tones, the central measures of orienting reveal very important differences in waveforms and topography.
Fig. 7.
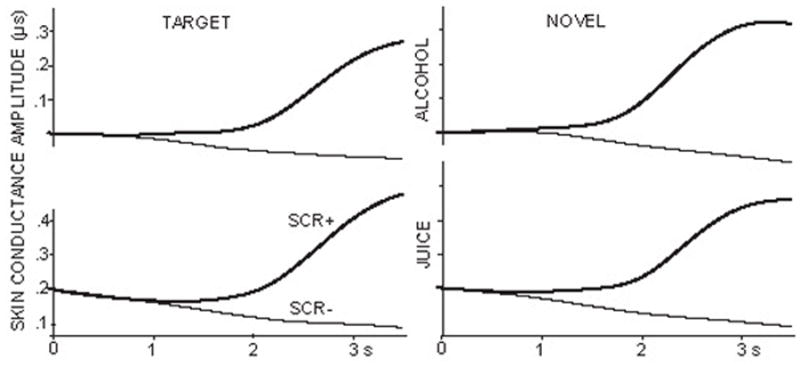
Average skin conductance waveforms to target and novel stimuli in alcohol and placebo conditions.
Both target and novel stimuli evoked a P3b, maximal over parietal sites and peaking at 340 ms. The analysis of P3b average amplitudes measured within 320–380 ms latency window revealed an interaction between the factors of beverage and SCR, F1/6 = 7.6, P < 0.03, and a triple interaction between the stimulus type, SCR and electrode sites, F3/18 = 4.1, P < 0.05. These interactions were due to alcohol-induced attenuation of P3b that was more pronounced for SCR− to target tones, but was about equal for novel tones on SCR+ vs. SCR− trials. The target P3b was the largest over parietal sites. In contrast to arousal-related nature of P3a suggestive of its relationship to autonomic orienting, no such dependence was seen for P3b. Given a small sample size, we examined a possibility that a P3b association with arousal was overlooked due to a Type II error. The estimated power of detecting a difference between the SCR+ and SCR− in placebo condition was 0.06 at alpha < 0.05. In contrast, a significant arousal-related P3a difference of 9.4 μV to novel sounds was detected in spite of the small sample size with power = 0.56, at alpha < 0.05. Nevertheless, confirmatory studies are needed before the association between the P3b and sympathetic arousal can be ruled out as implausible.
DISCUSSION
A dissociation between behavioral and physiological measures was observed in this study. Whereas the moderately low alcohol dose had no effect on reaction times or performance accuracy, it altered central measures of brain activity indexed by ERPs. Inebriation resulted in a significant but very small increase in N1 latency, it increased N2 amplitude and attenuated both P3a and P3b subcomponents of the Late Positive Complex. Attenuating effects of alcohol on P3a were most pronounced, but not limited to the frontal areas, whereas the P3b decrement was the largest over centro-parietal region. The results obtained in the present study confirm previously reported similar general effects of alcohol-induced attenuation of endogenous components in oddball paradigms (Porjesz and Begleiter, 1981, 1985; Jääskeläinen et al., 1996). A more reliable effect on the LPC as compared to the earlier components is a general finding (Salamy and Williams, 1973; Teo and Ferguson, 1986) especially at lower alcohol doses (Jääskeläinen et al., 1999). All of the observed effects were due to the alcohol inebriation as none of the behavioral or physiological measures were affected by expectancy regarding the beverage content.
The most striking effect of the moderately low dose of alcohol used in the current study was the attenuation of the P3a component selectively evoked by novel tones on the trials accompanied by autonomic arousal. Previous studies have suggested that the large LPC results from a superposition of at least two distinct components, P3a and P3b, differing in task correlates, latency and topography in auditory (Ford et al., 1976; Friedman et al., 1993; Halgren et al., 1995a, b; Knight, 1984; Roth, 1973; Squires et al., 1975), visual (Courchesne et al., 1975; Knight, 1997) and somatosensory modalities (Yamaguchi and Knight, 1991). Indeed, a LPC with two peaks to rare stimuli has been observed in previous studies (Polich et al., 1985; Polich, 1988; Johnson and Donchin, 1985). In the present study, the grand average waveforms (Fig 3), reveal bifurcated peaks corresponding in latency to the P3a (at 280 ms) and P3b (at 340 ms). A contribution of this study is its concurrent measurement of the autonomic arousal and central ERPs. This approach permitted a clear distinction between a large, anteriorly distributed arousal-related P3a evoked by novel tones, and the posteriorly distributed P3b which did not show a clear relationship to autonomic arousal. A large P3a with fronto-central distribution was evoked only on those novel trials that also elicited an orienting response and it was abolished by mild alcohol intoxication. Thus, the differential effects of the pharmacological challenge have further underlined the distinction between the two subcomponents of the LPC. These results indicate that alcohol has adverse effects on the integration of autonomic arousal and cognitive processing during involuntary orienting.
The P3a is evoked by stimuli that demand processing because of their potential biological significance, such as novel and rarely occurring stimuli regardless of whether they are overtly task-relevant. It appears to be involuntary as it does not require active attention, it is modality-nonspecific and related to autonomic arousal. All these features suggest a possible role of the P3a as a central index of orienting to incidental and biologically salient stimuli, reflecting activation of the cortico-limbic arousal system (Squires et al., 1975 Courchesne et al., 1975; Knight, 1984; Knight & Scabini, 1998). However, due to a small sample size, these results need to be viewed as preliminary and are in need of a replication.
Available data from studies employing oddball paradigms support the physiological and functional distinction between the involuntary orienting to potentially significant environmental events and the voluntary attention to task-relevant stimuli. It has been shown that prefrontal lesions result in significant P3a reductions to novel stimuli (Knight, 1984; Knight, 1997; Knight and Scabini, 1998). Consistent with this evidence, we have observed alcohol-induced reduction of arousal-related P3a over the fronto-central region. Intracerebral recordings have indeed found generators of activity with the same latency and task correlates as the scalp P3a in dorsolateral prefrontal, orbitofrontal, gyrus rectus and anterior cingulate cortices (Wood and McCarthy, 1985; Alain et al., 1989; Smith et al., 1990; Baudena et al., 1995; Halgren et al., 1995a,b).
A high-level control system with rich and bidirectional anatomical connections with most levels of the neuraxis is necessary to integrate a multitude of changes that are implicated during orienting. They range from phasic arousal increase, sensory input facilitation, selective inhibition of the processing of irrelevant stimuli and suppression of activity, to changes in posture and preparations for complex motor program executions (Lynn, 1966). The frontal lobes in conjunction with posterior regions (Mesulam, 1990) are implicated in integration of specific (event encoding) and non-specific (arousal) aspects of orienting through their close relationship with the nucleus reticularis thalami and, thereby with midbrain reticular activation system (Scheibel, 1980; Skinner and Yingling, 1977). The importance of the frontal lobes in autonomic-central integration is confirmed by the absence of SCR-ORs to anticipatory events in patients with prefrontal lesions (Bechara et al., 1996) and in primates after frontal or amygdala lesions (Kimble, Bagshaw and Pribram, 1965; Bagshaw and Benzies, 1968). Thus, due to the very rich interconnections of the frontal lobes and limbic structures it is probable that the frontal lobes exert many different types of modulatory and integrative influences on autonomic and somatic activity during orienting of attention to potentially significant stimuli. The P3a might reflect a stage at which autonomic processes are engaged in the general framework of response preparation and integrated with central processing stream of stimulus significance.
Lyytinen, Blomberg and Näätänen (1992) recorded skin conductance responses in parallel with ERPs during an auditory sequence of frequent and infrequent tones differing in pitch and rise time. A tendency towards larger P3a response was observed on trials differing in rise-time on which SCRs were also evoked. The tones were incidental to the situation and were ignored as the subjects were focused on solving a demanding intelligence test. Similarly, a larger P3a (earlier part of the LPC), but not P3b was observed to rare target stimuli on trials accompanied with SCRs (Bahramali et al., 1997) or skin sympathetic nerve activity (Ito et al., 1996). None of those studies used unexpected novel sounds. In contrast, active attention to the stimuli was required in the current study. It is plausible that the P3a observed here was a manifestation of a normal, temporary “attention shift” (Näätänen, 1990) to a novel stimulus that was sufficiently deviant and thereby potentially significant. Overall, these results indicate that the physiological system subserving processing of the novel, unexpected stimuli is particularly susceptible to acute alcohol effects, in agreement with other evidence (Grillon et al., 1995; Jääskeläinen et al., 1996).
Non-surprisingly, repeated but task relevant rare target tones may bypass the “orienting-to-novelty” stage and are processed by the event-integration network indexed by P3b. P3b can be distinguished from the P3a by its longer latency, more posterior scalp distribution, and task correlates such as stimulus relevance, probability and dependence on attention. Depth recordings have found that activity with latency and task correlates similar to the scalp-P3b is largest in the hippocampus (Halgren et al., 1980; McCarthy et al., 1989; Halgren et al., 1995a, b), but local generation is also well established in other multimodal association cortices that generally subserve high-level supramodal associations derived from declarative, semantic and primary memories (Halgren, 1990). Alcohol-induced attenuation of P3b observed in the present study indicates that alcohol impairs the function of a widepread system that includes cognitive contextual integration.
The potential significance of these results derives from the evidence about the importance of orienting and vigilance for car driving. It has been suggested that intoxicated experienced drivers can adequately execute overlearned motor programs (Huntley, 1973). However, impairments in divided attention (Koelega, 1995), detection of novel moving stimuli (Nicholson et al., 1995), and traffic hazards (West et al., 1993) can be seen even at low BALs. Thus, it is likely that when some unexpected situations demanding a swift and non-practiced reaction arise, drunk drivers do not orient to them properly, do not make correct decisions and do not select or execute a motor program with sufficient precision so as to avoid an accident.
In summary, moderately low alcohol dose resulted in a slight increase in N1 latency, increased N2 amplitude and reduced LPC amplitude. Concurrent recording of electrodermal responsivity (as an index of the autonomic arousal) ERPs indicating central stimulus processing, confirmed other evidence about the distinction between P3a and P3b as subcomponents of the late positivity. The fronto-central P3a (280 msec latency) was evoked by unique novel tones embedded in an oddball sequence of frequent standard and rare target tones but only on those trials that also evoked autonomic orienting. In contrast, elicitation of a posteriorly maximal P3b (340 msec latency) by target tones was not correlated with arousal. Alcohol intoxication significantly attenuated the P3a evoked by novel tones on trials accompanied by arousal responses and also attenuated P3b. This evidence suggests the P3a as a central index of orienting to novel, potentially significant stimuli. Moderately low doses of alcohol exert adverse effects on multiple brain systems concerned with sustained and phasic attentional processes and cognitive integration.
Acknowledgments
Supported by NS18741(NIH), UCLA and Sigma-Xi Society. We thank Kerin Asher for help in data collection. Dedicated to J & V. This study was conducted while the first author was with the Department of Psychology, University of California, Los Angeles.
Contributor Information
Ksenija Marinkovic, MGH-NMR Center, Harvard Medical School, 149 13th Street, Rm. 2301, Charlestown, MA 02129
Eric Halgren, MGH-NMR Center, Harvard Medical School, Radiology, University of Utah, INSERM E9926, Marseilles, France
Irving Maltzman, Psychology Dept., Franz Hall, Univ. of California, Los Angeles Los Angeles, CA 90095-1563
References
- Alain C, Richer F, Achim A, Saint-Hilaire JM. Human intracerebral potentials associated with target, novel and omitted auditory stimuli. Brain Topography. 1989;1:237–245. doi: 10.1007/BF01129601. [DOI] [PubMed] [Google Scholar]
- Bagshaw MH, Benzies S. Multiple measures of the orienting reaction and their dissociation after amygdalectomy in monkeys. Experimental Neurology. 1968;20:175–187. doi: 10.1016/0014-4886(68)90090-3. [DOI] [PubMed] [Google Scholar]
- Bahramali H, Gordon E, Lim CL, Li W, Lagopoulos J, Leslie J, Rennie C, Meares RA. Evoked related potentials associated with and without an orienting reflex. Neuroreport. 1997;8:2665–2669. doi: 10.1097/00001756-199708180-00006. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Feldmann S, Gordon E, Cocker KI, Rennie C. Elicitation and habituation of the electrodermal orienting response in a short interstimulus interval paradigm. International Journal of Psychophysiology. 1993;15:247–53. doi: 10.1016/0167-8760(93)90008-d. [DOI] [PubMed] [Google Scholar]
- Baudena P, Heit G, Clarke JM, Halgren E. Intracerebral potentials to rare target and distractor auditory and visual stimuli: 3. Frontal cortex. Electroencephalography and Clinical Neurophysiology. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Becker DE, Shapiro D. Directing attention toward stimuli affects the P300 but not the orienting response. Psychophysiology. 1980;17:385–389. doi: 10.1111/j.1469-8986.1980.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Campbell KB, Lowick BM. Ethanol and event-related potentials: the influence of distractor stimuli. Alcohol. 1987;4:257–63. doi: 10.1016/0741-8329(87)90021-8. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Rosler F, Ruchkin D, Siddle D. Cognition and event-related potentials: II. The orienting reflex and P300. In: Karrer R, Cohen J, Tueting P, editors. Brain and Information: Event-Related Potentials. Academy of Sciences; New York: 1984. pp. 39–57. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. Hodder & Stoughton, Sevenoaks; England: 1975. [Google Scholar]
- Ford JM, Roth WT, Kopell BS. Attention effects on auditory evoked potentials to infrequent events. Biological Psychology. 1976;4:65–77. doi: 10.1016/0301-0511(76)90031-4. [DOI] [PubMed] [Google Scholar]
- Friedman D, Hakerem G, Sutton S, Fleiss J. Effects of stimulus uncertainty on the pupillary dilation response and the vertex evoked potential. Electroencephalography and Clinical Neurophysiology. 1973;34:475–484. doi: 10.1016/0013-4694(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Gough HG. Theory and measurement of socialization. Journal of Consulting Psychology. 1960;24:23–30. [Google Scholar]
- Grillon C, Sinha R, O’Malley SS. Effects of ethanol on the processing of low probability stimuli: And ERP study. Psychopharmacology. 1995;119:455–465. doi: 10.1007/BF02245862. [DOI] [PubMed] [Google Scholar]
- Halgren E. Human evoked potentials. In: Boulton AA, Baker GB, Vanderwolf C, editors. Neurophysiological Techniques –Applications to Neural Systems. Humana; Clifton, NJ: 1990. pp. 147–275. [Google Scholar]
- Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: Gazzaniga M, editor. The Cognitive Neurosciences. MIT Press; Cambridge Mass: 1995. pp. 1137–1151. [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liégeois-Chauvel C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: 1. Superior temporal plane and parietal lobe. Electroencephalography and Clinical Neurophysiology. 1995a;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: 2. Medial, lateral and posterior temporal lobe. Electroencephalography and Clinical Neurophysiology. 1995b;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Squires NK, Wilson CL, Rohrbaugh JW, Babb TL. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Hull JG, Bond CF., Jr Social and behavioral consequences of alcohol consumption and expectancy: A meta-analysis. Psychological Bulletin. 1986;99:347–360. [PubMed] [Google Scholar]
- Huntley MS. Alcohol influences upon closed-course driving performance. Journal of Safety Research. 1973;5:149–164. [Google Scholar]
- Huynh H, Feldt LS. Performance of traditional F tests in repeated measures designs under covariance heterogeneity. Communications on Statistical and Theoretical Mathematics. 1980;A9:61–74. [Google Scholar]
- Ito H, Sugiyama Y, Mano T, Okada H, Matsukawa T, Iwase S. Skin sympathetic nerve activity and event-related potentials during auditory oddball paradigms. Journal of Autonomic Nervous System. 1996;60:129–35. doi: 10.1016/0165-1838(96)00043-4. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Näätänen R, Sillanaukee P. Effect of acute ethanol on auditory and visual event-related potentials: A review and reinterpretation. Biological Psychiatry. 1996;40:284–291. doi: 10.1016/0006-3223(95)00385-1. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Schroger E, Näätänen R. Electrophysiological indices of acute effects of ethanol on involuntary attention shifting. Psychopharmacology (Berl) 1999;141:16–21. doi: 10.1007/s002130050801. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Donchin E. Second thoughts: multiple P300s elicited by a single stimulus. Psychophysiology. 1985;22:182–94. doi: 10.1111/j.1469-8986.1985.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Freeman PR, Harrell R. The effects of family history, sobriety length, and drinking history in younger alcoholics on P300 auditory-evoked potentials. Alcohol and Alcoholism. 1997;32:233–239. doi: 10.1093/oxfordjournals.alcalc.a008262. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Verbaten MN, Roelofs JW, Slangen JL. “Initial-” and “change-orienting reactions”: an analysis based on visual single-trial event-related potentials. Biological Psychology. 1989;28:199–226. doi: 10.1016/0301-0511(89)90001-x. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Bagshaw MH, Pribram KH. The GSR of monkeys during orienting and habituation after selective partial ablations of the cingulate and frontal cortex. Neuropsychologia. 1965;3:121–128. [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT. Distributed cortical network for visual stimulus detection. Journal of Cognitive Neuroscience. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. In: Jasper HH, Riggio S, Goldman-Rakic PS, editors. Epilepsy and the Functional Anatomy of the Frontal Lobe. Raven Press; New York: 1995. pp. 21–36. [PubMed] [Google Scholar]
- Koelega HS. Alcohol and vigilance performance: A review. Psychopharmacology (Berl) 1995;118:233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- Landauer AA, Howat P. Low and moderate alcohol doses, psychomotor performance and perceived drowsiness. Ergonomics. 1983;26:647–57. doi: 10.1080/00140138308963386. [DOI] [PubMed] [Google Scholar]
- Loveless NE. Event-related slow potentials of the brain as the expression of orienting function. In: Kimmel HD, van Olst EH, Orlebeke JF, editors. The Orienting Reflex in Humans. Erlbaum; Hillsdale: 1979. pp. 77–100. [Google Scholar]
- Lynn R. Attention, Arousal and the Orientation Reaction. Pergamon Press; Oxford: 1966. [Google Scholar]
- Lyytinen H, Blomberg AP, Näätänen R. Event-related potentials and autonomic responses to a change in unattended auditory stimuli. Psychophysiology. 1992;29:523–34. doi: 10.1111/j.1469-8986.1992.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Maltzman I. The OR and significance. Pavlovian Journal of Biological Science. 1990;25:111–121. doi: 10.1007/BF02974265. [DOI] [PubMed] [Google Scholar]
- Mills KC, Neal EM, Peed-Neal I. Handbook for Alcohol Education: The Community Approach. Ballinger Publishing Company; Cambridge, MA: 1983. [Google Scholar]
- Marinkovic K, Halgren E, Klopp J, Maltzman I. Alcohol effects on movement-related potentials: A measure of impulsivity? Journal of Studies on Alcohol. 2000;61:24–31. doi: 10.15288/jsa.2000.61.24. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Intracranial recordings of endogenous ERPs in humans. Electroencephalography and Clinical Neurophysiology Supplement. 1987;39:331–337. [PubMed] [Google Scholar]
- McCarthy G, Wood CC, Williamson PD, Spencer DD. Task-dependent field potentials in human hippocampal formation. Journal of Neuroscience. 1989;9:4253–4268. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Monteiro MG, Schuckit MA. Populations at high alcoholism risk: recent findings. Journal of Clinical Psychiatry. 1988;49:3–7. [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Näätänen R, Gaillard AWK. The orienting reflex and the N2 deflection of the event-related potential (ERP) In: Gaillard AWK, Ritter W, editors. Tutorials in Event-Related Potential Research: Endogenous Components. North-Holland; Amsterdam: 1983. pp. 119–141. [Google Scholar]
- Nicholson ME, Andre JT, Tyrrell RA, Wang M, Leibowitz HW. Effects of moderate dose alcohol on visual contrast sensitivity for stationary and moving targets. Journal of Studies on Alcohol. 1995;56:261–266. doi: 10.15288/jsa.1995.56.261. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychology. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Horvath TB, Roth WT, Clifford ST, Kopell BS. Acute and chronic effects of ethanol on event-related potentials. In: Begleiter H, editor. Biological Effects of Alcohol. Plenum Press; New York: 1980. pp. 625–639. [DOI] [PubMed] [Google Scholar]
- Polich J. Bifurcated P300 peaks: P3a and P3b revisited? Journal of Clinical Neurophysiology. 1988;5:287–294. [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. Effects of age on the P300 component of the event-related potential from auditory stimuli: peak definition, variation, and measurement. Journal of Gerontology. 1985;40:721–726. doi: 10.1093/geronj/40.6.721. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human evoked brain potentials and alcohol. Alcoholism: Clinical and Experimental Research. 1981;5:304–317. doi: 10.1111/j.1530-0277.1981.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Brain dysfunction and alcohol. In: Kissin B, Begleiter H, editors. The Pathogenesis of Alcoholism. Plenum Press; New York: 1983. pp. 415–483. [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and the Brain. Plenum Press; New York: 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford; New York: 1987. pp. 45–63. [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 207–247. [Google Scholar]
- Rohrbaugh JW. The orienting reflex: Performance and central nervous system manifestations. In: Parasuraman R, Davies DR, editors. Varieties of Attention. Academic Press; New York: 1984. pp. 323–373. [Google Scholar]
- Rohsenow DJ, Marlatt GA. The balanced placebo design: Methodological considerations. Addictive Behaviors. 1981;6:107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Roth WT. Auditory evoked responses to unpredictable stimuli. Psychophysiology. 1973;10:125–138. doi: 10.1111/j.1469-8986.1973.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Roth WT. A comparison of P300 and skin conductance response. In: Gaillard AWK, Ritter W, editors. Tutorials in Event-Related Potential Research: Endogenous Components. North-Holland; Amsterdam: 1983. pp. 177–199. [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Salamy A, Williams HL. The effects of alcohol on sensory evoked and spontaneous cerebral potentials in man. Electroencephalography and Clinical Neurophysiology. 1973;35:3–11. doi: 10.1016/0013-4694(73)90126-0. [DOI] [PubMed] [Google Scholar]
- Salamy JG, Wright JR, Faillace LA. Changes in average evoked responses using abstention in chronic alcoholics. Journal of Nervous and Mental Disease. 1980;168:19–25. doi: 10.1097/00005053-198001000-00005. [DOI] [PubMed] [Google Scholar]
- Scheibel AB. Anatomical and physiological substrate of arousal: A view from the bridge. In: Hobson JA, Brazier MAB, editors. The Reticular Formation Revisited. Raven Press; New York: 1980. pp. 55–66. [Google Scholar]
- Simons RF, Rockstroh B, Elber T, Fiorito E, Lutzenberg W, Birbaumer N. Evocation and habituation of autonomic and event-related potential responses in a nonsignal environment. Journal of Psychophysiology. 1987;1:45–59. [Google Scholar]
- Skinner JE, Yingling CD. Central gating mechanisms that regulate event-related potentials and behavior: A neural model for attention. In: Desmedt JE, editor. Attention: Voluntary Contraction and Event-Related Potentials. Progress in Clinical Neurophysiology. Karger; Basel: 1977. pp. 30–69. [Google Scholar]
- Smith ME, Halgren E, Sokolik M, Baudena P, Mussolino A, Liégeois-Chauvel C, Chauvel P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalography and Clinical Neurophysiology. 1990;76:235–248. doi: 10.1016/0013-4694(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Pergamon Press; New York: 1963. [Google Scholar]
- Sokolov EN. The neuronal mechanisms of the orienting reflex. In: Sokolov EN, Vinogradova OS, editors. Neuronal Mechanisms of the Orienting Reflex. Lawrence Erlbaum; Hillsdale: 1975. pp. 217–235. [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Tarter RE, McBride H, Buonpane N, Schneider DU. Differentiation of alcoholics: Childhood history of minimal brain dysfunction, family history, and drinking pattern. Archives of General Psychiatry. 1977;34:761–768. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- Teo RK, Ferguson DA. The acute effects of ethanol on auditory event-related potentials. Psychopharmacology (Berlin) 1986;90:179–184. doi: 10.1007/BF00181237. [DOI] [PubMed] [Google Scholar]
- West R, Wilding J, French D, Kemp R, Irving A. Effect of low and moderate doses of alcohol on driving hazard perception latency and driving speed. Addiction. 1993;88:527–532. doi: 10.1111/j.1360-0443.1993.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Woestenburg JC, Verbaten MN, Slangen JL. Stimulus information and habituation of the visual event-related potential and the skin conductance reaction under task-relevance conditions. Biological Psychology. 1983;16:225–240. doi: 10.1016/0301-0511(83)90026-1. [DOI] [PubMed] [Google Scholar]
- Wood CC, McCarthy G. A possible frontal lobe contribution to scalp P300. Society for Neuroscience Abstracts. 1985;11:879. [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to Linear Models and Experimental Design. Harcourt Brace Jovanovich; San Diego: 1990. [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalography and Clinical Neurophysiology. 1991;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]
- Zeiner AR, Kegg PS. Menstrual cycle and oral contraceptive effects on alcohol pharmacokinetics in Caucasian females. In: Galanter M, editor. Currents in Alcoholism. Grune and Stratton; New York: 1981. pp. 47–56. [PubMed] [Google Scholar]


