Abstract
The present study examined developmental changes in the ability to recognize face parts. In Experiment 1, participants were familiarized with whole faces and given a recognition test with old and new eyes, noses, mouths, inner faces, outer faces, or whole faces. Adults were above chance in their recognition of the eye and mouth regions. However, children did not naturally encode and recognize face parts independently of the entire face. In addition, all age groups showed comparable inner and outer face recognition, except for 8- to 9-year-olds who showed a recognition advantage for outer faces. In Experiment 2, when participants were familiarized with eyes, noses, or mouths and tested with eyes, noses, or mouths, respectively, all ages showed above-chance recognition of eyes and mouths. Thirteen- to 14-year-olds were adult-like in their recognition of the eye region, but mouth recognition continued to develop beyond 14 years of age. Nose recognition was above chance among 13- to 14-year-olds, but recognition scores remained low even in adulthood. The present findings reveal unique developmental trajectories in the use of isolated facial regions in face recognition and suggest that featural cues (as a class) have a different ontogenetic course relative to holistic and configural cues.
Keywords: featural face processing, face recognition
Recognizing other members of one’s biological or social group is of paramount importance for many animals and humans. Given the importance of faces for humans, existing evidence suggests that adults are extremely sensitive to minor changes in facial structure (Ge, Luo, Nishimura, & Lee, 2003), and even newborns are somewhat biased to prefer faces or face-like stimuli (Fantz, 1963; Goren, Sarty, & Wu, 1975; Johnson & Morton, 1991; Macchi Cassia, Simion, & Umiltà, 2001; Maurer & Young, 1983; Mondloch et al., 1999; Simion, Valenza, Umiltà, & Barba, 1998). Impairments in face processing are associated with severe neurocognitive disorders such as autism (see Behrmann, Thomas, & Humphreys, 2006, and Itier & Batty, 2009, for reviews). Given its importance, it is no wonder that a great deal of research has been devoted to understanding the developmental trajectory of face recognition in children.
The existing research has shown that three types of information are important for face recognition (for a review, see Lee, Anzures, Quinn, Pascalis, & Slater, 2011). One type of information is the isolated featural information of a face such as its eyes, nose, and mouth. Another is configural information – the spatial relation information among the individual isolated facial features (Freire & Lee, 2003; Maurer, Le Grand, & Mondloch, 2002), and the third is holistic information –the facial gestalt that fuses featural and configural information into an unbroken whole (Tanaka & Farah, 1993). The use of these three types of facial information in face recognition has been shown to be characterized by unique developmental trajectories. However, much of the research that has investigated the development of face recognition has been devoted primarily to highlighting changes in the use of holistic and configural face processing. In contrast, how featural face recognition develops remains largely unknown. Research regarding the importance of the eye and mouth regions during emotion processing (Adams & Kleck, 2003, 2005; Smith, Cottrell, Gosselin, & Schyns, 2005; Tonks, Williams, Frampton, Yates, & Slater, 2007) and the importance of the mouth region in speech processing (Buchan, Paré, & Munhall, 2007, 2008; Desjardins & Werker, 2004; Dodd, 1979; Hardison, 2003; Hazan, Sennema, Iba, & Faulkner, 2005; Helfer, 1997; Kuhl & Meltzoff, 1982; Lansing & McConkie, 2003; Nakano et al., 2010; Patterson & Werker, 1999, 2003) suggest differences in the degree to which individual facial features are typically processed. These differences, in turn, allude to potentially unique developmental trajectories in processing individual facial features such that proficiency in processing the eye and mouth regions might emerge at an earlier age relative to proficiency in processing the nose region. Thus, the main objective of the present study is to examine developmental changes in featural recognition from childhood to adulthood.
Holistic face processing
Holistic face processing is often measured with the use of a composite face task whereby participants are asked to judge whether parts of two faces (e.g. the top parts) are identical or different while ignoring the other parts of the faces (e.g. the bottom parts). In the composite face task, the parts of the face that participants are told to ignore are always different (e.g. the bottom parts), and the top and bottom parts of the face pairs are either aligned (as a normal face would appear) or misaligned. Adults find it more difficult to correctly judge that the face parts are identical in the aligned condition because it is difficult for them to process one part of the face in isolation from the rest of the face (Carey & Diamond, 1994; Young, Hellawell, & Hay, 1987). This so-called composite face effect has also been found in children. Children at 10 years of age and younger show evidence of holistic face processing by making more errors and producing slower responses in their identity judgments for the top portion of aligned faces relative to their identity judgments for the top portion of misaligned faces (Carey & Diamond, 1994; de Heering, Houthuys, & Rossion, 2007; Macchi Cassia, Picozzi, Kuefner, Bricolo, & Turati, 2009; Mondloch, Pathman, Maurer, Le Grand, & de Schonen, 2007). Further evidence suggests that this composite face effect has already reached adult levels by 4 years of age (de Heering et al., 2007).
Holistic face processing has also been investigated using the part–whole recognition task. During this task, participants learn whole faces and are subsequently asked to identify familiar facial features when presented in the context of an entire face or when presented in isolation. Holistic face processing is inferred from better recognition of facial features in the whole face recognition task than in the isolated features recognition task, presumably because features are encoded relative to the entire face rather than in isolation. Studies have found that children indeed encode faces holistically in that they show greater accuracy in their recognition of individual facial features when presented in the context of an entire face than when presented in isolation (Pellicano & Rhodes, 2003; Pellicano, Rhodes, & Peters, 2006; Seitz, 2002; Tanaka, Kay, Grinnell, Stansfield, & Szechter, 1998). Consistent with findings regarding the composite face effect in children, this whole–part advantage in recognition is adult-like by 4 years of age (Pellicano & Rhodes, 2003; Pellicano et al., 2006).
Configural face processing
Contrary to the use of holistic face processing, children at 4 years of age demonstrate only chance performance in a recognition task involving configural changes for familiarized faces (Mondloch, Leis, & Maurer, 2006) and familiar peers’ faces (Mondloch & Thomson, 2008). Although face recognition based on configural changes improves during childhood and is above chance by 6 years of age (Baudouin, Gallay, Durand, & Robichon, 2010; Freire & Lee, 2001; Mondloch, Le Grand, & Maurer, 2002), the ability to use configural information to recognize faces does not reach the adult level even by 14 years of age (Mondloch, Geldart, Maurer, & Le Grand, 2003; Mondloch et al., 2002; Mondloch, Le Grand, & Maurer, 2003).
Processing of internal facial regions
In contrast to the abundance of studies that have examined the development of configural and holistic face processing, the developmental trajectory of recognizing relatively isolated internal facial regions has received less attention. Although it has been argued that featural face processing may be easier and matures earlier than configural face processing (Freire & Lee, 2001; Mondloch et al., 2002; but see Quinn & Tanaka, 2009), it remains largely unknown how featural face recognition itself develops. Ten-year-olds have been reported to perform like adults or slightly worse than adults in a short-term recognition task in which faces were presented sequentially and participants were asked to decide whether the two faces were the same or different (Mondloch et al., 2002; Mondloch, Robbins, & Maurer, 2010). When the faces were different, they were shown with different eyes and mouths. However, simultaneous changes to two isolated features do not allow an examination of the potential differential use of individual internal facial regions in recognition. Indeed, Hay and Cox (2000) found that 6-year-olds showed better recognition of the eyes relative to the mouth and nose regions, as well as better recognition of the mouth relative to the nose region. Nine-year-olds also showed better recognition of the eyes relative to the mouth and nose regions, but comparable recognition of the mouth and nose regions (Hay & Cox, 2000).
Consistent with findings regarding differential processing of the internal facial features in 6- and 9-year-olds (Hay & Cox, 2000), existing research suggests that social interactions may involve differential processing of the eye, nose, and mouth regions. Processing of the eye region has been implicated in studies that have examined emotion processing (Adams & Kleck, 2003, 2005; Smith et al., 2005). In addition, processing of the mouth region has been implicated in emotion (Smith et al., 2005) as well as speech processing (Buchan et al., 2007, 2008; Desjardins & Werker, 2004; Dodd, 1979; Kuhl & Meltzoff, 1982; Lansing & McConkie, 2003; Nakano et al., 2010; Patterson & Werker, 1999, 2003). Thus, the use of facial cues in social interactions favours processing of the eye and mouth regions over processing of nose regions. Such differences may cultivate unique developmental trajectories in the ability to encode and recognize individual internal facial regions.
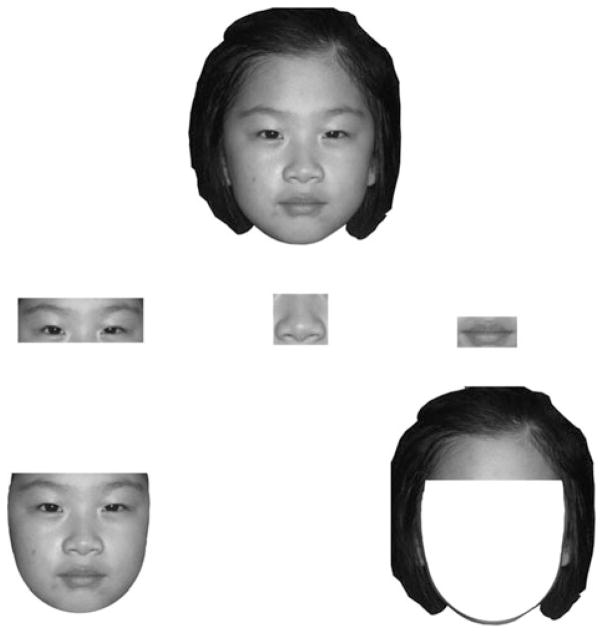
To examine the much overlooked developmental trajectory of recognizing relatively isolated internal facial regions, we familiarized 8- to 9-year-olds, 13- to 14-year-olds, and young adults with a set of photographs containing the whole faces of different individuals. After familiarization with the whole faces, participants were randomly assigned to one of six recognition test conditions: (i) recognizing only the eyes of the original faces; (ii) recognizing only the noses of the original faces; (iii) recognizing only the mouths of the original faces; (iv) recognizing only the inner parts of the original faces without the hair and external contour; (v) recognizing only the outer parts of the original faces without the eyes, nose, mouth, or cheeks; or (vi) recognizing the original whole faces (see Figure 1 for examples). Participants were not told in advance which stimulus format they were going to receive during the recognition test, nor were they informed that they may be required to recognize only parts of the original faces. It should be noted that recognition of the eyes only condition was not limited to featural face recognition because our set of eye stimuli included natural variability in the spacing between the eyes and the spacing between the eyes and eyebrows (see Figure 1). Thus, recognition in the eyes only condition involved recognition of the eye region as isolated from the rest of the face.
Figure 1.
Example stimulus of an 8-year-old used in the whole face familiarization task/recognition test condition, and the eye, nose, mouth, inner face, and outer face recognition test conditions.
A whole face recognition condition was included to ensure that 6 s of familiarization with each face stimulus was sufficient for participants in each age group to learn the face. An inner face recognition condition was included to ensure that participants were attending to the internal regions of the faces. An outer face recognition condition was included because previous studies have alluded to newborns’ and young children’s greater reliance on the outer facial regions over the inner facial regions in their recognition of unfamiliar faces (Turati, Macchi Cassia, Simion, & Leo, 2006; Want, Pascalis, Coleman, & Blades, 2003). Thus, the present study also examined the development of inner and outer face recognition for unfamiliar faces. Our experimental design in which participants were not informed that they would be given a recognition test with only portions of the original faces allowed us to examine how well participants naturally encode and recognize face parts. It was hypothesized that eye and mouth recognition would be above chance and adult-like at an earlier age relative to nose recognition.
EXPERIMENT 1
Method
Participants
Four hundred fifty-seven participants took part in the experiment. They included 164 8- to 9-year-olds (M = 8.41, SD = 0.49, 79 males), 123 13- to 14-year-olds (M = 13.77, SD = 0.43, 47 males), and 170 adults between 18 and 26 years of age (M = 20.95, SD = 1.62, 69 males). All participants were Han Chinese recruited from a metropolitan city in China.
Stimuli
For each participant age group, the stimuli consisted of 20 colour whole face photographs (10 males) of Chinese faces in a frontal pose and matched to the age of the participants (i.e. a total of 60 whole faces). The face stimuli were matched in age for each participant age group to control for any potential own-age face recognition biases that may form from differential experience with individuals from different age groups (Anastasi & Rhodes, 2005; Bäckman, 1991; Kuefner, Macchi Cassia, Picozzi, & Bricolo, 2008). The 60 faces (20 from each age group) were rated by a group of adults to ensure that the faces in each age group were comparable in distinctiveness (p values >.05). Five additional versions of each of the whole face photographs were created: (i) eyes; (ii) nose; (iii) mouth; (iv) inner face (a U shape adjacent to the cheek and jaw regions was used to create the contour for the inner face stimuli); and (v) outer face (see Figure 1).
Procedure
Participants were asked to complete a computerized face recognition task. During the learning phase, participants were familiarized with 10 photographs of whole faces (five males) from their respective age groups (e.g. adults viewed adult faces, adolescents viewed adolescent faces, etc.). Faces were presented one at a time, and each face was presented for 6 s. The learning phase was immediately followed by a recognition test. Participants were randomly assigned to one of six recognition test conditions: (i) eyes (N = 77); (ii) nose (N = 74); (iii) mouth (N = 80); (iv) inner face (N = 71); (v) outer face (N = 70); or (vi) whole face (N = 85). However, participants were not informed prior to the recognition test which test condition they would receive, nor were they informed that they may be presented with only a portion of the faces at test. Those in the whole face recognition condition were shown the same images of the familiarized faces at test. During the recognition test, participants were shown 20 images in sequential presentation – 10 images from the learning phase and 10 new images. Participants were asked to indicate whether each image was old or new via different key presses. Each test image remained on the screen for a maximum of 10 s or until participants made their response. The images were presented in a randomized order.
Results and Discussion
Recognition of internal facial regions
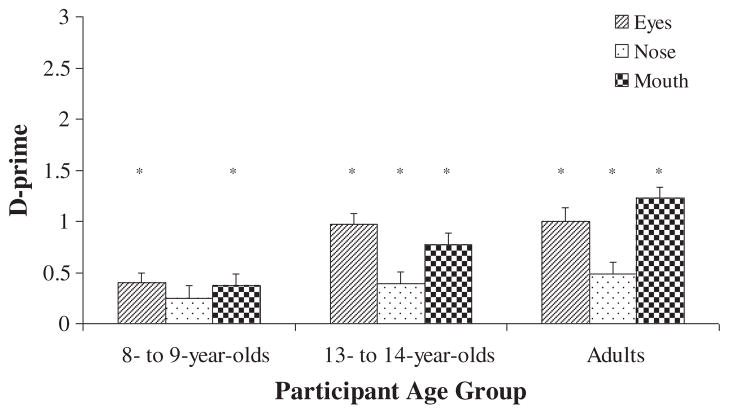
D-prime scores were computed from participants’ recognition of each internal facial region. The d-prime scores were used as the dependent variable in one-sample t-tests to determine whether participants could discriminate between old and new internal facial regions at above-chance levels. Results revealed that 8- to 9-year-olds could not discriminate between old and new internal facial regions (p values >.05). Thirteen- to 14-year-olds showed chance-level discrimination between old and new eyes (p >.05), and a trend towards above-chance discrimination between old and new noses and mouths (p values = .06). Adults showed above-chance discrimination between old and new eyes and mouths (p values <.05), and chance-level discrimination between old and new noses (see Figure 2).
Figure 2.
Participants’ d-prime scores for whole face, inner face, outer face, eye, nose, and mouth recognition after familiarization with whole faces. * indicates above-chance recognition.
Additional analyses of participants’ criterion scores showed a trend towards a more conservative response bias among adults relative to 8- to 9-year-olds (p = .02 compared to the adjusted alpha value of .017; see Table 1). There were no other age-related differences in response bias, and there were no differences in response biases for recognition of the eye, nose, and mouth regions (p values >.05).
Table 1.
Mean criterion scores for each participant age group and recognition condition (SD in brackets)
| Participant age group | Eyes | Nose | Mouth | Inner faces | Outer faces | Whole faces | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 8- to 9-year-olds | 0.047 (0.338) | −0.151 (0.266) | −0.136 (0.366) | −0.029 (0.417) | 0.005 (0.351) | 0.290 (0.412) |
| 13- to 14-year-olds | −0.069 (0.368) | 0.087 (0.352) | −0.075 (0.435) | 0.041 (0.287) | −0.084 (0.195) | 0.081 (0.330) | |
| Adults | 0.113 (0.265) | −0.009 (0.171) | 0.083 (0.279) | 0.185 (0.331) | 0.182 (0.277) | 0.135 (0.342) | |
| Experiment 2 | 8- to 9-year-olds | −0.002 (0.336) | −0.109 (0.475) | −0.218 (0.537) | |||
| 13- to 14-year-olds | 0.094 (0.321) | −0.170 (0.320) | −0.093 (0.388) | ||||
| Adults | 0.072 (0.332) | 0.004 (0.234) | 0.180 (0.371) |
Overall, the recognition of relatively isolated internal facial features revealed differences in the developmental trajectories of participants’ recognition of the eyes, nose, and mouth regions of unfamiliar faces. Recognition of the eye region only reached above-chance levels in adulthood. Recognition of the nose region approached above-chance levels at 13 to 14 years of age but deteriorated to chance levels during adulthood. Recognition of the mouth region began to reach above-chance levels by 13 to 14 years of age and was maintained into adulthood.
Inner and outer face recognition
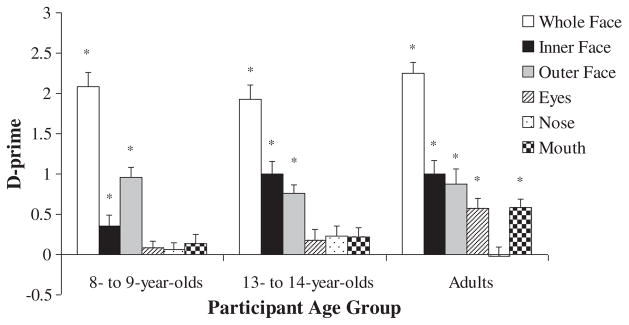
D-prime scores were computed for each participant and were used as the dependent variables in one-sample t-tests to determine whether participants could discriminate between old and new inner and outer faces at above-chance levels. Participants from each age group showed above-chance discrimination between old and new inner faces (p values <.05). Thus, participants from all age groups were attending to the internal facial regions as evident by their proficiency in recognizing inner faces. Participants from each age group also showed above-chance discrimination between old and new outer faces (p <.01).
To assess potential differences in participants’ recognition of inner and outer faces, an ANOVA was conducted with participants’ d-prime scores for inner and outer faces as the dependent variable. A preliminary analysis revealed no significant main effect of, or interactions with, participant gender. The follow-up 3 (participant age group: 8- to 9-year-olds, 13- to 14-year-olds, young adults) × 2 (stimulus test condition: inner faces, outer faces) ANOVA revealed a significant interaction between participant age group and stimulus test condition, F(2, 129) = 5.78, partial η2= .08, p <.05. Post-hoc analyses showed that 8- to 9-year-olds were better in their recognition of outer faces relative to their recognition of inner faces, t(53) = 3.33, p <.05 (see Figure 2). In contrast, 13- to 14-year-olds and young adults showed comparable recognition of inner and outer faces (p values >.05). Additional analyses of participants’ criterion scores revealed that 8- to 9-year-olds, 13- to 14-year-olds, and adults showed comparable response biases in their inner and outer face recognition (p values >.05; see Table 1).
Better outer face than inner face recognition among 8- to 9-year-olds in the present study is consistent with previous findings reported by Want et al. (2003) regarding an external face advantage in 5- to 7-year-olds’ recognition of unfamiliar faces (see Turati et al., 2006, for similar findings in newborns). This finding suggests that poor featural recognition (i.e. eyes, nose, and mouth) among the youngest age group may be at least partially due to their greater reliance on the external facial features relative to the internal facial features of unfamiliar faces. Comparable inner and outer face recognition among adults in the present study also replicates previous findings by Ellis, Shepherd, and Davies (1979) and Want et al. (2003). In addition, the present study shows that the adult-like pattern of comparable inner and outer face recognition for unfamiliar faces is evident by 13 to 14 years of age.
Whole face recognition
One-sample t-tests were conducted with participants’ whole face recognition d-prime scores as the dependent variable. Results revealed that participants from each age group were able to discriminate between old and new whole faces at above-chance levels (p values <.001; see Figure 2), thereby confirming that 6 s of familiarization with each face was sufficient for participants in each age group to learn the faces. An analysis of participants’ criterion scores also showed no differences in whole face recognition biases across the participant age groups (p >.05; see Table 1). Thus, recognition of face parts after familiarization with whole faces in the other conditions would reflect participants’ natural encoding and recognition of parts of the whole faces. Eight- to 9-year-olds and 13- to 14-year-olds were comparable with adults in their whole face recognition performance (p values >.05), suggesting that any age-related differences in the other recognition test conditions would most likely reflect differences in encoding and recognizing face parts with minimal influence of age-related differences in memory abilities.
Overall, Experiment 1 showed that despite a proficiency in inner face recognition among participants from all age groups, there were nonetheless age-related differences in the recognition of relatively isolated internal facial regions. Such differences may be at least partially due to 8- to 9-year-olds’ greater reliance on the outer rather than the inner portions of unfamiliar faces. Contrary to predictions, the eye and mouth regions did not receive better processing than the nose region until adulthood.
It is important to note that the individual developmental trajectories in recognizing relatively isolated internal facial regions in Experiment 1 are a reflection of the way in which individuals naturally encode whole faces and their subsequent recognition memory of parts of those faces. Thus, it appears that prior to adulthood, children do not naturally encode and recognize relatively isolated internal facial regions when they encounter whole faces. Instead, children may be relying more on processing faces holistically – an ability comparable with adults by 4 years of age (de Heering et al., 2007; Pellicano & Rhodes, 2003; Pellicano et al., 2006). Thus, Experiment 2 was conducted to examine the development of recognition of relatively isolated internal facial regions when participants were asked to encode face parts and subsequently recognize those face parts. That is, in Experiment 2, the familiarization and test images were matched (e.g. familiarization with eyes and recognition test with eyes). It was hypothesized that eye and mouth recognition would be above chance and adult-like at an earlier age relative to nose recognition.
EXPERIMENT 2
In Experiment 2, participants were familiarized with eyes, noses, or mouths, and then given a recognition test with eyes, noses, or mouths, respectively.
Method
Participants
Two hundred and thirty-one participants took part in the experiment. They included 74 8- to 9-year-olds (M = 8.49, SD = 0.50, 44 males), 69 13- to 14-year-olds (M = 13.59, SD = 0.50, 24 males), and 88 adults between 20 and 24 years of age (M = 22.15, SD = 0.93, 43 males). All participants were Han Chinese recruited from a metropolitan city in China, and none had participated in Experiment 1.
Stimuli
The stimuli were the same photographs of eyes, noses, and mouths used in Experiment 1.
Procedure
The procedures were the same as those used in Experiment 1, except that participants were familiarized with eyes, noses, or mouths, and then given a recognition test with eyes, noses, or mouths, respectively. Participants were randomly assigned to one of the three conditions.
Results and Discussion
D-prime scores were computed for each participant and used as the dependent variable in one-sample t-tests to determine whether participants could discriminate between old and new isolated facial features at above-chance levels. Results revealed that all age groups were able to discriminate between old and new eyes and mouths at above-chance levels (p values <.05). Thirteen- to 14-year-olds and adults were also able to discriminate between old and new noses at above-chance levels (p values <.05), and 8- to 9-year-olds showed a trend towards above-chance nose recognition (p = .07); see Figure 3.
Figure 3.
Participants’ d-prime scores for eye, nose, and mouth recognition after familiarization with eyes, noses, and mouths, respectively. * indicates above-chance recognition.
Developmental changes in the recognition of isolated facial regions were examined via separate ANOVAs for eye, nose, and mouth regions, with d-prime scores as the dependent variables. The ANOVAs revealed a significant main effect of participant age group in the recognition of the eye and mouth regions, F(2, 75) = 8.67, p <.001, η2 = .19, and F(2, 76) = 15.61, p <.001, η2 = .29, respectively. However, the ANOVA for nose recognition revealed no developmental changes (p >.05). Follow-up post-hoc analyses with sequential Bonferroni corrections showed that adults and 13- to 14-year-olds were significantly better than 8- to 9-year-olds in their recognition of the eye region (p values <.01). In addition, adults were significantly better than 8- to 9-year-olds and 13- to 14-year-olds in their recognition of mouths (p values <.05), and 13- to 14-year-olds were, in turn, better than 8- to 9-year-olds in their recognition of the mouth region (p <.05). There was also a significant main effect for participant gender in the recognition of the eye region in that female participants (M = 0.97, SD = 0.59) were better than male participants (M = 0.59, SD = 0.67), F(1, 72) = 3.86, p = .05, η2 = .05. No other main effects of participant gender nor interactions with participant gender were significant (p values >.05).
An analysis of participants’ criterion scores showed that adults were comparable with 13- to 14-year-olds in their response bias in mouth recognition, but adults were more conservative in their mouth recognition judgments compared with 8- to 9-year-olds (p <.01). Criterion scores for the eye and nose regions were comparable across all age groups (p values >.05; see Table 1).
Overall, Experiment 2 revealed developmental changes in recognition when participants were familiarized with a given set of isolated facial regions followed by a recognition test with old and new facial regions. As predicted, above-chance recognition of eyes and mouths was evident at an earlier age – by 8 to 9 years of age – compared with above-chance recognition of noses, which was evident at 13 to 14 years of age. All age groups were comparable in their recognition of noses –recognition scores that remained consistently low with age rather than improving slowly with age. In contrast, recognition of the eye and mouth regions continued to improve with age, with recognition of the eye region adult-like by 13 to 14 years of age, and recognition of the mouth region not quite adult-like at 13 to 14 years of age.
In addition, female participants were better in their recognition of the eye region than male participants. This gender difference may be due to gender differences in mutual gaze, with females tending to engage more in mutual gaze than males – a gender difference evident as young as 4 to 6 years of age that persists into adulthood (Exline, 1963; Kleinke, 1986; Levine & Sutton-Smith, 1973).
GENERAL DISCUSSION
The present study showed that children as young as 8 to 9 years of age are capable of recognizing isolated facial regions if they are asked to encode such isolated facial regions during familiarization. However, even 13- to 14-year-olds do not appear to naturally encode and recognize isolated facial regions when they initially encounter whole faces. Perhaps there is a greater reliance on holistic face processing prior to adulthood given its early maturity relative to featural and configural face processing.
In relation to other types of facial cues that are used to recognize faces, our results show that the processing of relatively isolated facial regions differs in its developmental trajectory from that of holistic face processing. Whereas holistic face processing is adult-like by 4 years of age (de Heering et al., 2007; Pellicano & Rhodes, 2003; Pellicano et al., 2006), results from Experiment 1 suggest that after the natural encoding of faces (i.e. after familiarization with whole faces), the recognition of the eye region is only at chance even at 13 to 14 years of age, and the recognition of the mouth region is only beginning to approach above-chance levels at 13 to 14 years of age. When the familiarization and test images are matched, adult-like recognition of the eye region is evident at 13 to 14 years of age, and recognition of the mouth region continues to improve beyond 13 to 14 years of age.
Whereas holistic face processing is mature at an earlier age, the ability to encode and recognize the eye region, in turn, appears to mature earlier than configural face recognition. Studies examining the use of configural cues in face recognition have shown immature performance at 13 to 14 years of age relative to adults (Mondloch et al., 2003; Mondloch et al., 2002; Mondloch et al., 2003). In contrast, the present study showed that 13- to 14-year-olds are adult-like in their recognition of the eye region when asked to encode eye regions. However, similar to the development of configural face processing abilities, recognition of the mouth region has yet to reach adult levels at 13 to 14 years of age. Thus, the overall ability to encode and recognize isolated facial regions continues to improve beyond 13 to 14 years of age. However, the encoding and recognition of different isolated facial regions show distinct developmental trajectories. In addition, despite some proficiency in encoding and recognizing isolated facial regions prior to adulthood, when children encounter whole faces, they do not appear to encode face parts independently of one another.
The findings from both experiments suggest the importance of the eyes and mouth and the less prominent role of the nose region in featural face recognition. When asked to encode whole faces, adults were above chance in their recognition of the eye and mouth regions, but were only at chance in their recognition of noses. When asked to encode and recognize isolated facial regions, nose recognition scores were low and showed no major improvements with age. In contrast, recognition of the eye region improved until 13 to 14 years of age, and recognition of the mouth region continued to improve beyond 13 to 14 years of age. This pattern of results may arise from the social importance of the eye and mouth regions in relation to emotion processing (Adams & Kleck, 2003, 2005; Smith et al., 2005; Tonks et al., 2007) and speech processing (Buchan et al., 2007, 2008; Desjardins & Werker, 2004; Dodd, 1979; Hardison, 2003; Hazan et al., 2005; Helfer, 1997; Kuhl & Meltzoff, 1982; Lansing & McConkie, 2003; Nakano et al., 2010; Patterson & Werker, 1999, 2003). Given cultural differences in face scanning (Blais, Jack, Scheepers, Fiset, & Caldara, 2008; Liu et al., 2011; Wheeler et al., 2011), future studies should verify whether the developmental changes in the present study with Chinese participants are also found among Caucasian participants. However, considering that overt measures of attention to different areas of faces as measured by eye-tracking can be independent of holistic face processing (de Heering, Rossion, Turati, & Simion, 2008), differential attention to isolated facial features may, in a similar way, be independent of featural face recognition. Indeed, eye-tracking studies have shown the importance of the nose region or the centre of own-race faces among Chinese infants and adults (Blais et al., 2008; Fu, Hu, Wang, Quinn, & Lee, 2012; Liu et al., 2011). Nonetheless, the present study along with findings by Ge et al. (2008) revealed that Chinese children and adults are generally more proficient in their recognition of the eyes and mouth relative to their recognition of noses. In addition, the prominent role of the eyes and mouth relative to the nose region in featural face recognition has also been found among Caucasian children (Hay & Cox, 2000). These findings taken together suggest that the nose region may only serve as the reference point, such that the information from other major parts of the face (e.g. both eyes and mouth) can be readily and optimally processed and integrated – an intriguing hypothesis awaiting empirical verification.
One limitation of the study is that the images used to test participants’ recognition were either cropped from the same images used during familiarization (Experiment 1) or the exact same images used during familiarization (Experiment 2). A more rigid test of the recognition of isolated facial regions would have used different images across the familiarization and recognition phases of the study. This would have ensured that recognition of isolated facial regions was based on the identity of the familiarized faces independent of low-level visual cues (e.g. brightness, colour, etc.).
In addition, our findings can be extended by further exploring the developmental changes between the age groups tested in the present study. For example, additional age groups between 8 to 9 years of age and 13 to 14 years of age and between 13 to 14 years of age and adulthood can be tested on our tasks. Such additional studies would provide a more comprehensive overview of how the recognition of isolated facial regions develops with age.
Overall, the present study has shown that different featural cues have unique developmental trajectories in face recognition. Furthermore, the use of featural cues in face recognition is different in its developmental trajectory relative to the use of both holistic and configural information. The present study, along with previous findings, suggests that the ability to recognize faces indeed has a protracted developmental course. This protracted course specifically reflects the gradual development of the ability to process not only face configural but also featural information. It remains unresolved as to whether such protracted development is specific to face recognition or if it is a more general issue concerning the recognition of any type of visual object. This question awaits answers with specifically designed future studies.
Acknowledgments
This research was supported by a National Institutes of Health (NIH) Grant R01 HD046526 to Kang Lee, Paul C. Quinn, Olivier Pascalis, James W. Tanaka, and Alan M. Slater. This research was also supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) grant and a National Natural Science Foundation of China (NSFC) grant to Kang Lee, and a National NSFC grant (31070908) and a Zhejiang Provincial NSFC grant (Y2100970) to Liezhong Ge.
References
- Adams RB, Kleck RE. Perceived gaze direction and the processing of facial displays of emotion. Psychological Science. 2003;14:644–647. doi: 10.1046/j.0956-7976.2003.psci_1479.x. [DOI] [PubMed] [Google Scholar]
- Adams RB, Kleck RE. Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5:3–11. doi: 10.1037/1528-3542.5.1.3. [DOI] [PubMed] [Google Scholar]
- Anastasi JS, Rhodes MG. An own-age bias in face recognition for children and older adults. Psychonomic Bulletin & Review. 2005;12:1043–1047. doi: 10.3758/bf03206441. [DOI] [PubMed] [Google Scholar]
- Bäckman L. Recognition memory across the adult life span: The role of prior knowledge. Memory & Cognition. 1991;19:63–71. doi: 10.3758/bf03198496. [DOI] [PubMed] [Google Scholar]
- Baudouin J, Gallay M, Durand K, Robichon F. The development of perceptual sensitivity to second-order facial relations in children. Journal of Experimental Child Psychology. 2010;107:195–206. doi: 10.1016/j.jecp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Blais C, Jack RE, Scheepers C, Fiset D, Caldara R. Culture shapes how we look at faces. PloS One. 2008;3:e3022. doi: 10.1371/journal.pone.0003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JN, Paré M, Munhall KG. Spatial statistics of gaze fixations during dynamic face processing. Social Neuroscience. 2007;2:1–13. doi: 10.1080/17470910601043644. [DOI] [PubMed] [Google Scholar]
- Buchan JN, Paré M, Munhall KG. The effect of varying talker identity and listening conditions on gaze behavior during audiovisual speech perception. Brain Research. 2008;1242:162–171. doi: 10.1016/j.brainres.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Diamond R. Are faces perceived as configurations more by adults than by children? Visual Cognition. 1994;1:253–274. [Google Scholar]
- Desjardins RN, Werker JF. Is the integration of heard and seen speech mandatory for infants? Developmental Psychobiology. 2004;45:187–203. doi: 10.1002/dev.20033. [DOI] [PubMed] [Google Scholar]
- Dodd B. Lip-reading in infants: Attention to speech presented in- and out-of synchrony. Cognitive Psychology. 1979;11:478–484. doi: 10.1016/0010-0285(79)90021-5. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Shepherd JW, Davies GM. Identification of familiar and unfamiliar faces from internal and external features: Some implications for theories of face recognition. Perception. 1979;8:431–439. doi: 10.1068/p080431. [DOI] [PubMed] [Google Scholar]
- Exline RV. Explorations in the process of person perception: Visual interaction in relation to competition, sex, and need for affiliation. Journal of Personality. 1963;31:1–20. [Google Scholar]
- Fantz RL. Pattern vision in newborn infants. Science. 1963;140:296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K. Face recognition in 4- to 7-year-olds: Processing of configural, featural, and paraphernalia information. Journal of Experimental Child Psychology. 2001;80:347–371. doi: 10.1006/jecp.2001.2639. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K. Person recognition by young children: Configural, featural, and paraphernalia processing. In: Pascalis O, Slater A, editors. The development of face processing in infancy and early childhood. New York: Nova Science Publishers; 2003. pp. 191–205. [Google Scholar]
- Fu G, Hu CS, Wang Q, Quinn PC, Lee K. Adults scan own- and other-race faces differently. PloS One. 2012;7:e37688. doi: 10.1371/journal.pone.0037688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Anzures G, Wang Z, Kelly DJ, Pascalis O, Quinn PC, Lee K. An inner face advantage in children’s recognition of familiar peers. Journal of Experimental Child Psychology. 2008;101:124–136. doi: 10.1016/j.jecp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Luo J, Nishimura M, Lee K. The lasting impression of Chairman Mao: Hyperfidelity of familiar-face memory. Perception. 2003;32:601–614. doi: 10.1068/p5022. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PYK. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:545–549. [PubMed] [Google Scholar]
- Hardison DM. Acquisition of second-language speech: Effects of visual cues, context, and talker variability. Applied Psycho Linguistics. 2003;24:495–522. [Google Scholar]
- Hay DC, Cox R. Developmental changes in the recognition of faces and facial features. Infant and Child Development. 2000;9:199–212. [Google Scholar]
- Hazan V, Sennema A, Iba M, Faulkner A. Effect of audiovisual perceptual training on the perception and production of consonants by Japanese learners of English. Speech Communication. 2005;47:360–378. [Google Scholar]
- de Heering A, Houthuys S, Rossion B. Holistic face processing is mature at 4 years of age: Evidence from the composite face effect. Journal of Experimental Child Psychology. 2007;96:57–70. doi: 10.1016/j.jecp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- de Heering A, Rossion B, Turati C, Simion F. Holistic face processing can be independent of gaze behaviour: Evidence from the composite face illusion. Journal of Neuropsychology. 2008;2:183–195. doi: 10.1348/174866407x251694. [DOI] [PubMed] [Google Scholar]
- Helfer KS. Auditory and auditory–visual perception of clear and conversational speech. Journal of Speech, Language, and Hearing Research. 1997;40:432–443. doi: 10.1044/jslhr.4002.432. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and Biobehavioral Reviews. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Morton J. Biology and cognitive development: The case of face recognition. Cambridge, MA: Basil Blackwell, Inc; 1991. [Google Scholar]
- Kleinke CL. Gaze and eye contact: A research review. Psychological Bulletin. 1986;100:78–100. [PubMed] [Google Scholar]
- Kuefner D, Macchi Cassia VM, Picozzi M, Bricolo E. Do all kids look alike? Evidence for an other-age effect in adults. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:811–817. doi: 10.1037/0096-1523.34.4.811. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982;218:1138–1141. doi: 10.1126/science.7146899. [DOI] [PubMed] [Google Scholar]
- Lansing CR, McConkie GW. Word identification and eye fixation locations in visual and visual-plus-auditory presentations of spoken sentences. Perception & Psychophysics. 2003;65:536–552. doi: 10.3758/bf03194581. [DOI] [PubMed] [Google Scholar]
- Lee K, Anzures G, Quinn PC, Pascalis O, Slater A. Development of face processing expertise. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. Handbook of face processing. Oxford: Oxford University Press; 2011. pp. 753–778. [Google Scholar]
- Levine MH, Sutton-Smith B. Effects of age, sex, and task on visual behavior during dyadic interaction. Developmental Psychology. 1973;9:400–405. [Google Scholar]
- Liu S, Quinn PC, Wheeler A, Xiao N, Ge L, Lee K. Similarity and difference in the processing of same- and other-race faces as revealed by eye tracking in 4- to 9-month-olds. Journal of Experimental Child Psychology. 2011;108:180–189. doi: 10.1016/j.jecp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi Cassia V, Picozzi M, Kuefner D, Bricolo E, Turati C. Holistic processing for faces and cars in preschool-aged children and adults: Evidence from the composite effect. Developmental Science. 2009;12:236–248. doi: 10.1111/j.1467-7687.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V, Simion F, Umiltà C. Face preference at birth: The role of an orienting mechanism. Developmental Science. 2001;4:101–108. [Google Scholar]
- Maurer D, Young RE. Newborn’s following of natural and distorted arrangements of facial features. Infant Behavior & Development. 1983;6:127–131. [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Thomson K. Limitations in 4-year-old children’s sensitivity to the spacing among facial features. Child Development. 2008;79:1513–1523. doi: 10.1111/j.1467-8624.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal of Experimental Child Psychology. 2003;86:67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Early visual experience is necessary for the development of some – but not all – aspects of face processing. In: Pascalis O, Slater A, editors. The development of face processing in infancy and early childhood: Current perspectives. New York: Nova Science Publishers, Inc; 2003. pp. 99–117. [Google Scholar]
- Mondloch CJ, Leis A, Maurer D. Recognizing the face of Johnny, Suzy, and me: Insensitivity to the spacing among features at 4 years of age. Child Development. 2006;77:234–243. doi: 10.1111/j.1467-8624.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Lewis TL, Budreau DR, Maurer D, Dannemiller JL, Stephens BR, Kleiner-Gathercoal K. Face perception during early infancy. Psychological Science. 1999;10:419–422. [Google Scholar]
- Mondloch CJ, Pathman T, Maurer D, Le Grand R, de Schonen S. The composite face effect in six-year-old children: Evidence of adult-like holistic face processing. Visual Cognition. 2007;15:564–577. [Google Scholar]
- Mondloch CJ, Robbins R, Maurer D. Discrimination of facial features by adults, 10-year-olds, and cataract-reversal patients. Perception. 2010;39:184–194. doi: 10.1068/p6153. [DOI] [PubMed] [Google Scholar]
- Nakano T, Tanaka K, Endo Y, Yamane Y, Yamamoto T, Nakano Y, Kitazawa S. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2935–2943. doi: 10.1098/rspb.2010.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson ML, Werker JF. Matching phonetic information in lips and voice is robust in 4.5-month-old infants. Infant Behavior & Development. 1999;22:237–247. [Google Scholar]
- Patterson ML, Werker JF. Two-month-old infants match phonetic information in lips and voice. Developmental Science. 2003;6:191–196. [Google Scholar]
- Pellicano E, Rhodes G. Holistic processing of faces in preschool children and adults. Psychological Science. 2003;14:618–622. doi: 10.1046/j.0956-7976.2003.psci_1474.x. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Rhodes G, Peters M. Are preschoolers sensitive to configural information in faces? Developmental Science. 2006;9:270–277. doi: 10.1111/j.1467-7687.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Tanaka JW. Infants’ processing of featural and configural information in the upper and lower halves of the face. Infancy. 2009;14:474–487. doi: 10.1080/15250000902994248. [DOI] [PubMed] [Google Scholar]
- Seitz K. Parts and wholes in person recognition: Developmental trends. Journal of Experimental Child Psychology. 2002;82:367–381. doi: 10.1016/s0022-0965(02)00106-6. [DOI] [PubMed] [Google Scholar]
- Simion F, Valenza E, Umiltà C, Barba BD. Preferential orienting to faces in newborns: A temporal-nasal asymmetry. Journal of Experimental Psychology Human Perception and Performance. 1998;24:1399–1405. doi: 10.1037//0096-1523.24.5.1399. [DOI] [PubMed] [Google Scholar]
- Smith ML, Cottrell GW, Gosselin F, Schyns PG. Transmitting and decoding facial expressions. Psychological Science. 2005;16:184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology. 1993;46A:225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Kay JB, Grinnell E, Stansfield B, Szechter L. Face recognition in young children: When the whole is greater than the sum of its parts. Visual Cognition. 1998;5:479–496. [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Slater A. Assessing emotion recognition in 9–15-year-olds: Preliminary analysis of abilities in reading emotion from faces, voices and eyes. Brain Injury. 2007;21:623–629. doi: 10.1080/02699050701426865. [DOI] [PubMed] [Google Scholar]
- Turati C, Macchi Cassia V, Simion F, Leo I. Newborns’ face recognition: Role of inner and outer facial features. Child Development. 2006;77:297–311. doi: 10.1111/j.1467-8624.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Want SC, Pascalis O, Coleman M, Blades M. Recognizing people from the inner or outer parts of their faces: Developmental data concerning ‘unfamiliar’ faces. British Journal of Developmental Psychology. 2003;21:125–135. [Google Scholar]
- Wheeler A, Anzures G, Quinn PC, Pascalis O, Omrin DS, Lee K. Caucasian infants scan own- and other-race faces differently. PloS One. 2011;6:e18621. doi: 10.1371/journal.pone.0018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Hellawell D, Hay DC. Configurational information in face perception. Perception. 1987;16:747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]