Abstract
The Barcelona Clinic Liver Cancer (BCLC) staging system is the method currently used to stage hepatocellular carcinoma (HCC) and therefore plays an important role in deciding on an appropriate course of treatment. BCLC takes into consideration the extent of the disease as well as patient factors such as hepatic function and performance status. However, it does not propose solutions for all clinical situations. Although radiotherapy (RT) is not included in the BCLC guidelines, the potent local antitumor effect of RT should be considered seriously as a part of the treatment strategy. Novel RT technologies introduced during the last decade have made it possible to deliver higher doses of radiation to the tumor while avoiding damage to critical normal tissues adjacent to the tumor. Because of the growing interest in using RT for HCC patients unfit for or progressed beyond standard treatments, the role of RT for HCC patients needs to be specified within the BCLC staging system. Curative RT can be used for patients with either very early or early stage BCLC; focal high dose RTs, such as stereotactic body RT, are especially useful. Intermediate or advanced stage disease confined to the liver can be managed safely and effectively by localized RT in conjunction with other treatment modalities such as transarterial chemoembolization or concurrent or adjuvant chemotherapy. In this review, the efficacy of RT in each BCLC stage of HCC will be discussed.
Key Words: Hepatocellular carcinoma, Radiotherapy, Treatment guidelines
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related death worldwide [1]. Hepatic function is included in many staging systems for HCC because it is widely accepted that the prognosis of HCC is impacted by hepatic function, in addition to tumor-related factors [2, 3, 4, 5, 6]. One of the most widely used staging systems is the Barcelona Clinic Liver Cancer (BCLC) system [2]. One major advantage of BCLC is that it provides both tumor staging and treatment recommendations. However, one disadvantage is that the advanced stage disease consists of heterogeneous disease phenotypes with variable prognosis for which there is a single recommended treatment—sorafenib. The majority of HCC patients die of intrahepatic tumor progression; therefore, liver-directed therapies are essential for improving clinical outcomes regardless of the stage [7]. In practice, standard curative local treatment modalities such as liver resection, radiofrequency ablation (RFA), and liver transplantation are offered to a limited number of patients [8]. When these options are limited, transarterial chemoembolization (TACE) is widely used in Asian countries for local control of the disease [9, 10, 11, 12, 13]. Nevertheless, local progression or intrahepatic recurrence is common after these therapies [14].
Despite its well-established local antitumor effect, radiotherapy (RT) for HCC has long been overlooked by physicians because even after delivery of sub-therapeutic radiation doses, fatal hepatic injury may develop [15, 16]. With recent developments in RT technology, it is possible to deliver precisely focused, high-dose radiation to partial volumes of the liver [17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]. This has been incorporated into the National Comprehensive Cancer Network Guidelines, version 2.2012, for HCC, which recommends RT as a locoregional therapy for all tumors irrespective of location [30]. Moreover, RT is considered appropriate for unresectable, locally advanced HCC with hepatic function of Child–Pugh class (CP class) A or B, which is supported by evidence level II in the practice guidelines of the Korean Liver Cancer Study Group [31].
In this review, we will discuss the specific indications for RT in HCC according to the BCLC staging system.
BCLC Very Early Stage Or Early Stage
The treatment of choice for small, solitary HCC at very early BCLC stage and with no portal hypertension is a partial liver resection, whereas liver transplantation is considered for cases with portal hypertension or early BCLC stage and with no contraindication to transplant. For HCC patients at an early BCLC stage and with an inoperable condition, including those refusing surgery, local ablation such as RFA or percutaneous ethanol injection therapy is recommended. However, the lesions near the hepatic dome or adjacent to the main portal vein can be difficult to target with RFA or may be heated improperly because of the heat sink phenomenon and are also susceptible to procedure-related complications [32]. Empirical use of RT for these lesions has resulted in a substantial response (fig. 1).
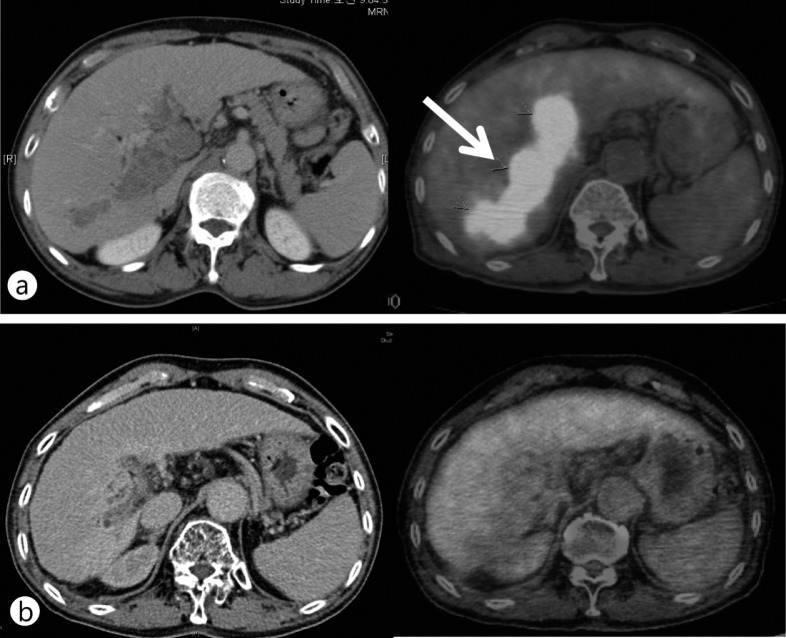
Fig. 1.
A HCC (black dotted circle and white arrow) at the liver dome and near a great vessel. A total of 90 Gy of hypofractionated RT in 18 fractions was delivered. This patient was disease-free 2 years after RT. (a) Computed tomography (CT) image before RT, (b) CT image 2 years after RT.
Several reports of RT in these patient populations have been published recently. Updated results from the French RTF-1 trial, a prospective phase II trial including CP class A or B cirrhotic patients with small size HCC unsuitable for curative treatments, showed an 80% complete response (CR) rate and 12% partial response (PR) rate after administering 33 fractions of 3-dimensional conformal RT (3D CRT) at 66 Gy [33]. Grade 3 toxicities were observed in 19% patients but were all asymptomatic. Grade 4 toxicities were observed in 22% patients, and all patients had hepatic function of CP class B. Kwon et al. [34] reported the results of stereotactic body RT (SBRT) for 42 HCC patients with tumors <100 cc who were ineligible for local ablation or surgical resection; total doses of 30–39 Gy were administered in 3 fractions. The overall in-field CR and PR rates were 59.6 and 26.2%, respectively, and the 1- and 3-year overall survival rates were 92.9 and 58.6%, respectively. Patients with smaller tumors (<32 cc) had superior in-field progression-free and overall survival rates, and major toxicity was observed in only 1 patient. A prospective, phase I dose escalation study of SBRT for primary HCC conducted at Indiana University reported the 1- and 2-year overall survival rates of 75 and 60%, respectively [35]. The radiation dose was escalated to 48 Gy (16 Gy/fraction) in Child-Turcotte-Pugh's class A patients. For Child-Turcotte-Pugh's class B patients, a regimen of 5 fractions starting at 40 Gy (8 Gy/fraction) was administered; 1 patient experienced progressive liver failure. The Child-Turcotte-Pugh score was the only factor related to more than a single grade 3 or greater liver toxicity event or death within 6 months. Takeda et al. [36] suggested that a combination of TACE and SBRT could be considered for solitary tumors <100 cc that are not close to the gastrointestinal tract or kidneys. According to their preliminary report, all patients were alive at the end of a median follow-up of 20 months. Fifty percent of the patients showed CR and 44% showed stable disease. No serious treatment-related toxicity was observed. Seo et al. [37] also reported on the toxicity and efficacy of SBRT after TACE for the treatment of localized HCC. They administered SBRT at 33–57 Gy in 3 or 4 fractions, according to the tumor volume (median, 40.5 cc). Three months after SBRT, they reported a 2-year overall survival rate of 61.4%, local progression-free survival rate of 66.4%, and local response rate of 63%. They found the high radiation dose to be independently related to survival. They also reported a decline in hepatic function in 6 patients (16%) and Grade 3 musculoskeletal toxicity in 1 patient (2.7%).
Taken together, the studies reviewed above suggest that SBRT could be used as a curative treatment modality for selected patients, either alone or in combination with TACE. In cases with large tumors or tumors close to radiosensitive organs, efforts should be made to deliver sufficient dose within an acceptable range of expected toxicities [38]. In addition, conventional fraction schedules or precise RT techniques, such as intensity-modulated RT (IMRT), breath control, and tumor tracking should be considered [39, 40].
BCLC Intermediate Stage HCC
Although TACE is recommended for the BCLC intermediate stage on the basis of studies reporting a survival benefit [11, 41, 42], the beneficial effects are limited to cases with vascular shunting, recanalization around the tumor, or multiple feeding vessels [43, 44]. In addition, efficacy is decreased and procedural morbidity is increased in tumors >10 cm [45]. These effects are well documented in the pathological evidence from patients who underwent resection after TACE [43, 44]. Even after complete necrosis of the main tumor, the presence of microsatellite lesions and microvascular invasion around the tumor may cause subsequent local recurrence [46]. To overcome these limitations, TACE is typically performed repeatedly; however, the final outcome in the majority of patients is outgrowth of HCC that is refractory to TACE.
Sergio et al. [47] found that patient survival was affected by elevation in angiogenic factors and invasive parameters observed after incomplete TACE. Therefore, the antivascular and antitumor activities of RT can ameliorate the limitations of TACE [48]. After encouraging results of combination treatment of TACE and radiation [20], Seong et al reported in more detail the treatment outcomes of patients who underwent TACE alone or TACE plus RT [21]. Of 105 patients with tumors of >5 cm in diameter, TACE was incomplete in 73 patients (69.5%). Of these, 38 patients received local RT and 35 patients received repeated TACE. The 2-year survival rate of patients receiving RT was significantly higher than that of patients receiving repeated TACE (37% vs. 14%, respectively, p = 0.001). The survival benefit was greater in patients with large tumors (>8 cm). Meta-analysis of 17 trials involving 1476 patients of unresectable HCC showed that TACE and RT combination therapy significantly improved 1-, 2-, 3-, and 5-year survival rates compared with TACE alone [49]. Choi et al. [50] reported the outcomes of 16 HCC patients with tumors >5 cm (median, 9 cm) who had received TACE and RT combination therapy followed by hepatic resection. TACE was performed three times on average, and the median radiation dose was 45 Gy. The degree of tumor necrosis was >90% in 87.5% of the patients. Five patients survived >2 years, and 2 of these patients survived >5 years; median survival time was 13.3 months. This study showed the possibility of long-term survival in patients who became eligible for tumor resection following TACE and RT combination therapy. Fig. 2 shows images of tumors in patients with incomplete TACE and RT, who eventually achieved CR.
Fig. 2.
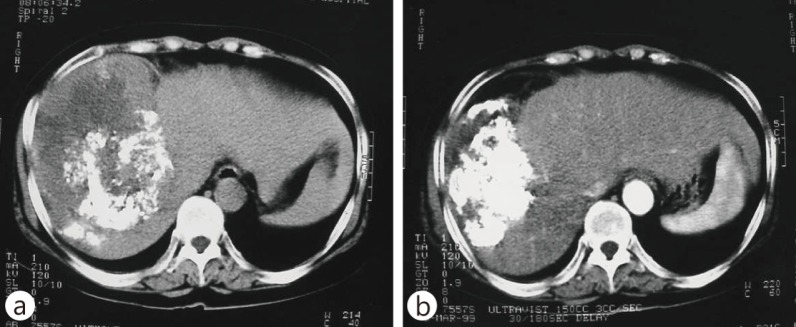
A HCC following incomplete TACE. A total of 54 Gy of conventional RT in 30 fractions was delivered. This patient gained complete response after RT. (a) CT image after TACE and before RT, (b) CT image after RT.
BCLC Advanced Stage HCC—Intrahepatic Lesion
The BCLC advanced stage consists of heterogeneous disease categories, including portal vein invasion, lymph node metastasis, and distant metastasis in patients with performance 0–2. Sorafenib is an orally available multikinase inhibitor that blocks the serine-threonine kinase Raf-1 as well as the activity of receptor tyrosine kinases of vascular endothelial growth factor receptors. Sorafenib is recommended as the standard treatment in the BCLC guidelines on the basis of demonstration of improved survival in advanced HCC patients in phase III randomized trials in United States and Asia-Pacific region [51, 52]. However, the recommendations regarding sorafenib in the Asian-Pacific region have some differences [31, 53]. At the workshop on the multidisciplinary management of nonresectable HCC under the 1st Asia-Pacific Primary Liver Cancer Expert Meeting, although there was a consensus agreement to recommend sorafenib for HCC with extrahepatic spread [54], the strategies to treat either HCC confined to the liver in the BCLC advanced stage or patients who stopped sorafenib because of adverse events or disease progression remained unfinalized.
For patients with disease confined to the liver but with portal vein invasion and thrombosis (PVT), promising objective response rates after RT combined with other modalities have been reported [55, 56, 57, 58]. Han and Seong et al. [59] reported the outcome of localized concurrent chemoradiotherapy (CCRT) with intra-arterial chemotherapy followed by hepatic arterial infusion chemotherapy in advanced HCC patients with portal vein invasion and well-reserved hepatic function. An objective response was observed in 18 of 40 patients (45%), and the 3-year overall survival rate was 24.1%. Fig. 3 shows the tumor response in a patient with massive portal vein invasion who received CCRT and gained CR with a survival of >2 years. Yoon et al. [60] reported 1- and 2-year survival rates of 42.5 and 22.8%, respectively, in 412 patients with portal vein invasion receiving TACE or transarterial tumor chemoinfusion followed by RT for PVT. Median 40 Gy (range, 21–60 Gy) in 2–5 fractions was delivered. Overall and PVT objective response rates were 27.9 and 85.6%, respectively. There is no agreement on the radiation field for treating HCC with PVT, specifically regarding whether to include the tumor and PVT or the PVT alone. The published reports show a slight survival benefit when the radiation field includes the tumor and PVT [59, 61, 62, 63, 64]; however, further investigation is needed. To identify the optimal RT technique for the typical advanced intrahepatic HCC, a large mass with vascular invasion, Lee et al. [58] compared tumor coverage and normal organ doses of 3D CRT, linac-based IMRT, and helical tomotherapy for the delivery of 60 Gy in 30 fractions”. Helical tomotherapy achieved the best tumor coverage as well as lower dose to the remaining normal liver, whereas linac-based IMRT showed better stomach sparing in cases of separated lesions in both liver lobes. This study [65] suggests that tumor location should be considered when determining the RT technique.
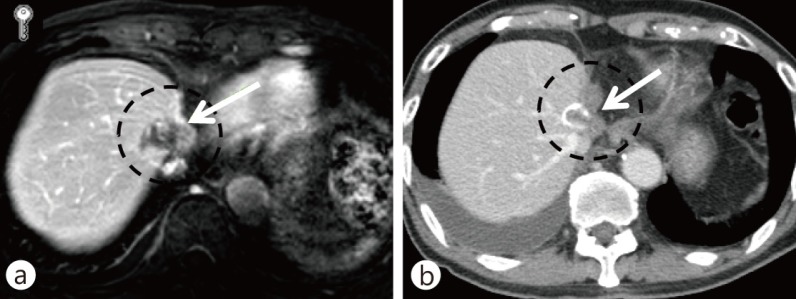
Fig. 3.
A HCC with portal vein thrombosis (white arrow). The patient was treated with concurrent intra-arterial chemotherapy and IMRT by helical tomotherapy. A total of 50 Gy of radiation in 20 fractions was delivered. After treatment, the patient received a year of adjuvant intra-arterial chemotherapy. The patient was disease-free 2 years after RT. (a) Positron emission tomography (PET)CT images before RT, (b) PET-CT images 2 years after RT.
BCLC Advanced Stage HCC—Extrahepatic Lesion
In patients with BCLC advanced stage extrahepatic disease, RT effectively controlled local lesions. For HCC with lymph node metastases, Yoon et al. [66] reported an overall response rate to RT of 76%. In addition, they showed that the total radiation dose, time dose fractionation values, and the biologically effective dose are all important factors related to response. They also suggest using radiation doses of >45 Gy to achieve a significant response (response rate of 93% vs. 57%, p = 0.003). Gastrointestinal bleeding or ulceration was observed in 15.7% of their patients, and toxicities of >grade 3 were observed in 5.9% patients.
A history of chronic gastritis or ulceration, with the inclusion of the whole circumference of the gastric antrum, was an important factor for the development of bleeding [66]. Some studies have analyzed prognostic factors in patients receiving RT for HCC with lymph node metastases. After adjustments using multivariate analysis, one study reported that CP class B and the presence of symptoms were significantly associated with inferior overall survival [67], whereas another study found that the absence of other concurrent distant metastasis and controllable primary HCC were significant [68].
RT is also effective in patients with localized distant metastases [69]. HCC metastases are frequently found in the skeletal system, particularly vertebrae, and are accompanied with severe pain, neuropathic pain, and possible spinal cord compression. In patients with solitary paraspinal metastases, RT can effectively achieve disease control that frequently translates into long-term survival. However, precision technology is strongly recommended to spare the radiosensitive spinal cord whilst delivering a sufficiently high-dose radiation for tumor control. Intensity modulated technology is useful (fig. 4).
Fig. 4.
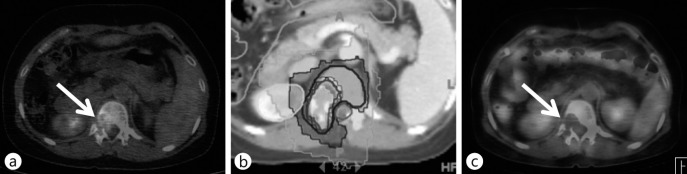
A HCC with solitary spinal metastasis (white arrow). Hypofractionated IMRT by helical tomotherapy was performed to protect the spinal cord and kidney. The patient was disease-free 2 years after RT. (a) PET-CT images before RT, (b) axial isodose distribution of RT, (c) PET-CT images 2 years after RT.
BCLC Terminal Stage HCC
To determine a detailed treatment plan for patients with distant metastases, the systemic tumor burden and disease symptoms should be considered. Effective supportive care and full symptomatic palliation are important for patients in the BCLC terminal stage. Therefore, regardless of the location, tumor lesions inducing pain or specific symptoms are an indication for RT.
Seong et al. [70] reported an overall response rate of 73% in HCC patients receiving palliative RT for painful bone metastasis. Patients receiving >43 Gy in a biological effective dose had a response rate of 96%, and objective reduction of tumor size was observed in 13 of the 15 available sites. Nakamura et al. [71] evaluated the therapeutic effects of RT on spinal HCC metastases by retrospective analysis of 24 ambulatory patients and reported an ambulatory rate of 85% after 3 months and 63% after 6 months, a local progression-free survival rate of 53% after 3 months and 47% after 6 months. They suggested that a biological equivalent dose of 39–50.7 Gy (median 44.8 Gy) was not sufficiently low to prevent paralysis and that dose escalation with a highly precise radiation technique should be evaluated further.
Despite the rarity of brain metastasis from HCC—0.9% of 6,919 patients [72], these lesions are an important cause of morbidity and mortality and are associated with extremely poor survival. In a retrospective analysis of 62 patients with brain metastasis, 17 were treated with only whole-brain RT (WBRT), 10 with only gamma knife surgery (GKS), 6 patients with only surgical resection, and 5 patients with surgical resection followed by WBRT. The median survival time was only 6.8 weeks (95% confidence interval, 3.8–9.8 weeks) after a diagnosis of brain metastasis was made. Treatment modality (resection or GKS and/or WBRT vs. steroid alone), number of brain lesions, and hepatic function were all associated with survival with statistical significance.
Conclusions
RT is applicable for HCC patients at each BCLC stage (fig. 5). For patients at a very early or early stage of BCLC, RT may be an alternative to surgery, especially SBRT. However, for patients with tumors >5 cm or tumors that are very close to radiosensitive organs, conventional RT should be used to deliver safe treatment. For the intermediate stage, RT combined with TACE could have a greater benefit over repeated TACE. Advanced stage disease confined to the liver can be managed effectively by localized RT in combination with other treatment modalities, such as concurrent or adjuvant chemotherapy. In addition, RT can provide good palliation of symptoms in patients with extrahepatic metastases. Further clinical studies using novel radiation technologies and multidisciplinary approaches are necessary to specify treatment options for HCC patients.
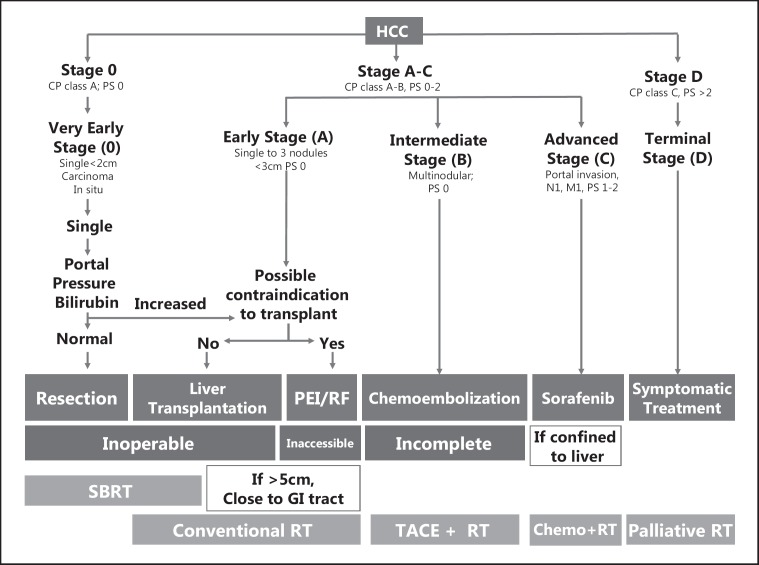
Fig. 5.
RT according to the Barcelona Clinic Liver Cancer stage. Patients who cannot receive recommended treatment at each stage can be managed using RT. Solitary tumors distant from the gastrointestinal tract and kidneys with tumors volume <100 cc are indicated for stereotactic body RT. PS = performance status; PEI = percutaneous ethanol injection; RF = radiofrequency.
Acknowledgement
This work was supported by the National R&D program grant for cancer control, the Ministry of Health and Welfare (0620390).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 3.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.The cancer of the liver italian program (clip) investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (clip score): Its value and limitations, and a proposal for a new staging system, the japan integrated staging score (jis score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 7.Dawson LA. Overview: Where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol. 2011;21:241–246. doi: 10.1016/j.semradonc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 9.Lee IJ, Seong J. Radiotherapeutic strategies in the management of hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):123–133. doi: 10.1159/000333275. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, Miyayama S, Takashima T, Unoura M, Kogayashi K. Small hepatocellular carcinoma: Treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 13.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, Takeda T, Yoneda N, Notsumata K, Toya D, Tanaka N, Mitsui T. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: Relationship between local tumor recurrence and visualization of the portal vein with iodized oil. Journal of vascular and interventional radiology. J Vasc Interv Radiol. 2007;18:365–376. doi: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Masuda T, Beppu T, Ishiko T, Horino K, Baba Y, Mizumoto T, Hayashi H, Okabe H, Horlad H, Doi K, Okabe K, Takamori H, Hirota M, Iyama K, Baba H. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008;15:589–595. doi: 10.1007/s00534-007-1288-4. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane AM, Murray-Lyon IM, Brinkley DM, Williams R. Quadruple chemotherapy versus radiotherapy in treatment of primary hepatocellular carcinoma. Cancer. 1977;40:609–614. doi: 10.1002/1097-0142(197708)40:2<609::aid-cncr2820400203>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 17.Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990;19:1041–1047. doi: 10.1016/0360-3016(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence TS, Ten Haken RK, Kessler ML, Robertson JM, Lyman JT, Lavigne ML, Brown MB, DuRoss DJ, Andrews JC, Ensminger WD, et al. The use of 3-d dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 19.Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, Lawrence TS. A phase i trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39:1087–1092. doi: 10.1016/s0360-3016(97)00550-6. [DOI] [PubMed] [Google Scholar]
- 20.Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO, Kim GE. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393–397. doi: 10.1016/s0360-3016(98)00415-5. [DOI] [PubMed] [Google Scholar]
- 21.Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver international: official journal of the International Association for the Study of the Liver. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 22.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, Nam HR, Ahn YC, Huh SJ. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Bea SH, Kim MS, Cho CK, Kim KB, Lee DH, Han CJ, Park SC, Kim YH. Feasibility and efficary of stereotactic abla-tive radiotherapy for barcelona clonical liver cancer-c heoatocellular carcinoma. Liver Caner. 2012;1:112. [Google Scholar]
- 24.Jiang GL. Hepatic proliferation after irradiation injuries. Liver Cancer. 2012;1:120. [Google Scholar]
- 25.Park JH, Yoon SM, Lim YS, Kim SY. J.Y. S, Kim KM, Lee HC, Park G, Kim JH: A two-week schedule of hypo-fractionated radiotherapy in patients with small hepatocellular carcinom: Efficacy and clinical outcomes. Liver Cancer. 2012;1:131. [Google Scholar]
- 26.Terashima K, Demizu Y, Hashimoto N, Mima M, Araya M, Jin D, Fujii O, Niwa Y, Fuwa N. Particle radiotherapy using proton or carbon ion beams for hepatocelllular carcinoma with portal vein tumor thrombus extending to the main trunk or major branches. Liver Cancer. 2012;1:135. [Google Scholar]
- 27.Yoon HI, Lee IJ, Pyun HO, Han KH, Seong J. Matched pair analysis comparing the clinical outcome of hy-pofractionated radiotherapy with tomotherapy and 3-dimensional conventional raditherapy in locally advanced hepatocellular carcinoma. Liver Cancer. 2012;1:137. [Google Scholar]
- 28.Yoon SM, Lim YS, Kim SY, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS, Park JH, Kim JH. Stereotactic body radiation therapy for primary or recurrent hepatocellular carcinoma: Long-term patient outcomes. Liver Cancer. 2012;1:138. [Google Scholar]
- 29.Yu JL, Park HC, Yoon SM, Kim TH, Seong J, Jang HS, Kay CS, Kim CY, Chie EK, Kim JH, Kim MS, Choi Y. Multi-center validation study of the prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy: Comparison among the okuda, clip, cupi and jis staging systems (krog 11-05) Liver Cancer. 2012;1:138. [Google Scholar]
- 30.National Comprehensive Cancer Network. Nccn clinical practive guidelines in oncology – hepatobiliary cancers, 2012, 2012. [DOI] [PMC free article] [PubMed]
- 31.Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 32.Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: Pros and cons. Gut and liver. 2010;4(Suppl 1):S113–118. doi: 10.5009/gnl.2010.4.S1.S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, Merle P. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies-mature results of the french phase ii rtf-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, Johnstone PA. Phase i feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Takahashi M, Kunieda E, Takeda T, Sanuki N, Koike Y, Atsukawa K, Ohashi T, Saito H, Shigematsu N, Kubo A. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: Preliminary results for efficacy and toxicity. Hepatology research: the official journal of the Japan Society of Hepatology. 2008;38:60–69. doi: 10.1111/j.1872-034X.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- 37.Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, Han CJ, Park SC, Lee BH, Kim YH, Lee DH. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 38.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura K, Shirato H, Seppenwoolde Y, Shimizu T, Kodama Y, Endo H, Onimaru R, Oda M, Fujita K, Shimizu S, Miyasaka K. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2003;56:221–228. doi: 10.1016/s0360-3016(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Limdo H, Paik SW, Yoo BC, Koh KG, Lee JH, Choi MS, Park W, Park HC, Huh SJ, Choi DH, Ahn YC. Predictive factors of gastroduodenal toxicity in cirrhotic patients after three-dimensional conformal radiotherapy for hepatocellular carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;93:302–306. doi: 10.1016/j.radonc.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 42.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai M, Okamura J, Kuroda C. Transcatheter chemo-embolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer. 1984;54:387–392. doi: 10.1002/1097-0142(19840801)54:3<387::aid-cncr2820540303>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 44.Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemo-embolization for hepatocellular carcinoma. Cancer. 1993;71:62–65. doi: 10.1002/1097-0142(19930101)71:1<62::aid-cncr2820710111>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Thornton RH, Covey A, Petre EN, Riedel ER, Maluccio MA, Sofocleous CT, Brody LA, Getrajdman GI, D'Angelica M, Fong Y, Brown KT. A comparison of outcomes from treating hepatocellular carcinoma by hepatic artery embolization in patients younger or older than 70 years. Cancer. 2009;115:5000–5006. doi: 10.1002/cncr.24556. [DOI] [PubMed] [Google Scholar]
- 46.Kudo M. Real practice of hepatocellular carcinoma in japan: Conclusions of the japan society of hepatology 2009 kobe congress. Oncology. 2010;78(Suppl 1):180–188. doi: 10.1159/000315740. [DOI] [PubMed] [Google Scholar]
- 47.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (tace) in hepatocellular carcinoma (hcc): The role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 48.Baker DG, Krochak RJ. The response of the microvascular system to radiation: A review. Cancer Invest. 1989;7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 49.Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, Zhang RM. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: A systematic review and metaanalysis. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:184–194. doi: 10.1016/j.radonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Choi SB, Kim KS, Park YN, Choi JS, Lee WJ, Seong J, Han KH, Lee JT. The efficacy of hepatic resection after neoadjuvant transarterial chemoembolization (tace) and radiation therapy in hepatocellular carcinoma greater than 5 cm in size. J Korean Med Sci. 2009;24:242–247. doi: 10.3346/jkms.2009.24.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 52.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the asia-pacific region with advanced hepatocellular carcinoma: A phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 53.Arii S, Sata M, Sakamoto M, Shimada M, Kumada T, Shiina S, Yamashita T, Kokudo N, Tanaka M, Takayama T, Kudo M. Management of hepatocellular carcinoma: Report of consensus meeting in the 45th annual meeting of the japan society of hepatology (2009) Hepatol Res. 2010;40:667–685. doi: 10.1111/j.1872-034X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 54.Park HC, Seong J, Tanaka M, Zeng ZC, Lim HY, Guan S, Bae SH, Tak WY. Multidisciplinary management of nonresectable hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):134–140. doi: 10.1159/000333276. [DOI] [PubMed] [Google Scholar]
- 55.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 56.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, Sun HC, Wang BL, Zhang JY, Jiang GL, Wang YQ. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 57.Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 58.Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Han KH, Seong J, Kim JK, Ahn SH. Lee do Y, Chon CY: Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003. doi: 10.1002/cncr.23684. [DOI] [PubMed] [Google Scholar]
- 60.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, Suh DJ. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: Long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 61.You CR, Jang JW, Kang SH, Bae SH, Choi JY, Yoon SK, Choi IB, Lee DH, Chun HJ, Choi BG. efficacy of transarterial chemolipiodolization with or without 3-dimensional conformal radiotherapy for huge hcc with portal vein tumor thrombosis. Korean J Hepatol. 2007;13:378–386. doi: 10.3350/kjhep.2007.13.3.378. [DOI] [PubMed] [Google Scholar]
- 62.Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 63.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 64.Kim JS, Han KH, Lee DY, Seong JS, Youn YH, Cheong JY, Ahn SH, Chon CY, Moon YM. concurrent chemo-ra-diation therapy for advanced hepatocellular carcinoma with portal vein thrombosis. Taehan Kan Hakhoe chi = Korean J Hepatol. 2002;8:71–79. [PubMed] [Google Scholar]
- 65.Lee IJ, Seong J, Koom WS, Kim YB, Jeon BC, Kim JH, Han KH. Selection of the optimal radiotherapy technique for locally advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2011;41:882–889. doi: 10.1093/jjco/hyr053. [DOI] [PubMed] [Google Scholar]
- 66.Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, Chung YW, Lee YS, Seo DJ. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer research and treatment: official journal of Korean Cancer Association. 2004;36:79–84. doi: 10.4143/crt.2004.36.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K, Chie EK, Kim W, Kim YJ, Yoon JH, Lee HS, Ha SW. Absence of symptom and intact liver function are positive prognosticators for patients undergoing radiotherapy for lymph node metastasis from hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:729–734. doi: 10.1016/j.ijrobp.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 68.Park YJ. Lim do H, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Nam HR, Oh DR, Park W, Ahn YC, Huh SJ: Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006;41:1099–1106. doi: 10.1007/s00535-006-1895-x. [DOI] [PubMed] [Google Scholar]
- 69.Asahara T, Dohi K, Hino H, Nakahara H, Katayama K, Itamoto T, Shimamoto F, Honke Y. A case of hepatocellular carcinoma with bone metastasis responding to radiotherapy after successful hepatectomy of primary lesion. Hiroshima J Med Sci. 1999;48:35–39. [PubMed] [Google Scholar]
- 70.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver international: official journal of the International Association for the Study of the Liver. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura N, Igaki H, Yamashita H, Shiraishi K, Tago M, Sasano N, Shiina S, Omata M, Makuuchi M, Ohtomo K, Nakagawa K. A retrospective study of radiotherapy for spinal bone metastases from hepatocellular carcinoma (hcc) Jpn J Clin Oncol. 2007;37:38–43. doi: 10.1093/jjco/hyl128. [DOI] [PubMed] [Google Scholar]
- 72.Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, Yoo NC. Brain metastases from hepatocellular carcinoma: Prognostic factors and outcome: Brain metastasis from hcc. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]