Abstract
Background
Adherence to surveillance recommendations for patients at risk of developing hepatocellular carcinoma (HCC) is influenced by several factors, including the etiology of chronic liver disease.
Aim
The aim of this study was to analyze whether tumor stage at diagnosis and prognosis differ in patients with alcohol-related HCC compared to those with chronic viral hepatitis-related HCC.
Patients and Methods
Medical records of 650 patients diagnosed with HCC between 1994 and 2011 were analyzed retrospectively. Groups were formed from patients having either alcohol abuse or viral hepatitis (chronic hepatitis B or C virus infection) as the only known HCC risk factors. Demographic data (age and gender), tumor stage at diagnosis, survival, liver function [Child–Pugh–Turcotte (CPT) score] in patients with liver cirrhosis, complications of liver cirrhosis, and serologic parameters were compared between the two groups.
Results
A total of 393 HCC cases (male 84%, median age 65 years) were identified, with alcohol abuse as the causative factor in 76.8% and chronic viral hepatitis in 23.2%. In patients with alcohol abuse, 278 (92.1%) were diagnosed with liver cirrhosis (CPT A 49.3%, CPT B 31.1%, CPT C 9.6%), while in patients with viral hepatitis, 84 (92.3%) suffered from liver cirrhosis (CPT A 59.3%, CPT B 23.1%, CPT C 8.8%). Tumor stage in patients with alcohol abuse was Barcelona Clinic Liver Cancer (BCLC) C in 43.7%, BCLC B in 30.5%, and BCLC A in 14.6%. Patients with viral hepatitis showed a trend toward diagnosis at an earlier tumor stage (BCLC B 35.2%, BCLC C 34.1%, BCLC A 22.2%). Etiology of liver cirrhosis did not significantly influence survival in intermediate and advanced tumor stages, but BCLC-A patients with alcohol-related disease demonstrated prolonged survival compared to patients with viral hepatitis.
Conclusion
Tumor stage at diagnosis of HCC is influenced by the etiology of underlying chronic liver disease and is more progressed in patients having a disease with alcoholic etiology. Majority of HCC patients are not diagnosed at a curable stage, which underlines the need for specialized care for all patients with chronic liver disease, independent of etiology and consequent adherence to current surveillance guidelines.
Key Words: BCLC, HCC, Prognosis, Risk factors, Tumor stage
Introduction
Worldwide hepatocellular carcinoma (HCC) is most frequently related to chronic hepatitis B virus (HBV) or chronic hepatitis C virus (HCV) infection. Even in Northern Europe, a low endemic area for viral hepatitis, more recent data indicate a rise in the viral etiology of HCC [1, 2, 3, 4]. Alcohol abuse and non-alcoholic fatty liver disease (NAFLD) are the two other dominant risk factors for HCC development, with different prevalence rates in different parts of the world.
Most cases of HCC develop in the context of liver cirrhosis, independent of the etiology.
Currently, there are no randomized controlled trials assessing the role of screening for patients at risk for developing HCC. However, surveillance strategies have been implemented as a result of indications from retrospective and cohort studies. Patients under surveillance are diagnosed with HCC at less advanced stages and are more likely to be eligible for potentially curative therapies [5]. Surveillance strategies applying semi-annual ultrasound are cost-effective [6, 7, 8, 9, 10, 11, 12] and are recommended for patients with liver cirrhosis irrespective of etiology [13].
For patients with HBV and HCV infection, surveillance is recommended by international guidelines [13, 14, 15] based on the lifetime risk of developing HCC for infected patients under certain conditions.
Prospective cohort studies revealed a 5–100-fold increase in the risk of developing HCC among subjects with chronic HBV infection [16]. In patients with chronic HBV infection, surveillance is not only recommended in case of liver cirrhosis with good or moderately impaired liver function (CPT A and B), but also in patients without cirrhosis in cases of active hepatitis or a family history of HCC [13].
Cross-sectional and case-control studies have shown a 5–20-fold increase in the risk for developing HCC in patients with chronic HCV infection compared to HCV-negative subjects [16]. Because the risk increases with progressive fibrosis and is highest in patients with liver cirrhosis, surveillance is currently recommended for patients with chronic HCV infection and advanced fibrosis (F3 according to METAVIR score) or cirrhosis in CPT stages A and B [13].
Heavy alcohol consumption (>80 g/day) over a period of more than 10 years increases the risk for HCC approximately 5-fold [17]. The annual incidence of HCC in patients with alcoholic liver cirrhosis is 1–2% [18]. Synergistic effects have been shown for alcohol consumption and viral hepatitis with respect to hepatocarcinogenesis.
NAFLD represents a hepatic manifestation of the metabolic syndrome and promotes hepatocarcinogenesis in part directly via insulin resistance and its subsequent inflammatory cascade [19] independent of liver cirrhosis [20, 21]; however, surveillance is currently not recommended for patients with metabolic syndrome without liver cirrhosis.
For patients with end-stage liver disease, independent of the cause of liver cirrhosis, surveillance is recommended only in those patients awaiting liver transplantation [13].
Several factors have an impact on adherence to surveillance, including the etiology of chronic liver disease and patient-related factors such as compliance and awareness of the risk. Factors related to the social and medical systems are of importance as well [22].
The aim of this study was to analyze a large, single-center cohort of patients to determine whether those with alcohol-related HCC differ from those with chronic viral hepatitis related-HCC with respect to tumor stage at diagnosis and prognosis.
Patients and Methods
The medical records of 650 patients admitted to the University Hospital of Magdeburg between February 1994 and January 2011 and diagnosed with HCC were analyzed retrospectively. The analysis included patients that were referred to the Department of Gastroenterology, Hepatology and Infectious Diseases; to the Department of Surgery; or to the Department of Radiology and Nuclear Medicine.
In 499 patients (76.77%) HCC was diagnosed histologically. In the remaining 151 patients (23.23%) the European Association for the Study of the Liver (EASL) criteria [23] and coincident findings in two different contrast-enhanced imaging modalities (ultrasound, computed tomography and/or magnetic resonance tomography) in combination with a significantly elevated level of alpha-fetoprotein (AFP) in the serum were applied.
By stratification of risk factors for the development of HCC, a subgroup of patients was identified in whom either alcohol abuse or viral hepatitis (chronic hepatitis B or C virus infection) were the only known risk factors. In these patients, besides demographic data (age and gender), tumor stage at diagnosis and liver function in terms of CPT score in those with liver cirrhosis, complications of liver cirrhosis, and serological parameters were analyzed.
Tumor stage was assessed on the basis of radiology reports and on histopathological criteria if available. Number and size of HCC nodules, as well as the presence of distant metastases, including lymph node involvement were recorded. If available, information on the patency of the portal vein was also analyzed. Barcelona Clinic Liver Cancer (BCLC) classification [24] was used to define tumor stage, liver function was assessed by CPT score, and clinical performance status of the patient was also assessed.
Statistical Analyses
Descriptive statistical analysis was performed using SPSS 11.5.1. (IBM SPSS Data Collection, USA). Results for numerical data are provided as mean and standard deviation. For categorical data, results are specified as absolute numbers and percentages. A t-test was applied for comparison of survival times, chi-square test for comparison of categorical data, and Mann-Whitney U test for non-categorical data. P < 0.05 was considered to be statistically significant. Cox analysis and the Kaplan Meier method were used for survival curve determinations.
Results
A total of 393 patients (84% male), with a median age of 65 years (17–85 years) were identified for study participation. Liver cirrhosis was diagnosed in 362 (92.1%) cases. Alcohol abuse was identified as a causative factor in 302 (76.8%) cases and chronic viral hepatitis in 91 (23.2%) cases.
A total of 92.1% of alcohol-related HCC patients, and 57.1% of chronic viral hepatitis patients were male (p < 0.05).
Of patients with alcohol-induced liver disease, 278 (92.1%) were diagnosed with liver cirrhosis. Liver function was well preserved (CPT A) in 49.3% of these patients, while in 31.1% liver function was moderately impaired (CPT B), and 9.6% presented with liver cirrhosis at CPT C. Ascites was diagnosed in 128 (42.4%) patients with alcohol-related liver disease, and 116 (38.4%) were diagnosed with esophageal varices.
Conversely, 84 of 91 patients with HCC associated with viral hepatitis suffered from liver cirrhosis (92.3%) of which 59.3% were diagnosed with HCC at a good liver function stage of CPT A, while 23.1% presented with CPT B cirrhosis and 8.8% with decompensated cirrhosis at CPT C (table 1). Of these patients, 27 (29.7%) presented with ascites, and 32 (35.2%) were diagnosed with esophageal varices (table 1).
Table 1.
Liver function and clinical signs of portal hypertension in patients with HCC with respect to chronic liver disease etiology
| Alcohol |
Viral hepatitis |
|||
|---|---|---|---|---|
| n | % | n | % | |
| nd | 18 | 6.0 | 4 | 4.4 |
| CPT A | 149 | 49.3 | 54 | 59.3 |
| CPT B | 94 | 31.1 | 21 | 23.1 |
| CPT C | 29 | 96 | 8 | 8.8 |
| no liver cirrhosis | 12 | 4.0 | 4 | 4.4 |
| ascites | 128 | 42.4 | 27 | 29.7 |
| esophageal varices | 116 | 38.4 | 32 | 35.2 |
When comparing CPT classes between the two groups of patients there was no significant statistical difference, but patients with alcohol-related disease had a significantly higher point score than patients with viral hepatitis-related HCC (p = 0.04).
When analyzing laboratory characteristics of the two groups of patients, significantly higher concentrations of alkaline phosphatase and gamma-glutamyl-transferase in patients with alcoholic liver disease were observed. These patients also showed a trend towards higher serum bilirubin concentrations (table 2). Patients with viral hepatitis-associated HCC alternatively presented with significantly increased transaminases as a marker of active necroinflammation.
Table 2.
Laboratory characteristics with respect to chronic liver disease etiology
| parameter | Alcohol Mean (SD) | Viral hepatitis Mean (SD) | p |
|---|---|---|---|
| albumin (g/dl) | 36.59 (7.66) | 35.83 (7.23) | 0.584 |
| bilirubin [µmol/1] | 45.12 (89.33) | 31.32 (58.74) | 0.224 |
| quick (%) | 83.98 (18.81) | 86.70 (18.59) | 0.220 |
| ALAT (µmol/sl] | 1.24 (2.47) | 1.37 (1.11) | <0.001 |
| ASAT (µmol/sl] | 1.78 (2.46) | 1.94 (1.32) | <0.001 |
| AP (µmol/sl] | 6.25 (7.48) | 3.97 (2.91) | 0.024 |
| GGT (µmol/sl] | 5.60 (5.33) | 2.67 (2.56) | <0.001 |
| hemoglobin (mmol/1) | 8.05 (1.31) | 8.22 (1.17) | 0.520 |
| platelets (Gpt/1) | 206.98 (117.97) | 165.40 (107.75) | <0.001 |
| AFP (ng/ml) | 18306.31 (96435.68) | 16054.89 (104406.02) | 0.212 |
AFP exceeded the level of diagnostic significance (200 ng/dl) in 45.3% of patients with alcohol-related HCC and in 31.8% of patient with viral hepatitis-related HCC (p = 0.033). When analyzing the patients with significantly increased AFP levels in the serum and comparing the two groups stratified by BCLC stage at diagnosis there was no significant statistical difference between the patients with alcoholic liver disease and those with viral hepatitis.
As for tumor stage at diagnosis, majority of the patients with alcohol-induced liver disease were diagnosed at an advanced tumor stage (BCLC C, 43.7%), followed by intermediate (BCLC B, 30.5%) and early tumor (BCLC A, 14.6%) stages. Patients with HCC attributable to chronic viral hepatitis showed a trend toward an earlier tumor stage diagnosis, with 35.2% at intermediate stage and 34.1% at advanced stage. Almost every fourth patient was diagnosed at an early tumor stage (BCLC A, 22.2%, table 3).
Table 3.
Tumor stage at diagnosis of HCC with respect to chronic liver disease etiology (p = 0.186)
| Alcoholic liver disease |
Viral hepatitis |
|||
|---|---|---|---|---|
| BCLC class | n | % | n | % |
| A | 44 | 14.6 | 20 | 22.0 |
| B | 92 | 30.5 | 32 | 35.2 |
| C | 132 | 43.7 | 31 | 34.1 |
| D | 34 | 11.3 | 8 | 8.8 |
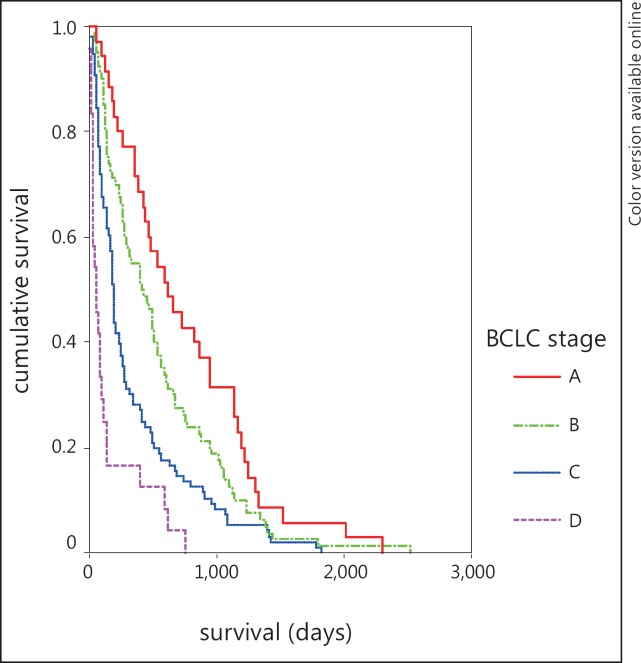
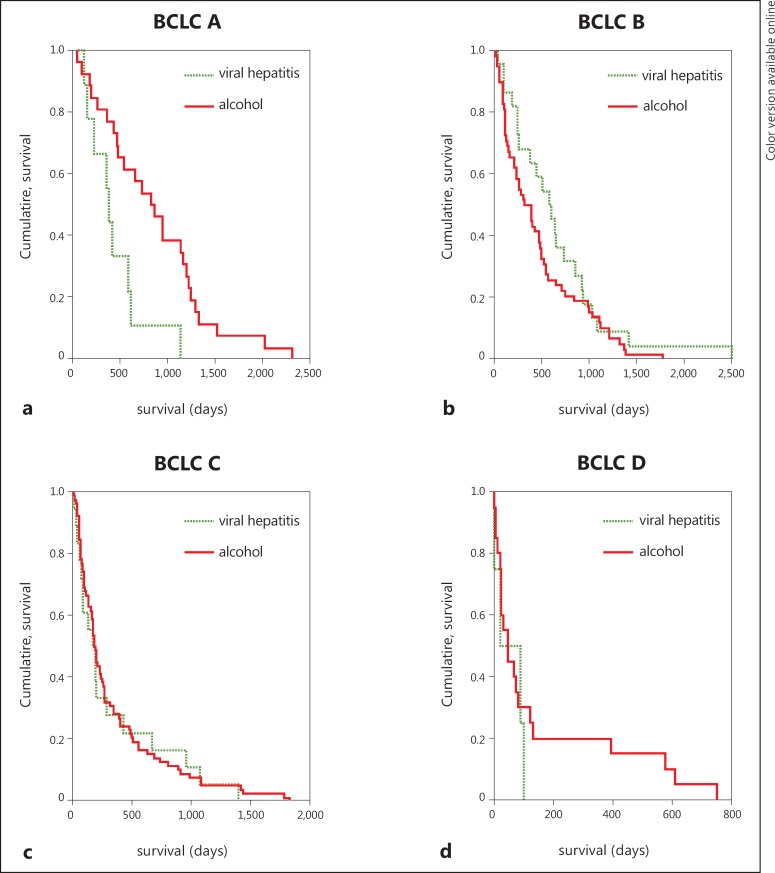
At the time of analysis, 235 patients had died. Advanced tumor stages were associated with shorter survival rates (fig. 1). Etiology of liver cirrhosis did not significantly influence survival in intermediate and advanced tumor stages (table 4, fig. 2 b-d). In BCLC stage A patients with alcohol-related disease survived significantly longer than those with chronic viral hepatitis (p = 0.046, fig. 2a).
Fig. 1.
Survival according to BCLC stage at diagnosis.
Table 4.
Survival with respect to tumor stage according to BCLC class and chronic liver disease etiology
| BCLC | Etiology | n | Mean (days) | 95% CI | t-test |
|---|---|---|---|---|---|
| A | alcohol | 26 | 862.5 | 641.9–1083.1 | p = 0.046 |
| viral hepatitis | 9 | 443.2 | 239.7–646.7 | ||
| 35 | 754.7 | 566.7–942.7 | |||
| B | alcohol | 58 | 487.1 | 376.3–597.9 | p = 0.109 |
| viral hepatitis | 22 | 676.4 | 445.5–907.2 | ||
| 80 | 539.2 | 434.3–644.0 | |||
| C | alcohol | 78 | 342.0 | 254.0–430.0 | p = 0.977 |
| viral hepatitis | 18 | 338.9 | 149.1–528.8 | ||
| 96 | 341.4 | 260.9–421.8 | |||
| D | alcohol | 20 | 159.3 | 57.9–260.7 | p = 0.403 |
| viral hepatitis | 4 | 58.5 | 10.1–107.0 | ||
| 24 | 142.5 | 51.9–233.1 | |||
Fig. 2.
Survival according to BCLC stage at diagnosis with respect to etiology.
Discussion
In patients with alcohol induced HCC, a diagnosis of HCC is more tardive compared with that of patients with chronic viral hepatitis. Complications of liver cirrhosis attributable to portal hypertension (ascites, esophageal varices) were more frequent in patients with alcoholic liver disease and HCC than in patients with viral hepatitis. A more advanced tumor stage combined with other complications of liver cirrhosis often leads to fewer treatment options.
There are several reasons for the delayed diagnosis of HCC in alcoholic patients. It is possible that patients with chronic alcohol abuse are diagnosed at advanced tumor stages because surveillance strategies do not effectively reach this target population. Factors related to the individual patient, e.g., awareness of high-risk status [25] as well as medical and social system factors frequently stigmatize patients addicted to alcohol and may play a role in this situation.
In the United States (US), less than every second patient with liver cirrhosis undergoes regular surveillance for HCC, varying in frequency according to cirrhosis etiology and medical specialty involved [26]. A survey of US gastroenterologists revealed that 79% are aware of the characteristics of high-risk patients that qualify for HCC screening [27] and 100% claim to follow the surveillance guidelines. However, when analyzing a community setting in the US, 40.6% of patients at high risk for HCC development did not receive sufficient surveillance [22]. An analysis of more than 1,800 US patients with HCC identified from the National Cancer Institute's (NCI) Surveillance Epidemiology and End Results (SEER) database revealed that less than 20% of patients with liver cirrhosis who developed HCC had received regular surveillance. Patients from urban areas and patients with higher income were more likely to receive regular screening [28].
In Europe, studies analyzing adherence to surveillance guidelines are lacking. Nevertheless, in spite of the explicit European screening guidelines introduced more than 10 years ago, the rate of patients diagnosed in early tumor stage is still low and has not improved over time, independent of liver disease etiology [3].
The challenge is not only to offer effective withdrawal therapies for patients addicted to alcohol but also to implement strategies for surveillance, which assists the patients to overcome inhibitions with regard to regular visits to the doctor. It is important to improve educational advertising concerning the consequences of chronic alcohol abuse.
Although current international guidelines do not recommend the use of AFP concentration as a tool in the surveillance of patients at risk for HCC development anymore, our data show that this information may still be helpful in those with alcoholic liver cirrhosis. Approximately every second patient with alcohol-related HCC presents with serum AFP concentrations exceeding 200 ng/dl.
Once HCC is diagnosed at an advanced stage, the disease prognosis is influenced by tumor stage and liver function, but not by etiology. However, in cases of early HCC (BCLC A) we observed a better prognosis for patients with alcohol-related disease than for those with chronic viral hepatitis. Ongoing necroinflammation observed in patients with viral hepatitis probably leads to faster progression of liver function impairment and a higher risk of de novo carcinogenesis compared with the arrest of progression of liver disease in alcoholic patients after withdrawal. This observation, although based on a very small number of patients, underlines the importance of treating chronic liver disease to prevent the development of de novo HCC in these patients. Recently, the International Liver Pathology Study group proposed to diagnose liver cirrhosis more comprehensively with respect to grade of activity and risk factors for malignancy [29]. This might even be more important for patients who have undergone curative treatment for early HCC to predict the outcome of individual patients. Randomized controlled trials to evaluate the effect of antiviral therapy in patients with HCV infection for secondary prevention of HCC are still not available [30], but elevated aspartate aminotransferase was identified as a negative prognostic factor in patients with advanced HCC [31].
There are few data comparing prognoses of HCC of different etiologies if tumor stage stratification at diagnosis is considered. It has been shown that the prognosis of patients with HCV-related HCC in a non-resectable stage is worse than that of other etiologies [32].
Most studies, comparing the prognosis of curatively treated HCC in patients with viral hepatitis to virus-negative HCC patients were performed in Asia. A retrospective analysis of a large Chinese cohort of patients with curatively resected HCC showed no significant differences between patients with hepatitis B- or C-related HCC and patients with non-hepatitis B- or C-related HCC with regard to survival, albeit the prognostic factors identified in these two groups of patients were different [33]. These data partly contradict a Japanese retrospective analysis revealing higher overall and progression-free survival for patients with HCC not attributable to HBV or HCV infection [34]. For patients with chronic HBV infection it has been shown that successful treatment of the infection prolongs survival of patients with non-resectable HCC treated by chemoembolization [35], and after curative treatment [36].
In HCC, treatment of the malignancy must be complemented by treatment for liver insufficiency and complications of portal hypertension particularly after curative treatment to prevent tumor recurrence.
Conclusion
Tumor stage at diagnosis of HCC is influenced by the etiology of underlying chronic liver disease. In patients with alcohol-related HCC, tumors are more likely to be detected at an advanced stage than in patients with chronic viral hepatitis-related HCC. However, independent of etiology, the majority of HCC patients are still not diagnosed at a curable stage. This problem underlines the need for specialized care for all patients with chronic liver disease and consequent adherence to current surveillance guidelines.
Diagnosis of HCC should not preclude the specialized and comprehensive treatment of the underlying chronic liver disease, especially in patients with chronic viral hepatitis. At more advanced tumor stages, the etiology of chronic liver disease does not influence HCC prognosis; however, stricter adherence to surveillance may allow for diagnosis of HCC at a curable stage.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner G, Kirovski G, Hebestreit A, Schölmerich J, Schlitt HJ, Stoeltzing O, et al. Epidemiology and survival of patients with hepatocellular carcinoma in Southern Germany. Int J Clin Exp Med. 2010;3:169–179. [PMC free article] [PubMed] [Google Scholar]
- 3.Hucke F, Sieghart W, Schöniger-Hekele M, Peck-Radosavljevic M, Müller C. Clinical characteristics of patients with hepatocellular carcinoma in Austria – is there a need for a structured screening program? Wien Klin Wochenschr. 2011;123:542–551. doi: 10.1007/s00508-011-0033-9. [DOI] [PubMed] [Google Scholar]
- 4.Borie F, Bouvier A, Herrero A, Faivre J, Launoy G, Delafosse P, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. 2008;98:505–509. doi: 10.1002/jso.21159. [DOI] [PubMed] [Google Scholar]
- 5.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin. Gastroenterol. Hepatol. 2011;9:617–23e1. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–476. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 7.Oka H, Kurioka N, Kim K, Kanno T, Kuroki T, Mizoguchi Y, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680–687. doi: 10.1002/hep.1840120411. [DOI] [PubMed] [Google Scholar]
- 8.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–846. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 9.Wong LL, Limm WM, Severino R, Wong LM. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320–325. doi: 10.1053/lv.2000.4875. [DOI] [PubMed] [Google Scholar]
- 10.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri M. Hepatocellular carcinoma: cost-effectiveness of screening. A systematic review. Risk management and healthcare policy. 2012;5:49–54. doi: 10.2147/RMHP.S18677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, et al. Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012;56:1089–1096. doi: 10.1016/j.jhep.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 13.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricke J, Seidensticker M, Mohnike K. Noninvasive diagnosis of hepatocellular carcinoma in cirrhotic liver: Current guidelines and future prospects for radiological imaging. Liver Cancer. 2012;1:51–58. doi: 10.1159/000339020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 19.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 20.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 21.Rosmorduc O, Fartoux L. HCC and NASH: how strong is the clinical demonstration? Clin Res Hepatol Gastroenterol. 2012;36:202–208. doi: 10.1016/j.clinre.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Wong CR, Garcia RT, Trinh HN, Lam KD, Ha NB, Nguyen HA, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54:2712–2721. doi: 10.1007/s10620-009-1015-x. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 25.Cho ER, Shin A, Choi KS, Lee H, Kim J. Factors associated with use of ultrasonography screening for hepatocellular carcinoma among hepatitis B or C carriers. Cancer Epidemiol. 2010;34:713–716. doi: 10.1016/j.canep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Patwardhan V, Paul S, Corey KE, Mazhar SM, Richter JM, Thiim M, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialists. Dig Dis Sci. 2011;56:3316–3322. doi: 10.1007/s10620-011-1836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma P, Saini SD, Kuhn LB, Rubenstein JH, Pardi DS, Marrero JA, et al. Knowledge of hepatocellular carcinoma screening guidelines and clinical practices among gastroenterologists. Dig Dis Sci. 2011;56:569–577. doi: 10.1007/s10620-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P, et al. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol. 2012;137:5–9. doi: 10.1309/AJCP2T2OHTAPBTMP. [DOI] [PubMed] [Google Scholar]
- 30.Michielsen P, Ho E, Francque S. Does antiviral therapy reduce the risk of hepatocellular carcinoma in patients with chronic hepatitis C? Minerva Gastroenterol Dietol. 2012;58:65–79. [PubMed] [Google Scholar]
- 31.Pinter M, Sieghart W, Hucke F, Graziadei I, Vogel W, Maieron A, et al. Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2011;34:949–959. doi: 10.1111/j.1365-2036.2011.04823.x. [DOI] [PubMed] [Google Scholar]
- 32.Abbas Z, Siddiqui A, Luck NH, Hassan M, Mirza R, Naqvi A, et al. Prognostic factors of survival in patients with non-resectable hepatocellular carcinoma: hepatitis C versus miscellaneous etiology. J Pak Med Assoc. 2008;58:602–607. [PubMed] [Google Scholar]
- 33.Li T, Qin L, Gong X, Zhou J, Sun H, Qiu S, et al. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: Clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence after resection. 2012. Cancer DOI: 10.1002/cncr.27697. [DOI] [PubMed]
- 34.Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Differences in long-term outcome and prognostic factors according to viral status in patients with hepatocellular carcinoma treated by surgery. J Gastrointest Surg. 2008;12:468–476. doi: 10.1007/s11605-007-0402-x. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda H, Kumada T, Tada T, Sone Y, Fujimori M. Transarterial chemoembolization for hepatitis B virus-associated hepatocellular carcinoma: improved survival after concomitant treatment with nucleoside analogues. J Vasc Interv Radiol. 2012;23:317–22e1. doi: 10.1016/j.jvir.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Jin YJ, Shim JH, Lee HC, Yoo D, Kim KM, Lim Y, et al. Suppressive effects of entecavir on hepatitis B virus and hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1380–1388. doi: 10.1111/j.1440-1746.2011.06776.x. [DOI] [PubMed] [Google Scholar]