Abstract
Liver cancer, the most common form of which is hepatocellular carcinoma (HCC), is one of the most deadly cancers worldwide. As of 2008, in men, HCC was the fifth most common cancer (approximately 450,000 new cases per year) and the second most frequent cause of death from cancer (around 416,000 deaths per year), whereas in women, it was the seventh most frequently diagnosed cancer (150,000 new cases per year) and the sixth most frequent cause of cancer deaths (140,000 deaths per year) [1]. Overall, HCC is the third leading cause of death from cancer globally [2, 3]. Worldwide, the incidence of HCC in males is more than twice that in females. The etiology of HCC is diverse; however, approximately 80% of HCCs occur secondary to chronic infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) [4]. The geographic distribution of HCC is such that the high-incidence regions of Eastern Asia and sub-Saharan Africa bear a disproportionate HCC burden, amounting to more than 80% of the global burden [4]. However, even in areas considered low-incidence regions—North America and Europe—the incidence of HCC is on the rise [4]. In the US, HCC incidence has risen more than threefold in the past 30 years, and it is now the ninth most frequent cause of death from cancer. The major reasons for the increased incidence of HCC in the US are the increasing prevalence of chronic HCV infection, increased immigration from high-incidence countries in Asia and Africa, and the increase in the number of individuals with cirrhosis due to obesity-related fatty liver disease. Most HCCs are diagnosed at an advanced stage for which there is no curative option. Sorafenib, the only agent specifically approved for HCC treatment, is of limited efficacy in this setting. Therefore, an urgent need for improved HCC therapy exists. In this review, we discuss the available data on the development and use of immunotherapy for HCC, with a particular focus on recent results and novel approaches.
Key Words: Hepatocellular Carcinoma, Immunotherapy, Liver Cancer, Liver Tolerance, Spontaneous Regression
Introduction
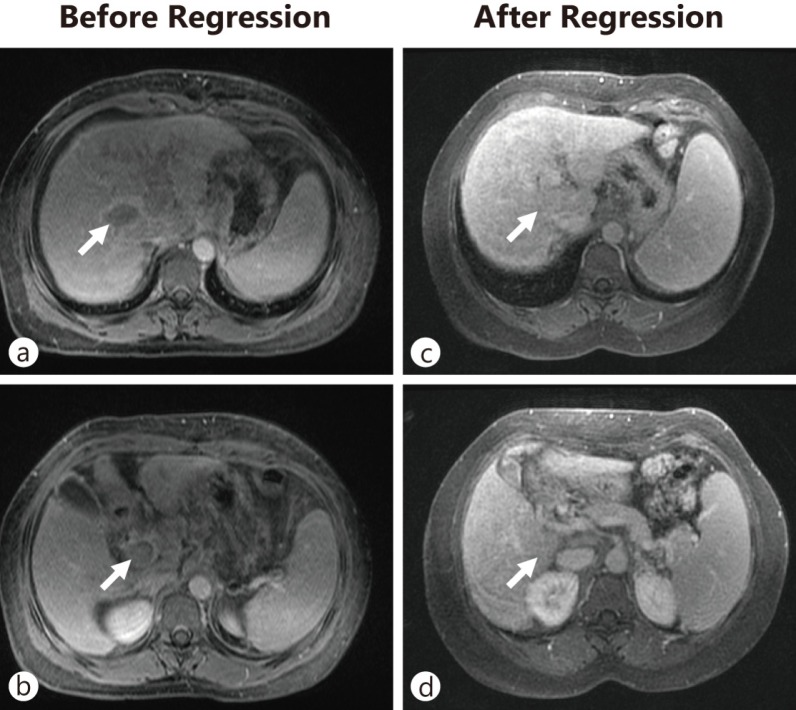
The incidence of hepatocellular carcinoma (HCC) continues to increase in the US and globally [1, 5]. In 2008 an estimated 748,300 new cases of liver cancer were diagnosed worldwide, and the mortality rate of liver cancer closely mirrored the incidence with 695,900 people dying of the disease [1]. This is largely because of the fact that the diagnosis is usually made at an advanced stage for which there are currently no highly effective treatments. Consequently, the number of deaths from HCC per year is almost identical to the number of new cases, reflecting a high case fatality rate and emphasizing the pressing need for the development of better treatment modalities [1]. In a 2006 population-based analysis in the US, Davila and El-Serag documented overall 1- and 3-year survival rates for US patients with HCC of only 20% and 5%, respectively, with a median survival of 8 months [6]. Chronic infection with hepatitis B and/or C virus (HBV, HCV) is the major cause of HCC worldwide. The World Health Organization (WHO) estimates that as of July 2012, about two billion people worldwide have been exposed to HBV, and 400 million people have chronic HBV infection (WHO Fact Sheet number 204). About 150 million people globally are infected with HCV (WHO Fact Sheet number 164). Chronic HBV and HCV infections progress through stages of increasing inflammation associated with fibrosis, and eventually result in cirrhosis, which predisposes individuals to HCC. In addition, HBV integration can predispose individuals to HCC in the absence of cirrhosis. Considering the extreme latency of HCC (3–4 decades after infection for HCV) and the previous HCV epidemics that occurred in the US in the 1960s, ‘70s, and ‘80s, the number of HCC cases with underlying HCV infection is expected to increase. The US Centers for Disease Control and Prevention estimate an annual incidence of new HCV infections of 25,000 and note that about 2.7 million Americans are infected with HCV and are at risk of developing HCC. The etiology of HCC is vast and includes other risk factors in addition to HCV/HBV infection. Furthermore, cofactors such as HIV infection and excessive alcohol consumption contribute to HCC pathogenesis. Current treatments for advanced HCC are at best minimally effective—the oral multikinase inhibitor sorafenib, the recommended therapeutic agent, extends life by only 3 months compared with placebo [7, 8]. This underlines the need for novel therapies and has spurred additional investigation of immunotherapy as a treatment strategy for HCC. The case for immunotherapy for HCC is made more clear because of the well-known phenomenon of spontaneous regression of advanced HCC, which suggests that enhanced immune activity is capable of inducing tumor clearance (fig. 1) [9].
Fig. 1.
Spontaneous regression of a HCC with portal vein invasion. A 43-year-old woman with chronic hepatitis C and cirrhosis presented with a 5-cm mass in the liver (a) with an associated tumor thrombus in the main, right, and left portal veins (b) and an alpha-fetoprotein level of 1548 ng/mL, consistent with HCC. Over the next year, the mass and portal vein tumor thrombus spontaneously regressed and the alpha-fetoprotein level decreased to 30 ng/mL. Observation over a 6-year follow-up period revealed no evidence of tumor recurrence (c, d).
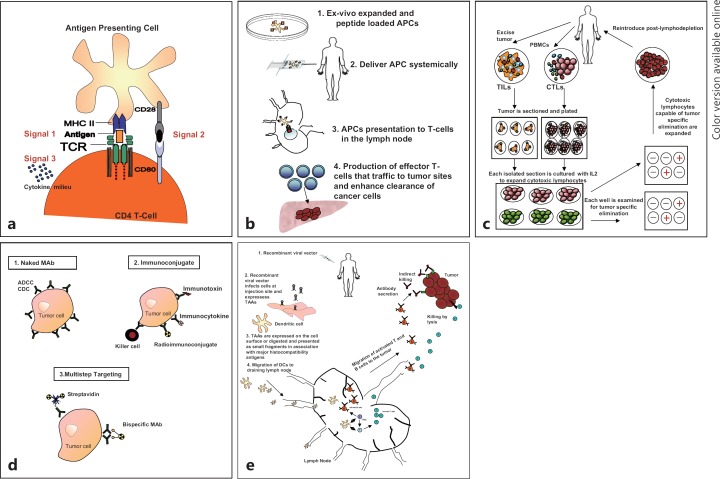
Harnessing of the immune system is increasingly being investigated as a central component of cancer treatment modalities. Cytokine-based tumor vaccines, cell-mediated vaccines, monoclonal antibodies, immune adjuvants, and prophylactic immune therapies have been successfully developed as potential or approved cancer therapies (table 1, see fig. 2). We argue in this article that the nascent field of HCC immunotherapy may provide pivotal advantages in the effort to improve HCC therapy. The strategy of adopting an immunocentric approach to HCC treatment to enhance established treatment modalities may be potentially more efficacious and less toxic. Two key elements must be appreciated and addressed in this strategy: (1) the HCC tumor antigen repertoire is both vast and, in many instances, unique because of mutations and aberrant expression profiles, and (2) reeducation of the host immune system may undermine the liver's inherent tolerogenicity. Moreover, when evading the tolerogenicity of the liver, effective immune therapies against HCC must employ extrahepatic priming of the immune system, such as in the lymph nodes or Peyer's patches, to facilitate antigen recognition and the mounting of a successful antitumor response. A multifaceted approach that employs immunotherapy in combination with other established paradigms in HCC therapy will, in our view, prove more effective in achieving disease regression and even cure.
Table 1.
Current cancer immune therapies
| Therapies | Indications (Trials) | Citations/Studies |
|---|---|---|
| Cytokine-based tumor vaccines | Hormone-naïve prostate cancer (Phase II) | 10 |
| Prophylactic vaccine and antiinflammatory therapies | HCC, Colorectal cancer | 11–13 |
| Cell-mediated vaccines | Metastatic melanoma, RCC (Phase II) | 14, 15 |
| Antigen-specific vaccines | Melanoma (Phase II), Ovarian carcinoma (Phase II) | 16, 17 |
| Monoclonal antibodies | Lymphoma, Leukemia | 18, 19 |
| Immune adjuvants | Metastatic melanoma (Phase II), Basal cell carcinoma (Phase I), Cutaneous T-cell lymphoma (Phase I) | 20–23 |
RCC = renal cell carcinoma.
Fig. 2.
Antigen presentation and immune therapy. (a) General concept of antigen presentation: Professional APC presenting an acquired antigen to a naïve CD4 T cell via the interaction between its peptide–MHC complex and the T-cell receptor (TCR, Signal 1). In the presence of a concomitant interaction between CD28 and CD80 (Signal 2), this will lead to T-cell activation. Signal 3: The characteristic composition of the cytokine milieu determines the type of effector CD4 T cell (regulatory T cell, or helper T cell, etc.) that will emerge. (b) Antigen presentation as a therapeutic strategy: (1) Selected antigens (Glypican-3, MAGE-A3, MAGE-A1) are loaded onto ex vivo APCs (e.g., dendritic cells). (2) The APCs are then delivered systemically. (3) APCs migrate to the lymph nodes, where their cargo antigens are presented tonaïve T cells, and activate them. (4) The activated T cells expand and migrate to the tumor, inducing tumor clearance. (c) Adoptive cell transfer: TILs or CTLs are isolated from patient specimens. The isolated cells are expanded by culturing with IL-2. The cells are then assayed for tumor-specific elimination. Cells with the unique ability to specifically eliminate tumors are expanded and reintroduced after lymphodepletion. These cells are capable of migrating to the tumor and have the potential to eliminate the tumor. (d) Antibody therapy: (1) Naked monoclonal antibodies (MAb) have been designed to specifically bind to receptors or surface molecules that are uniquely expressed by tumor cells and induce cell death via antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity. (2) Immunoconjugate: Antibodies have also been designed to carry payloads that are engineered to induce cell death. (3) Multistep targeting: Antibodies serve as an intermediary between the tumor cells and the targeting payloads. (e) Viral vectors: (1) recombinant viral vectors can be engineered to induce an immunologic response, and also to express selected tumor-associated antigens (TAAs). (2) Upon systemic infusion the viral vectors can selectively infect and induce tumor cell death. (3) The death of these tumor cells results in the release of tumor-specific peptide fragments that are taken up by APCs (i.e., dendritic cells) and expressed on the cell surface via their MHC. (4) Antigen-expressing APCs migrate to lymph nodes where they are exposed to and educate T and B lymphocytes. Educated T cells from the lymph nodes expand and home to the tumor where tumor cell lysis is induced.
The Inherently Immunosuppressive Microenvironment of The Liver (The Tolerogenic Liver)
The tolerogenicity of the liver to the vast array and constant flux of blood-derived antigens and bacterial molecules from the intestinal microbiota requires a plethora of immune-regulatory mechanisms in both healthy and diseased states. As the major detoxifying organ of the body, and because of its constant exposure to gut-derived materials via the portal venous blood, the liver is constantly capturing and eliminating toxins, pathogens, and harmless antigens without eliciting an immune response. To prevent a barrage of immune responses, the molecular architecture of the liver allows for a multipronged approach to immune surveillance. This is readily observed by the liver's role in portal venous tolerance and oral tolerance, the relatively low incidences of liver allograft rejection (minimal need for immune suppression after transplantation), and the tendency toward long-term microbial infections and tumor metastasis in the liver [24]. Liver allografts have also been reported to have an immunoprotective effect on simultaneously transplanted kidneys [25].
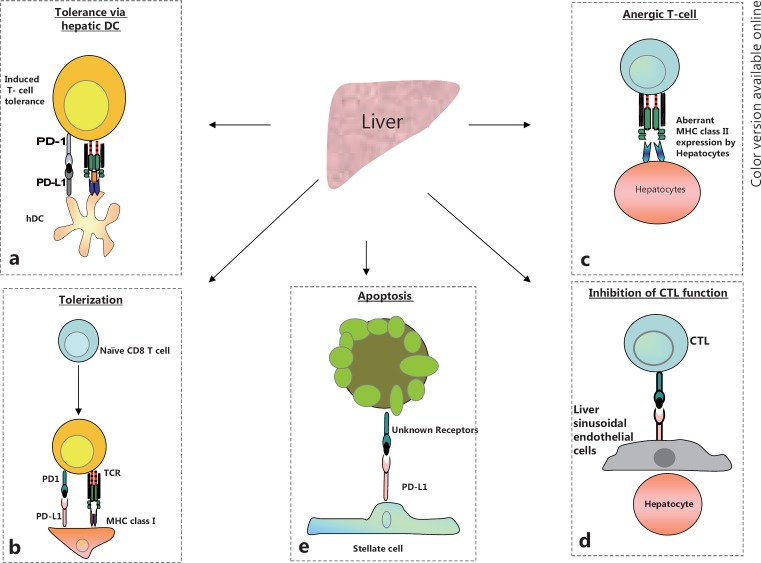
The liver's pathway to immune tolerance is multifaceted (see fig. 3a-e). Structurally, the liver is made up of repetitive functional units called hepatic lobules that are perfused by vascular supply structures referred to as sinusoidal vessels. These well-characterized networks of sinusoids allow for the mixing of portal venous and arterial blood in the liver before the blood flows into the metabolic units, the hepatocytes. Hepatocytes are shielded from direct interaction with the bloodstream by nonparenchymal liver cells: liver sinusoidal endothelial cells (LSECs) and stellate cells located in the space of Dissé. The liver also comprises other nonparenchymal cells including dendritic cells located predominantly in the periportal and pericentral area, Kupffer cells (resident macrophages found in the sinusoidal lumen), natural killer cells, natural killer T cells, and a loosely distributed population of liver-associated lymphocytes. Distinct hepatic regulatory pathways allow for the establishment of tolerance to innocuous antigens while enabling immunity to pathogens. The innate immune functions of nonparenchymal cells serve as a protective barrier between the hepatocytes and pathogens.
Fig. 3.
Pathways to immune-suppression within the liver: The liver is recognized as being “immune privileged.” Numerous hepatic antigen presenting cells orchestrate this immune evasion. (a) Induced T cell tolerance within the liver microenvironment via interaction between hepatic dendritic cells (hDCs) expressing the inhibitory ligand PD-L1 (b) Tolerization or neutralization of naïve lymphocytes occurs by the concomitant engagement of the PD-1 receptor and its ligand PD-L1 during initial antigen presentation via the MHC complex. (c) Aberrant expression of the MHC complex by hepatocytes in the diseased liver and the subsequent interaction of the MHC complex with the TCR, in the absence of the co-receptor CD80 and its ligand CD28, leads to T-cell anergy. (d) Expression of inhibitory molecules by liver sinusoidal endothelial cells (LSECs) results in the inhibition of CTLs. (e) Expression of the PD-L1 inhibitory molecule by hepatic stellate cells (HSCs) and their subsequent physical interaction with CTLs – via an unknown receptor – leads to loss of CTL function and CTL apoptosis.
Hepatocytes and other nonparenchymal cells serve a regulatory immune role by acting as local antigen-presenting cells (APCs) in well-defined anatomical niches. For instance, our own mRNA array analyses as well as reports by other researchers have shown that compared with benign adjacent tissues, major histocompatibility complex class (MHC) II is one of the most highly expressed genes in HCC tumors. The fact that diseased hepatocytes aberrantly express MHC class II molecules that interact with naïve T cells in the liver attests to the uniqueness and tolerogenicity of this organ [26, 27]. In normal liver physiology, after a liver insult, the MHC class II-expressing hepatocytes may engage naïve T cells in the liver microenvironment; however, because of the absence of suitable coactivator interactions, hepatocytes expressing MHC II molecules render naïve T cells in the liver anergic (see fig. 3c). This may be one mechanism by which HCCs evade the endogenous immune response. The liver is also composed of resident lymphocytes—natural killer cells, natural killer T cells, and classical T cells such as CD4+ T cells and CD8+ T cells—that may be rendered anergic during the initial progression of HCC [27]. Moreover, the interaction of cytotoxic T lymphocytes (CTLs) that may have migrated into the liver environment with MHC class II-expressing hepatocytes may induce apoptosis of the CTLs. To break this inherent tolerance and eliminate cancer cells, means of abating the tolerogenic characteristics of hepatic APCs need to be explored. As HCC originates and progresses within the hepatic parenchyma, a tolerogenic hepatic microenvironment that is incapable of adequately supporting effector lymphocyte function will need to be modified by adjuvant immunotherapeutics which enhance effector lymphocyte function, in order to limit and suppress tumor progression. In addition, T cells could be engineered to be resistant to the inhibitory effects of the tolerogenic hepatic microenvironment—this would greatly enhance intrahepatic effector T-cell function. Moreover, future immunotherapeutic strategies could include extrahepatic antigen presentation and lymphocyte priming, as well as liver-specific depletion of regulatory T cells.
Considering the Etiology of HCC to Design Effective Immunotherapeutics
There are numerous etiological factors that influence the incidence of HCC. This diverse etiology leads to unique geography-, race-, age- and gender-based manifestations of HCC. The vast majority of HCC cases have cirrhosis as the major risk factor (80–90% of patients with HCC) [4]. Chronic HBV and HCV infections and chronic alcohol exposure are the major promoters of cirrhosis [28]. Other risk factors for HCC include obesity, diabetes, steatohepatitis, smoking, and other causes of chronic liver disease. The cirrhotic liver is characterized by exhaustion of the liver's inherent regenerative propensity. The inflammed cirrhotic liver contains hepatocytes undergoing senescence within a genotoxic environment that high in reactive oxygen species and is conducive to the development of cancer. Within this genotoxic environment of inflammation and fibrogenesis, a proportion of hepatocytes become immortalized and primed for carcinogenesis. Liver cirrhosis is therefore crucial to the morbidity and mortality associated with HCC. The majority of HCC patients with HBV and HCV infection have cirrhosis secondary to chronic necroinflammation. This suggests a distinctive interaction with the host immune system that must be considered when designing effective immunotherapeutics. These viruses may also induce unique immunological fingerprints that must be factored into the design of any immune therapy regimen.
To develop effective immunotherapeutics, both the occurrence and extent of cirrhosis should be ascertained. Knowledge of HCC patient demographics, better understanding of the mechanisms underlying various HCC etiologies, and a fuller elucidation of the ensuing interplay between the specific liver microenvironment and the host immune system will lead to the development of better immunotherapeutic treatment modalities.
Immunotherapeutic Strategies with Potential Utility in HCC Therapy
The discipline of tumor immunotherapy is emerging as a viable weapon in the clinician's arsenal in the “war against cancer.” An effective immunotherapeutic modality must take advantage of the following considerations in order to mount an effective immune response against a tumor. First, tumors express antigens—mutant antigens and/or aberrantly expressed antigens—that are recognizable by the adaptive immune system. To exploit the unique antigenic profiles of cancer cells, tumor-associated antigens have been targeted through the isolation of antigen-specific human monoclonal antibodies or the design of humanized or chimeric monoclonal antibodies. Of these, tumor-targeted antibodies such as trastuzumab (Herceptin) for breast cancer therapy, rituximab (Rituxan) for lymphoma treatment, cetuximab (Erbitux) for metastatic colorectal cancer and advanced head and neck cancer, and bevacizumab (Avastin) for metastatic colorectal cancers and lung cancer have proven effective and are currently part of many established treatment modalities.
One tumor-associated antigen that has been identified and targeted for HCC immunotherapy is the membrane-bound heparan sulfate proteoglycan glypican-3 (GPC3). GPC3 is a carcinoembryonic antigen. It is uniquely overexpressed in HCC tumors in 70–81% of HCC patients, and patients with GPC3-positive HCCs have been shown to have markedly lower 5-year survival rates compared with patients with GPC3-negative HCCs. GPC3 is thus linked to a poor prognosis [29, 30]. The feasibility of GPC3 targeting for antibody or cell-based immunotherapy has been investigated in a number of studies. Regarding its use as a target for antibody therapies, data from Ishiguro et al. suggest that treatment with a humanized GPC3 antibody enhances the susceptibility of HCC to chemotherapy [31]. In preclinical studies, the combination of a humanized monoclonal antibody against GPC3 (hGC33) and sorafenib was proven to be more effective in inhibiting tumor progression than sorafenib alone. Phase I and phase II clinical trials of hGC33 for HCC are currently underway. The first trial is a monotherapeutic Phase II trial of hGC33 in patients with advanced or metastatic HCC (http://clinicaltrials.gov/ct2/show/NCT01507168). The second is a Phase I trial that aims to further elucidate the therapeutic benefit of combination therapy with hGC33 and sorafenib (http://clinicaltrials.gov/ct2/show/NCT00976170). Despite these initial investigations into the therapeutic benefits of anti-GPC3 antibodies, there remains a need to identify additional clinically targetable antigens, and much effort has been expended to identify and characterize HCC tumor antigens such as the “cancer/testis” antigens, which have been reported to have a unique expression profile in HCC tumors compared with adjacent liver tissues: MAGE-A1 is overexpressed in about 65% of HCCs, MAGE-A3 in about 70% of HCCs, and NY-ESO-1 in approximately 45% of HCCs [32]. However, more research needs to be conducted. Some of the questions that remain to be elucidated are as follows. (1) Which antigens are uniquely expressed by HCC, or are more immunogenic and could be exploited as targets for antibody therapy? (2) Is there an immunological distinction between HCC tumor antigens that are expressed intracellularly, on the cell surface, or secreted? (3) What are the HLA expression profile changes during HCC progression that would facilitate a more targeted immunotherapeutic approach? A better understanding of the antigenic profile of HCC would enable a more refined immunotherapeutic approach that could aid in the strategy of adoptive transfer of tumor-infiltrating lymphocytes (TILs) and the ex vivo priming of patient lymphocytes.
Emerging Strategies for Immune Checkpoint Blockade and Their Potential Relevance to HCC Therapy
Emerging evidence supports the contention that the tumor microenvironment provides protective niches that promote cancer cell evasion of an effective immune response [33]. The complex interactions among tumor cells, stromal cells (fibroblasts, regulatory immune cells, endothelial cells, and pericytes), soluble factors (transforming growth factor β, interleukin-10, and Fas), and membrane-attached molecules may favor tumor escape from the immune response [34]. Any successful host immune response against a tumor requires effective CTL activity. This, however, requires a well-orchestrated balance between positive and negative signals emanating from numerous T-cell coregulatory ligands and receptors.
Overexpression of immune inhibitory molecules in the tumor milieu has been associated with a negative prognosis [35, 36]. Overexpression of PD-L1 (also known as B7-H1 or CD274), which was originally described by Dong and colleagues and is one of the three members of the B7 family of T-cell costimulatory molecules [37], has been correlated with tumor aggressiveness and a poor prognosis [38, 39]. PD-1, a PD-L1 receptor, is expressed by stimulated B cells, T cells, and myeloid cells. The expression profile of PD-L1 is varied and includes dendritic cells, macrophages, T cells, B cells, and nonimmune cells. PD-L1 ligation of PD-1 is an immunosuppressive mechanism that has been usurped by tumor cells to evade immune surveillance (see figs. 3 a, 3b). These findings have led to the suggestion that the PD-L1 expression profile can be utilized as a tumor biomarker, and that therapeutic blockade of PD-L1 in the subset of patients who overexpress PD-L1 may be a viable immune therapeutic strategy. It has also been postulated that abrogation of the PD-L1 interaction may lead to enhanced clearance of HCV and HBV. This therapeutic strategy against PD-L1 could suppress both de novo tumorigenesis and HCC recurrence [40, 41]. The viability of this potential therapeutic approach is supported by findings from a trial of a PD-1-specific antibody (BMS-936558) in a population that included 296 patients with advanced solid tumors: treatment with the anti-PD-1 antibody resulted in objective responses in numerous solid tumors, including non-small cell lung cancer, melanoma, and renal cell cancer [42].
The therapeutic value of blocking the inhibitory signals emanating from the tumor microenvironment was first exploited by developing antibodies designed to block cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). CTLA-4 expression is restricted to CD8+ effector T cells and regulatory CD4+ T cells, and its stimulation regulates the initial amplitude of T-cell activation. CTLA-4 shares the ligands B7.1/CD80 and B7.2/CD86 with the T-cell costimulatory receptor CD28. B7.1/CD80 and B7.2/CD86 are frequently aberrantly expressed by tumors and other cells in the tumor microenvironment. Engagement of CTLA4 diminishes T-cell activation by sequestering the CD28 ligands. Anti-CTLA4 immunotherapy therefore enhances regulatory T-cell function. Ipilimumab, an anti-CTLA4 antibody, has been shown to extend survival of patients with metastatic melanoma, and has been approved by the US Food and Drug Administration for the treatment of advanced metastatic melanoma. However, there remains a need for further exploration of the molecular markers that predict a better therapeutic outcome in this as well as other patient populations [43]. Such therapeutic approaches seek to reconstitute the host immune response; they could prove extremely effective when used in combination with other treatment modalities.
Adoptive Cell Transfer(ACT) as a Therapeutic Strategy and Its Possible Application in HCC Immunotherapy
The adoptive transfer of autologous TILs and/or donor lymphocytes after allogeneic bone marrow or hematopoietic stem cell transplantation has been shown to be effective in the treatment of a number of solid tumors, including metastatic melanoma, neuroblastoma, and renal cell carcinoma [32, 44]. The best results have been achieved in studies employing ACT in combination with other chemotherapeutics or radiotherapeutics [32, 45]. ACT is based on the premise that lymphocytes that have successfully traversed through the tumor microenvironment have a unique ability to recognize the tumor and “home” back to it, and that this propensity can be harnessed by isolating, expanding, and reinfusing a large volume of such cells. First, TILs are extracted from the patient's resected tumor or from blood, and they are assessed for antitumor activity. Cells that possess antitumor activity are subsequently expanded and then infused into the patient after lymphodepletion, allowing them to preferentially home to the tumor and exert their antitumor activity. In an alternate strategy, cells can also be isolated from a suitable donor and subsequently engineered to recognize and eliminate tumor cells.
Current state-of-the-art ACT therapies utilize genetically engineered T-cell receptors with potent antitumor activity. Besides the benefit provided by infusion of a high number of tumor-specific T cells, an additional advantage of ACT therapy compared with other immunotherapeutic approaches is that the ex vivo activation of these cells avoids the immunosuppressive influence of the tumor microenvironment. When employed synergistically with therapies that eliminate immune suppressor cells such as regulatory T cells, the potency of ACT has been demonstrated to be superior to that of other treatment modalities [46]. ACT immunotherapy for metastatic melanoma treatment has achieved a complete response rate of up to 21.5%, which is superior to that of other approved therapeutics; for example, the chemotherapeutic agent decarbazine induces a 2.7% complete response rate [32]. In another study, 13% of metastatic melanoma patients who underwent ACT therapy after a nonmyeloablative lymphodepleting pretreatment experienced complete durable regression beyond 5 years [32]. ACT therapy combined with radiation therapy achieved complete tumor regression in 22% of previously heavily treated metastatic melanoma patients, in conjunction with an objective response rate of 52–72% depending on the dose of radiation therapy [45, 46]. These and other findings argue for the consideration of the development of ACT therapy for HCC treatment.
Therapeutic Vaccines and HCC Immunotherapy
The therapeutic vaccine approach to cancer immunotherapy, while intellectually appealing, has not been as successful as initially envisioned. The main challenge in advancing therapeutic cancer vaccines has been the development of an immune response against self because cancer cells arise from autologous cells. One approach to cancer vaccine therapy has been to utilize oncolytic viruses engineered to successfully lyse cancer cells and induce an antitumor immune response. While this approach has had mediocre results in the past, emerging research utilizing tumor-associated antigens expressed in a virus vector to immunize the host have been very successful.
The principles of therapeutic cancer vaccines are being elucidated in preclinical studies. Work by Dr. Richard Vile's group, among others, has furthered our molecular understanding of the design of an effective therapeutic cancer vaccine. Utilizing an immunogenic viral vector based on a vesicular stomatitis virus expressing a complete cDNA library constructed using RNA from normal tissues, Dr. Vile's group were able to induce tumor clearance in mouse models upon immunization [47]. While this approach needs to be further investigated and concerns about the risk of inducing autoimmunity should be addressed, this approach is promising. The antigenic repertoire that will elicit the most effective immunogenic response remains to be elucidated, and this will be crucial for the development of human therapies. As of 2012, there are over 300 clinical vaccine trials registered with NIH (ClinicalTrials.gov). Many of these trial modalities are being tested with other approved treatment protocols. Vaccines developed with mutated KRAS and administered in combination with gemcitabine for pancreatic cancer treatment led to a documented increase in median survival after R1 resection [48]. The expression of antigens that are uniquely or aberrantly expressed by tumor cells or the tumor microenvironment in viral vectors could therefore serve as a natural means of “rebooting” a patient's immune system.
Finally, recent immunotherapeutic strategies that seek to boost the immune system and specifically induce local inflammation by the infusion of cytokines, interferons and interleukins, have been successful [49, 50]. The premise of this approach is the use of cytokines as adjuvants. As a proof of principle, Kloess et al. investigated the cytotoxicity of donor-derived natural killer (dNK) cells from patients with stage IV neuroblastoma and reported that dNK cells that were cultured ex vivo in IL-2 had a more aggressive cytotoxic phenotype [51]. A phase I/II trial utilizing haploidentical stem cell transplantation in combination with the infusion of IL-2-activated allogeneic dNK cells is currently underway in high-risk stage IV neuroblastoma patients. The utilization of cytokines as adjuvants to established HCC treatment protocols could prove productive.
Concluding Remarks
HCC is an increasing cause of cancer-related mortality in the US, and the incidence of HCC is projected to increase in the years to come [52]. Current therapy for advanced HCC is grossly inadequate and as such further investigation into novel therapeutic approaches is necessary. The development of more animal models that closely reflect the different HCC subtypes would prove invaluable. The phenomenon of spontaneous HCC regression is well documented and current therapies, including the antiangiogenic multikinase inhibitor sorafenib and hepatic arterial chemoembolization, may contribute to disease stabilization in part through the induction of antitumor immune responses. Such therapeutic approaches take advantage of the strong association between tumor hypoxia and spontaneous HCC regression. However, another observation that has been documented in spontaneous HCC regression, namely systemic inflammation, remains to be effectively exploited. Combination therapeutic modalities that exploit both potential mechanisms may prove more efficacious. In order to develop an immunocentric therapeutic approach, further studies of the natural tolerogenicity of the liver are needed to help design immune therapies that can neutralize inhibitory molecules and mechanisms within the liver. The addition of anti-PD-L1, anti-PD-1, and anti-CTLA4 to current and future therapeutic modalities may enhance outcomes in HCC. Because lymphocytes must receive adequate costimulation from the tumor itself or from professional APCs that present tumor antigens in order to elicit an effective immune response, mechanisms that enhance antigen presentation in peripheral lymph nodes may also potentially be of value, perhaps through the use of systemic delivery of antigen-based therapies. Finally, it is critical to understand the role of the tumor environment in tumor immune escape from surveillance and to develop strategies to counteract this phenomenon.
Acknowledgements
We would like to dedicate this review article to the memory of Michael Bykov who on 6–30-2012 lost his fight against cancer. We would also like to thank Maja Radulovic for her assistance with the figures. This study was supported by grants from the National Institutes of Health to Lewis R. Roberts (CA128633 and CA165076) and Alexander G. Miamen (R25 GM 55252).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocelullar carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Davila JA, El-Serag HB. Racial differences in survival of Hepatocellular carcinoma in the United States: a population-based study. Clin Gastroenterol Hepatol. 2006;4:104–110. [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med. 2008;359:4. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. Epub 2008 Dec 16. [DOI] [PubMed] [Google Scholar]
- 9.Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford) 2012;14:500–505. doi: 10.1111/j.1477-2574.2012.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone naïve prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 11.Davis JP. Experience with hepatitis A and B vaccines. Am J Med. 2005;118:7s–15S. doi: 10.1016/j.amjmed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, et al. Hepatitis B vaccination and Hepatocellular carcinoma rates in boys and girls. JAMA. 2000;284:3040–3042. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- 13.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: systemic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 14.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-ativated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 21:884–890. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jager E. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA. 2012;109:5797–5802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, et al. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc. Natl. Acad. Sci. 2008;105:1650–55. doi: 10.1073/pnas.0707140104. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulla NE, Ninan MJ, Markowitz AB. Rituximab: current status as therapy for malignant and benign hematologic disorders. BioDrugs. 2012;26:71–82. doi: 10.2165/11599500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Foon KA, Mehta D, Lentzsch S, Kropf P, Marks S, Lenzner D, Pietragallo L, Sulecki M, Tarhini A, Boyiadzis M. Long-term results of chemoimmunotherapy with low-dose fludarabine, cyclophosphamide and high-dose rituximab as initial treatment for patients with chronic lymphocytic leukemia. Blood. 2012;119:3184–3185. doi: 10.1182/blood-2012-01-408047. [DOI] [PubMed] [Google Scholar]
- 20.Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, et al. Phase II trial of a Toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 22.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, et al. Phase I trial of Toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non-Hodgkin's lymphoma. Clin Cancer Res. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Girardi M, McAuley S, Schmalbach T. Cutaneous T-cell lymphoma (CTCL) responses to a TLR9 agonist CPG immunomodulator (CPG 7909), a phase I study. J Clin Oncol. 2004;22(14S):6600. [Google Scholar]
- 24.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 25.Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong T. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation. 2006;82:1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]
- 26.Herkel J, Jagemann B, Wiegard C, Lazaro JFG, Lueth S, Kanzler S, Blessing M, Schmitt E, and Lohse AW. MHC Class II-Expressing Hepatocytes Function as Antigen-Presenting Cells and Activate Specific CD4 T Lymphocytes. Hepatology. 2003;27:1079–1085. doi: 10.1053/jhep.2003.50191. [DOI] [PubMed] [Google Scholar]
- 27.Crispe IN, Giannandrea M, Klein I, John B, Sampson B. Wuensch: Cellular and molecular mechanisms of liver tolerance. 2006. Immunologic Reviews. 213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 28.Gurtsevitch VE. Human oncogenic viruses: Hepatitis B and hepatitis V viruses and their role in hepatocarcinogenesis. Biochemistry (Mosc) 2008;73:504–513. doi: 10.1134/s0006297908050039. [DOI] [PubMed] [Google Scholar]
- 29.Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, et al. Glypican-3 expression is correlated with poor prognosis in Hepatocellular carcinoma. Cancer Sci. 2009;100:1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16–25. doi: 10.1016/s0006-291x(03)00908-2. [DOI] [PubMed] [Google Scholar]
- 31.Ishiguro T, Kinoshita Y, Sugimoto M, et al. Anti-glypican3 antibody for treatment of human liver cancer. Proceedings of the 101st annual meeting of the AACR; 2010 April 17-21; Washington, DC.
- 32.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer-what clinicians need to know. Nat. Rev. Clin. Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Lienard D, Cerottini JC, Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen- experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RH, Kwon ED. Significance of B7-H1 overexpression in kidney cancer. Clin Genitourin Cancer. 2006;5:206–211. doi: 10.3816/CGC.2006.n.038. [DOI] [PubMed] [Google Scholar]
- 36.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;15:971–979. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61:101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 40.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 41.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian S, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:26. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodi FS, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottke, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med. 2011;17:854–859. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muscarella P, Wilfong LS, Ross SB, Richards DA, Raynov J, Fisher WE, Flynn PJ, Whiting SH, Rosemurgy A, Harrell FE, Jr, Mercaldo ND, Kosten S, Quiring J, Speyer S, Richman J, Ferraro J, Coeshott C, Cohn A, Rodell TC, Apelian D. A randomized, placebo controlled, double blind, multicenter phase 2 adjuvant trial of the efficacy, immunogeneicity, and safety of GI-4000 plus Gem vs Gem alone in patients with resected pancreas cancer with activating Ras mutations/survival and immunology analysis of the R1 Subgroup. J Clin Oncol. 30(Suppl) abstr #e14501, 2012. [Google Scholar]
- 49.Hofbauer GF, Baur T, Bonnet MC, Tartour E, Burg G, Berinstein NL, Dummer R. Clinical Phase I intratumoral administration of two recombinant ALVAC canarypox viruses expressing human granulocyte-macrophage colony-stimulating factor or IL-2: the transgene determines the composition of the inflammatory infiltrate. Melanoma Res. 2008;18:104–111. doi: 10.1097/CMR.0b013e3282f702cf. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg SA, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 51.Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, Passweg J, Klingebiel T, Schwabe D, Koehl U. IL-2 activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J ImmunoI. 2010;40:3255–3267. doi: 10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention's Recommendations and Reports August 17. 2012;61(RR04):1–18. [Google Scholar]